Abstract
Carnivorous plants have evolved modified leaves into the traps that assist in nutrient uptake from captured prey. It is known that the traps of carnivorous plants usually have lower photosynthetic rates than assimilation leaves as a result of adaptation to carnivory. However, a few recent studies have indicated that photosynthesis and respiration undergo spatio-temporal changes during prey capture and retention, especially in the genera with active trapping mechanisms. This study describes the spatio-temporal changes of effective quantum yield of photochemical energy conversion in photosystem II (ΦPSII) in response to ant-derived formic acid during its capture and digestion.
Key words: action potential, carnivorous plants, formic acid, photosynthesis, respiration, animal-plant interaction
Carnivorous plants have evolved their leaves into the modified structures called traps, which assist in nutrient uptake from prey bodies.1 The traps attract, catch and digest the animal prey; however, some species obtain substantial amount of nutrients from leaf litter (Nepenthes ampullaria), algae (Utricularia) or from faeces of tree shrew Tupaia montana (Nepenthes lowii, N. rajah, N. macrophylla) as a result of adaptive radiation with regard to nitrogen sequestration.2–5 Carnivorous plants are mainly restricted to sunny, moist and nutrient-poor environment, because only in this environment would the cost of producing traps be lower than the benefits gained from prey.6 From carbon metabolism point of view, the benefit is in term of increased rate of photosynthesis per unit leaf mass as a result of increased nitrogen concentration in the leaf or an increase in the total leaf mass that can be supported.6–8 The costs of carnivory include reduced rate of net photosynthesis (AN) in traps as a result of leaf adaptation to carnivory or increased rate of respiration (RD) as a result of extra energy requirements for attracting, capturing and digesting the prey.9 Whereas the reduced AN in the traps has been confirmed several times, the higher RD in traps is still ambiguous.9–12
Until now studies assessing the cost of carnivory have usually been confined to measurements of AN and RD in carnivorous traps vs. non-carnivorous leaves, to the construction costs and payback times for carnivorous organs or to the carbon costs of sticky mucilage secretion by glands.9–16 There is a growing body of evidences that prey-catching is active process involving spatio-temporal changes in AN and RD in traps, at least in carnivorous plants with active trapping mechanisms.17 First evidence, however not convincing, came from the work of Knight.9 She found that bladders of aquatic bladderwort Utricularia macrorhiza had a slightly greater RD (10%) than assimilation leaves, but these differences were not significant. Later Adamec found that RD of bladders in six Utricularia species was 75–200% greater than that in the leaves.18 The action of Utricularia bladder is one of the fastest movement in plant kingdom. When the trap of Utricularia is set, ready for trapping, it looks shrunken due to negative hydrostatic pressure. When the trapdoor is stimulated by prey it opens, sucks the water with prey and the door rapidly shuts. This firing process takes about 30 ms. Then the bladder restores its negative hydrostatic pressure by the removal of water from trap lumen through the glands. The resetting of bladders is a respiration-dependent process accompanied by the consumption of ATP.1,19,20 Adamec suggests that the bladders of Utricularia were in post-firing state and were therefore pumping water and is possible that their RD in this state was much higher than in their resting state.18 Adaptative changes in cytochrome c oxidase in the genus Utricularia may provide respiratory power for bladder function.21 The most famous carnivorous plant the Venus flytrap (Dionaea muscipula) also uses active trapping mechanism for prey capture. Recently, Hájek and Adamec published that the traps of D. muscipula had lower AN, whereas the RD in lamina and trap was comparable.12 This is in accordance with the classical interpretation of cost/benefit model of carnivory. However, in our previous study we have shown that trigger hair irritation in the open as well as in closed trap of Dionaea muscipula resulted in the rapid increase of RD and decrease of effective quantum yield of photochemical energy conversion in photosystem II (ΦPSII).17 We have suggested that this is a result of generation of action potentials upon trigger hair irritation.22–25
The link between electrical signals and inhibition of photosynthesis and stimulation of respiration has been described in several plant species, however it has not been known in carnivorous plants.26–30 Another genus of carnivorous plants that generates action potentials in response to mechanical irritation is sundew (Drosera). In the Dionaea traps, the action potential originates in any one of the six trigger hair and the potential propagates over the entire trap blade more rapidly across the lower (abaxial) surface. In the Drosera tentacle, action potentials are initiated by a receptor potential just below the swollen head of the tentacle and propagate only to its base and do not reach the leaf lamina.31–33 This is in accordance with the results that separated D. prolifera tentacles have many times greater RD in comparison with that of leaf lamina. This proves a very high metabolic and physiological activity of tentacles probably as a results of electrical irritability.11 It has been suggested that at least some of the energy connected with the rise of RD after action potential is utilized for the restoration of the state of ionic balance (i.e., restore the resting state).26 Except the electrical signals, chemical substances seem to be also effective in effecting photosynthesis in carnivorous plants. This study describes negative impact of ant-derived formic acid on effective quantum yield of photochemical energy conversion in PSII (ΦPSII), which is a sensitive indicator of plant photosynthetic performance.
We measured the chlorophyll fluorescence in response to prey capture (ant Lasius niger L.) by the leaf of Drosera capensis L. Ten one-year-old Drosera capensis L plants were grown in growth chamber at a irradiance 200 µmol m−2 s−1 photosynthetic active radiation (PAR), 14/10 h day/night cycle and daily temperature ∼25°C. Before the measurement, the plant was adapted to light intensity 100 µmol m−2 s−1 PAR for 10 minutes (time required for steady state values of chlorophyll fluorescence in light-adapted state, Ft). The actinic light was provided by fluorcam FC-1000 LC (Photon System Instruments, Czech Republic) using red emitting LED diodes (λ = 620 nm). The experiment started by application of first saturation pulse (4,000 µmol m−2 s−1 PAR, 800 ms duration, λ = 620 nm). Then (after 10 seconds) one ant (Lasius niger) was gently put on the D. capensis leaf. During the first two hours, saturation pulses were applied every three minute, thereafter every hour and later every 24 hour. After each saturation flash the visible pictures were taken by camera Nikon D60 (Nikon, Thailand). The ΦPSII, which indicates the proportion of the light absorbed by chlorophyll associated with photosystem II that is used in photochemistry, was calculated as (Fm′ - Ft)/Fm′.34,35 The experiment was repeated without ant's abdomen (the abdomen was cut by scalpel but ants survive and their moving was not affected). In the last experiment 1 µL 15 M formic acid (Fluka) was dropped on the leaf. All experiments were repeated four times independently and data presented are representative.
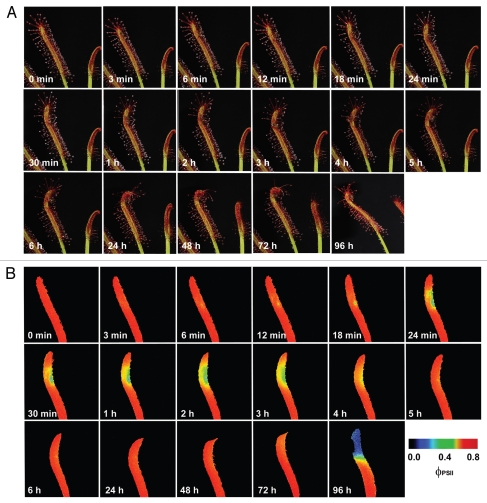
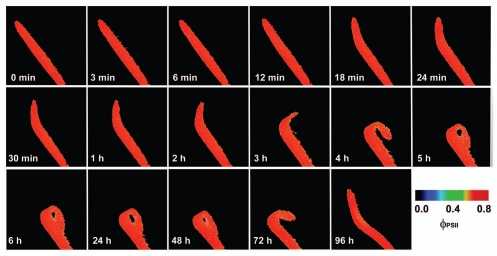
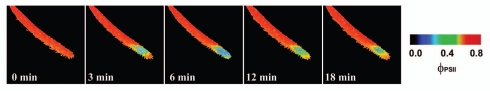
The inhibition of ΦPSII occurred within a few minutes after the ant was trapped by Drosera tentacles and then again after 96 hours (Fig. 1). Repeating the experiment without ant's abdomen had no negative impact on ΦPSII in spite of ant-induced leaf folding (Fig. 2). The most common substance in ant's abdomen is formic acid.36 This indicates that the inhibition of ΦPSII was caused first by the spraying of the formic acid on the leaf by the struggling ant and then by releasing the formic acid after 96 hours from ant's abdomen as a result of digestion. Therefore, the preliminary observation in Drosera mentioned at the end of discussion of our previous study was not associated with electrical signals.17 The inhibition was caused by the ant Lasius niger, which inhibits the ΦPSII in D. capensis by releasing the formic acid from its abdomen. This is consistent with the findings that propagation of action potentials in Drosera is restricted only to the tentacles and therefore had no effect on photosynthesis in leaf blade (Fig. 2). Further, application of 1 µL 15 M formic acid resulted in very similar effect like the living ant with intact abdomen (Fig. 3). The concentration of formic acid was chosen according to data that the venom of Formica rufa contains 5–17 M formic acid.36
Figure 1.
The visible response (A) and the response of effective quantum yield of photochemical energy conversion in photosystem II (ΦPSII, B) of Drosera capensis leaf to prey capture (intact ant Lasius niger). The ant was put on the leaf in time 10 seconds.
Figure 2.
The response of effective quantum yield of photochemical energy conversion in photosystem II (ΦPSII) of Drosera capensis leaf to prey capture (ant Lasius niger without abdomen). The ant without abdomen was put on the leaf in time 10 seconds. Note that no changes in ΦPSII occurred in spite of leaf folding.
Figure 3.
The response of effective quantum yield of photochemical energy conversion in photosystem II (ΦPSII) of Drosera capensis leaf to 1 µL of 15 M formic acid. The drop of formic acid was put on the leaf in time 10 seconds.
The production of formic acid by ants is thought to have evolved to improve capture of invertebrate prey and aid colony defence.37 Some examples document that negative effect of ant-derived formic acid on plant growth is not novel, but it has not been described in carnivorous plants previously. It is known that ants Myrmelachista schumanni use formic acid as a herbicide. The ants live inside the hollow stems of Duroia hirsuta, kill all plants other than their host plant by injecting formic acid into the leaves. By killing these others plants, the ants gain more nest sites and they create a single species stand of plants.38 Also, weaver ants (Oecophylla smaragdina) damage mango fruit by deposition of formic acid as a result of fighting between weaver colonies.39
The mechanism of action is very similar to the well known herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). Formic acid causes significant inhibition of the electron transfer on the acceptor side of photosystem II, particularly from plastoquinone A to plastoquinone B.40,41 From the data analysis of carnivorous plants published recently the genera with the highest proportions of ants in their diets are Brocchinia (90%), Nepenthes (73%) and Sarracenia (55%).42 All the mentioned genera have pitcher traps, with the permanent level of digestive fluid, in which the formic acid is diluted during digestion and thus the pitchers are probably prevented against its toxic effect. Captures of ants is much less frequent for sticky traps of Drosera (3.4%) and Pinguicula (0.5%); however it may have deteriorate effect.
Carnivorous plants are not just killers but are a fascinating group of plant. They do not just eat the animals but may form a complicated social network with them. Complicated animal-plant interaction, as has been described e.g. between carnivorous pitcher plant Nepenthes bicalcarata and ants may have direct impact on physiological processes similar as a well known relationship between acacia and ants.43 The possible impact of formic acid of ant species co-occurring with carnivorous plants in their natural habitat on photosynthesis remains to be elucidated.
Acknowledgements
This work was supported by grant VEGA 1/0040/09 from the Scientific Grant Agency of the Ministry of Education of the Slovak Republic.
Abbreviations
- AN
rate of net photosynthesis
- PAR
photosynthetic active radiation
- RD
rate of dark respiration
- ΦPSII
effective quantum yield of photochemical energy conversion in photosystem II
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11906
References
- 1.Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London, UK: Academic Press; 1989. [Google Scholar]
- 2.Moran JA, Clarke CM, Hawkins BJ. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. Int J Plant Sci. 2003;164:635–639. [Google Scholar]
- 3.Peroutka M, Adlassnig W, Volgger M, Lendl T, Url WG, Lichtscheidl IK. Utricularia: a vegetarian carnivorous plant? Plant Ecol. 2008;199:153–162. [Google Scholar]
- 4.Clarke CM, Bauer U, Lee CC, Tuen AA, Rembold K, Moran JA. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biol Lett. 2009;5:632–635. doi: 10.1098/rsbl.2009.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin L, Moran JA, Clarke C. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytol. 2010 doi: 10.1111/j.1469-8137.2009.03166.x. [DOI] [PubMed] [Google Scholar]
- 6.Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. Am Natur. 1984;124:479–497. [Google Scholar]
- 7.Farnsworth EJ, Ellison AM. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. J Ecol. 2008;96:213–221. [Google Scholar]
- 8.Pavlovic A, Singerová L, Demko V, Hudák J. Feeding enhances photosynthetic efficiency in the carnivorous pitcher plant Nepenthes talangensis. Ann Bot. 2009;104:307–314. doi: 10.1093/aob/mcp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight S. Costs of carnivory in the common bladderwort, Utricularia macrorhiza. Oecologia. 1992;89:348–355. doi: 10.1007/BF00317412. [DOI] [PubMed] [Google Scholar]
- 10.Pavlovic A, Masarovicová E, Hudák J. Carnivorous syndrome in Asian pitcher plants of the genus Nepenthes. Ann Bot. 2007;100:527–536. doi: 10.1093/aob/mcm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamec L. Dark respiration of leaves and traps of terrestrial carnivorous plants: are there greater energetic costs in traps? Cent Eur J Biol. 2010;5:121–124. [Google Scholar]
- 12.Hájek T, Adamec L. Photosynthesis and dark respiration of leaves of terrestrial carnivorous plants. Biologia. 2010;65:69–74. [Google Scholar]
- 13.Thorén LM, Tuomi J, Kåmåråinen T, Laine K. Resource avaibility affects investment in carnivory in Drosera rotundifolia. New Phytol. 2003;159:507–511. doi: 10.1046/j.1469-8137.2003.00816.x. [DOI] [PubMed] [Google Scholar]
- 14.Osunkoya OO, Daud SD, Di-Giusto B, Wimmer FL, Holige TM. Construction costs and physico-chemical properties of the assimilatory organs of Nepenthes species in northern Borneo. Ann Bot. 2007;99:895–906. doi: 10.1093/aob/mcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osunkoya OO, Daud SD, Wimmer FL. Longevity, lignin content and construction cost of assimilatory organs of Nepenthes species. Ann Bot. 2008;102:845–853. doi: 10.1093/aob/mcn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karagatzides JD, Ellison AM. Construction costs, payback times and the leaf economics of carnivorous plants. Am J Bot. 2009;96:1612–1619. doi: 10.3732/ajb.0900054. [DOI] [PubMed] [Google Scholar]
- 17.Pavlovic A, Demko V, Hudák J. Trap closure and prey retention in Venus flytrap (Dionaea muscipula) temporarily reduces photosynthesis and stimulates respiration. Ann Bot. 2010;105:37–44. doi: 10.1093/aob/mcp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamec L. Respiration and photosynthesis of bladders and leaves of aquatic Utricularia species. Plant Biol. 2006;8:765–769. doi: 10.1055/s-2006-924540. [DOI] [PubMed] [Google Scholar]
- 19.Sasago A, Sibaoka T. Water extrusion in the trap bladders of Utricularia vulgaris I. A possible pathway of water across the bladder wall. Bot Mag Tokyo. 1985;98:55–66. [Google Scholar]
- 20.Sasago A, Sibaoka T. Water extrusion in the trap bladders of Utricularia vulgaris II. A possible mechanism of water outflow. Bot Mag Tokyo. 1985;98:113–124. [Google Scholar]
- 21.Laakkonen L, Jobson RW, Albert VA. A new model for the evolution of carnivory in the bladderwort plant (Utricularia): Adaptive changes in cytochrome c oxidase (COX) provide respiratory power. Plant Biol. 2006;8:758–764. doi: 10.1055/s-2006-924459. [DOI] [PubMed] [Google Scholar]
- 22.Volkov AG, Adesina T, Jovanov E. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal Behavior. 2007;2:139–145. doi: 10.4161/psb.2.3.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkov AG, Adesina T, Jovanov E. Charge induced closing of Dionaea muscipula Ellis trap. Bioelectrochemistry. 2008;78:16–21. doi: 10.1016/j.bioelechem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Volkov AG, Carrell H, Markin VS. Biologically closed electrical circuits in Venus flytrap. Plant Physiol. 2009;149:1661–1667. doi: 10.1104/pp.108.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkov AG, Carrell H, Markin VS. Molecular electronics of the Dionaea muscipula trap. Plant Signal Behav. 2009;4:353–354. doi: 10.4161/psb.4.4.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dziubinska H, Trêbacz K, Zawadzki T. The effect of excitation on the rate of respiration in the liverwort Conocephalum conicum. Physiol Plant. 1989;75:417–423. [Google Scholar]
- 27.Koziolek C, Grams TEE, Schreiber U, Matyssek R, Fromm J. Transient knockout of photosynthesis mediated by electrical signals. New Phytol. 2003;161:715–722. doi: 10.1111/j.1469-8137.2004.00985.x. [DOI] [PubMed] [Google Scholar]
- 28.Lautner S, Grams TEE, Matyssek R, Fromm J. Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol. 2005;138:2200–2209. doi: 10.1104/pp.105.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser H, Grams TEE. Rapid hydropassive opening and subsequent active stomatal closure follow heat-induced electrical signals in Mimosa pudica. J Exp Bot. 2006;57:2087–2092. doi: 10.1093/jxb/erj165. [DOI] [PubMed] [Google Scholar]
- 30.Grams TEE, Lautner S, Felle HH, Matyssek R, Fromm J. Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ. 2009;32:319–326. doi: 10.1111/j.1365-3040.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 31.Williams SE, Pickard BG. Receptor potentials and action potentials in Drosera tentacles. Planta. 1972;103:193–221. doi: 10.1007/BF00386844. [DOI] [PubMed] [Google Scholar]
- 32.Williams SE, Pickard BG. Properties of action potentials in Drosera tentacles. Planta. 1972;103:222–240. doi: 10.1007/BF00386845. [DOI] [PubMed] [Google Scholar]
- 33.Williams SE, Pickard BG. The role of action potentials in the control of capture movements of Drosera and Dionaea. In: Skoog F, editor. Plant Growth Substances. Berlin-Heidelberg-New York: Springer; 1980. pp. 471–480. [Google Scholar]
- 34.Maxwell K, Johnson GN. Chlorophyll fluorescence-a practical guide. J Exp Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- 35.Rohácek K. Chlorophyll fluorescence parameters: the definition, photosynthetic meaning and mutual relationships. Photosynthetica. 2002;40:13–29. [Google Scholar]
- 36.ÓRourke, Fergus J. Formic acid production among the Formicidae. Ann Entomol Soc Am. 1950;43:437–443. [Google Scholar]
- 37.Bennett ATD, Lloyd MH, Cuthill IC. Ant-derived formic acid can be toxic for birds. Chemoecology. 1996;7:189–190. [Google Scholar]
- 38.Frederickson M, Greene M, Gordon D. Devil's gardens' bedevilled by ants. Nature. 2005;437:495–496. doi: 10.1038/437495a. [DOI] [PubMed] [Google Scholar]
- 39.Peng RK, Christian K. Determination and management of weaver ant Oecophylla smaragdina (Fabricius) (Hymenoptera: Formicidae), marks on mango fruit in the northern Territory of Australia. Int J Pest Manage. 2009;55:27–30. [Google Scholar]
- 40.Xu CH, Taoka S, Crofts AR, Govindjee Kinetic characteristics of formate formic acid binding at the plastoquinone reductase site in spinach thylakoids. Biochim Biophys Acta. 1991;1098:32–40. [Google Scholar]
- 41.Xiong J, Minagawa J, Crofts AR, Govindjee Loss of inhibition by formate in newly constructed photosystem II D1 mutants D1-R257E and D1-R257M, of Chlamydomonas reinhardtii. Biochim Biophys Acta. 1998;1365:473–491. doi: 10.1016/s0005-2728(98)00101-7. [DOI] [PubMed] [Google Scholar]
- 42.Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants—Darwin's ‘most wonderful plants in the world. J Exp Bot. 2009;60:19–42. doi: 10.1093/jxb/ern179. [DOI] [PubMed] [Google Scholar]
- 43.Merbach MA, Zizka G, Fiala B, Merbach D, Booth W, Maschwitz U. Why a carnivorous plants cooperates with an ant—selective defense against pitcher-destroying weevils in the myrmecophytic pitcher plant Nepenthes bicalcarata Hook F. Ecotropica. 2007;13:45–56. [Google Scholar]