Abstract
Plant genomes code for channels involved in the transport of cations, anions and uncharged molecules through membranes. Although the molecular identity of channels for cations and uncharged molecules has progressed rapidly in the recent years, the molecular identity of anion channels has lagged behind. Electrophysiological studies have identified S-type (slow) and R-type (rapid) anion channels. In this brief review, we summarize the proposed functions of the R-type anion channels which, like the S-type, were first characterized by electrophysiology over 20 years ago, but unlike the S-type, have still yet to be cloned. We show that the R-type channel can play multiple roles.
Key words: R-type anion channel, nitrate, sulphate, guard cell, action potential
Anion channels play a central role in signal transduction, nutrient transport and cell turgor regulation.1 By far, their function was particularly well investigated in the guard cells of stomata using a combination of electrophysiological, pharmacological and genetic tools. In this system, anion channel activation was shown to be one of the limiting steps in the loss of cell turgor leading to stomatal closure.2 In algal cells, anion channels were shown to contribute to membrane excitability through the generation of action potential.1,3
With the burst of molecular biology in the nineties, the genes coding for plant ion channels started to be unveiled. The first channel gene to be cloned in plant was the shaker-like potassium channel identified in a yeast functional expression screen.4,5 More than ten years later, TaALMT1 and AtCLCa were characterized as the first members of two important anion channel families.6,7 This growing group of newly identified channels, accounting for electrophysiological activity described long ago, includes the MSLs anion selective mechanosensitive channels.8 Recently, the well known S-type channel has been finally recognized to be encoded by members of the SLAC1 (and other SLAH) family (Slow Anion Channel-Associated 1).9 In agreement with electrophysiological data,10–13 it requires phosphorylation by a Protein Kinase in order to be functional.14,15 In contrast, the molecular identity of the R-type anion channel remains unknown. Therefore, this candidate, which has been functionally known since twenty years, remains the next challenge for plant channel physiologists.
Patch-Clamp Characterization of the R-Type Anion Channel
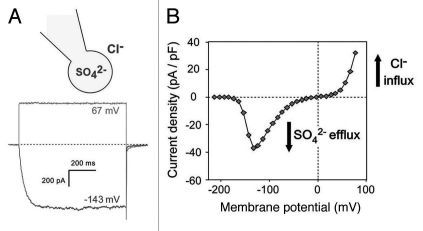
The R-type anion channel name comes from the rapidity to accommodate its gating to the membrane potential. This rapidly activating anion efflux channel presents a current-voltage relationship with a V-shape (Fig. 1). At physiological membrane potentials (−200 to −150 mV), R-type channels are closed while a depolarization elicits channel opening (voltage activation) and thus favoring anion effluxes (Fig. 1). The activation zone is under control of internal nucleotide and external anion concentrations. The voltage-dependent activation/deactivation of the current occur in the milliseconds range and were shown to be triggered by the exit/entry of a nucleotide in the channel pore.16 Another feature of this channel is its steep voltage dependency meaning that any variation in the membrane voltage will be sensed and thus amplified in a feed forward mechanism.
Figure 1.
Fast kinetic activation and voltage dependency of the R-type channel recorded in Arabidopsis suspension cells. (A) Activation kinetics in the hundred millisecond range with a pulse protocol from −213 mV to −143 mV and in the millisecond range with a pulse protocol from −213 mV to +67 mV are observed. (B) I–V curve obtained from a plot of the steady state current elicited by 1.5-s pulses from −213 mV to +77 mV with 10 mV increment, using a holding potential of −213 mV. In the ionic condition used inward current correspond to a sulphate efflux while outward current correspond to chloride influx. Redrawn from reference 27.
As for the majority of plant plasma membrane anion channels, the R-type channel is highly permeable to the common anion, nitrate (NO3−). Interestingly, the bivalent anion sulphate (SO42−), which is usually considered to be weakly permeant through membranes, has a similar permeability in the channel pore. To our knowledge, it is a unique property among plant channels. To a lesser extent the R-type channel is also selective for chloride, carbonate and malate.11 Strikingly, sulphate is not only highly permeant in the pore, but also exerts a strong positive regulatory effect by preventing the run-down of the channel activity. This high sulphate permeability together with this positive regulatory mechanism suggests that the R-type channel could function in the cell sulphate homeostasis. In Vicia faba, except for sulphate which has not been investigated, the rapid anion channel GCAC1 (Guard Cell Anion Channel 1) presents the same permeability sequence as its Arabidopsis counterpart.17
A second type of anion channel with slow activation/deactivation kinetics (S-type) and a weak voltage dependency have also been shown to coexist with the R-type on the plasma membrane of several cell types.18–20 Based on guard cell protoplast characterization, it was proposed that despite different features (gating characteristics, pharmacology) R- and S-type could be two gating modes of a single protein.21 Further studies performed on Arabidopsis hypocotyl protoplasts showed a strong sulphate permeability for the R-type channel while this anion is non-permeant for the S-type. This suggests that the two channels have different pore structures arguing for a different protein for each type. The recent molecular identification of the S-type channel has given genetic proof that the R- and S-type channels are separate entities.9 The Arabidopsis knock out mutants deficient in the SLAC channel still retain the R-type channel activity in the guard cells.
R-Type Channel Activity was Monitored in All Plant Cells
With the adaptation of the patch clamp technique to plant protoplasts, the R-type channel activity was first described by Keller and coworkers in Vicia faba guard cells.22 This opened the door to numerous studies leading to a detailed characterization of the channel regulations.22 Later on, the R-type channel was characterized on the plasma membrane of tobacco suspension cells where it was shown to be regulated by auxin.24 With the emergence of Arabidopsis as a model, R-type was localized and characterized in protoplasts generated from hypocotyl cells,25 from epidermal cells of the root growing apex,23 from epidermal root cells of the elongation zone26 and from the guard cells.12 Additionally, cell suspension culture derived from young Arabidopsis seedlings has proven to be a suitable system for studying R-type channel functions.27
Many Putative Functions for a Single Channel Activity
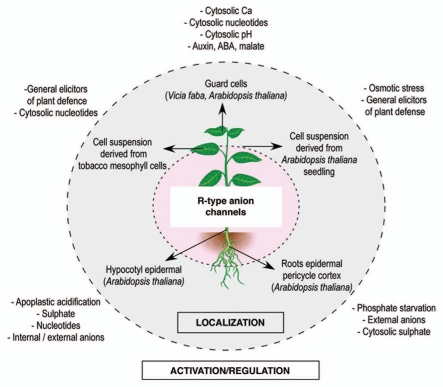
The multiple locations of the channel raise the question whether it might have different roles according to the tissue, or if it has a fundamental but general common function in the whole plant. In order to address this question we have reviewed the proposed functions and regulation elements of the R-channel according to the organ and plant type in which it was characterized (Fig. 2).
Figure 2.
Multiple localization and activation/regulation modes of the R-type anion channel.
R-Type Channel in Guard Cells
Different effectors such as malate, CO2 or auxin have been shown to modulate the voltage-dependency of R-type channel and for some of them the stomata aperture.17,28 More recently, Levchenko and coworkers reported that the Vicia faba R-type channel is activated by ABA without preceding Ca2+ signal.29 Genetic data coming from the patch-clamp study of mutants impaired in ABA signal transduction30 or of the mutant slac19, suggested that the S-type anion channel is more clearly involved in stomata closing31 while no real function has been assigned to the R-type channel belonging to this cell type.
R-Type Channel Function in Root
Plant roots not only absorb nutrients and water, but also actively interact with the rhizosphere. Plants release inorganic and organic compounds to modify the root environment and increase the availability of some nutrients (e.g., P), detoxify elements (e.g., Al) and also alter the rhizosphere population of microorganisms. Organic acids such as citrate and malate are excreted by roots to chelate the Ca2+, Fe3+ and Al3+ which form insoluble P compounds. This chelation process increases P availability to plants. Diatloff and coworkers showed that an R-type channel was responsible for this organic acid efflux and that this channel was specifically detected when roots were starved of P.26 In addition, a separate R-type channel was shown to be responsible for the efflux of inorganic ions such as sulphate, chloride and nitrate. This futile cycling of nutrients has puzzled plant scientists who have only been able so far to speculate at the physiological relevance of this efflux. The cloning of genes coding for this interesting R-type channel will allow the physiological relevance of nutrient efflux from roots to be studied in more detail.
R-Type Channel Function during Pathogen Interaction
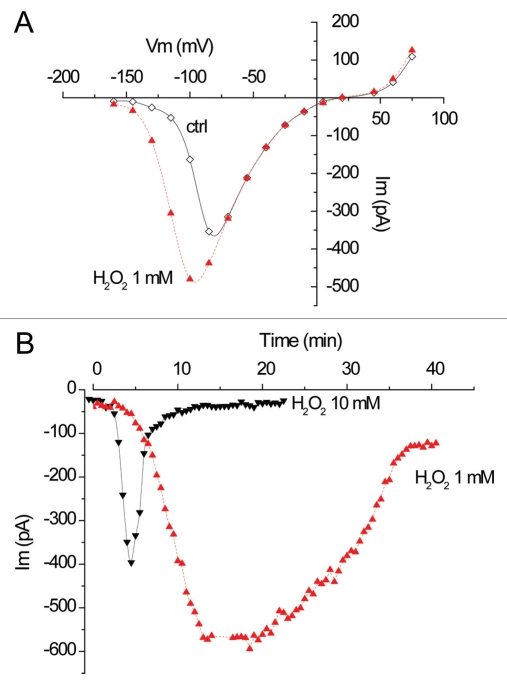
Activation of anion efflux in plants in response to pathogens or to elicitors derived from pathogens has been known for many years,32–34 but no anion channel has been identified as participating to this early response. Using a pharmacological approach on the Arabidopsis cell suspension system, we recently proposed that the R-type channel could be a major player in the signaling pathway leading to ROS (Reactive Oxygen Species) emission in response to PAMP (pathogen-associated molecular patterns) perception.27 This ROS production has among other an antimicrobial effect, limiting pathogen development and favoring plant cell survival during challenging. In such a system where R-type channel activity influence ROS production we might expect that the channel is itself regulated by ROS. Additional results obtained in the laboratory on the R-type channel of hypocotyl cells show that exogenous H2O2 (Fig. 3A) shifts the voltage threshold activation towards negative potential. For a holding potential of −130 mV, close to the resting potential of a plant membrane, the outward current will thus increase upon H2O2 treatment, enhancing anion efflux and membrane depolarization (Fig. 3B). This regulation is transient and the initial voltage dependency is recovered after 20–40 min. These data reinforce the link between ROS and R-type channel and even indicate a transient activating loop between these two components. Note that this regulation is cell type specific: it could be observed in hypocotyl cells but not in guard cells from A. thaliana.12
Figure 3.
Effect of exogenous application of H2O2 on the R-type anion channel in Arabidopsis hypocotyl protolasts. (A) H2O2 shifts the activation potential of the fast anion channel current. Representative I–V curves of the fast anion current before (◊) and 15 min after ( ) treatment with 1 mM H2O2. The potential for half maximal activation was shifted towards negative potentials by −20 ± 5 mV (n = 5) and −13 ± 7 mV (n = 4) upon treatment with 1 and 10 mM H2O2, respectively. From a holding potential of −160 mV, 0.8 sec depolarizing pulses were applied to the hypocotyl protoplast membrane with an increment of −15 mV. (B) Amplitude of fast anion current at −130 mV after treatment with 1 (
) treatment with 1 mM H2O2. The potential for half maximal activation was shifted towards negative potentials by −20 ± 5 mV (n = 5) and −13 ± 7 mV (n = 4) upon treatment with 1 and 10 mM H2O2, respectively. From a holding potential of −160 mV, 0.8 sec depolarizing pulses were applied to the hypocotyl protoplast membrane with an increment of −15 mV. (B) Amplitude of fast anion current at −130 mV after treatment with 1 ( ) or 10 mM (▾) H2O2 (representative examples). Upon treatment with 1 mM H2O2, a maximal shift was observed after 20 min and recovery was almost complete after 40 min (n = 3). With 10 mM H2O2, maximal shift was observed after 10 min and full recovery was observed after 20 min (n = 2). Experimental conditions: solutions as in reference 16. The pipette solution contained 10 mM ATP. Experiments were performed under continuous bath perfusion with 1 M KCl bath reference electrode.
) or 10 mM (▾) H2O2 (representative examples). Upon treatment with 1 mM H2O2, a maximal shift was observed after 20 min and recovery was almost complete after 40 min (n = 3). With 10 mM H2O2, maximal shift was observed after 10 min and full recovery was observed after 20 min (n = 2). Experimental conditions: solutions as in reference 16. The pipette solution contained 10 mM ATP. Experiments were performed under continuous bath perfusion with 1 M KCl bath reference electrode.
R-Type Channel and Cellular Homeosatsis
The fast anion channel (GCAC1) of Vicia faba guard cells requires intracellular nucleotides for its activity.35 Nucleotides providing voltage-independent regulation have been proposed to be allosteric regulators of the channel protein. A different mode of regulation by ATP through cytosolic protein phosphorylation has been described for the voltage-dependent anion channel of tobacco-cell suspension.24 A kinase inhibitor turns the channel into a voltage-independent mode indicating that phosphorylation controls the voltage dependence of the tobacco channel. In Arabidopsis thaliana hypocotyl cells, nucleotides are also required to maintain the steep voltage dependence of the channel25 but, in contrast to tobacco cells, ADP and non-hydrolysable nucleotides are also effective, indicating that phosphorylation is not involved.36 This biophysical mechanism for the gating of the R-type channel by nucleotides was elucidated by Colcombet and coworkers.16 The voltage-dependent closure of the channel at hyperpolarized membrane potentials results from voltage-dependent occlusion of the channel pore by intracellular nucleotides. All these studies provide a link between R-type channel and cell nucleotide status and therefore couple the metabolic status of the cell and its membrane excitability. Interestingly, in response to a decrease in the metabolic charge of the cell under hypoxia, a depolarization signal would be generated.37
Is R-Type Channel a Component of Fast Electrical Signaling?
Action potential (AP) which has been extensively studied in animal nerve cells has also been observed in various plant species such as Helianthus, Lupinus, Lycopersicon and Mimosa.38 In fact, APs seem to be present in any type of plant ranging from herbaceous monocot to trees and algae. In 2001, AP was described for the first time in Arabidopsis.39 In the giant algae, Chara, the different conductance steps involved in AP have been elucidated.3 A calcium entry is the initiating event of AP then an activation of anion conductance would produce the depolarization phase and the repolarization would be due, as for nerve cells, to an increase of potassium conductance. Up to now ion channels involved in the genesis and propagation of the plant AP are unknown but they should present characteristics and current-voltage (I–V) shape close to those encountered in excitable animal cells. The I–V relationship of the sodium current responsible of AP in excitable cells and historically in the squid giant axon shows a V shape which resemble that of the R-type channel. Hence, sodium inward current of animal cells would be equivalent of inward anion current of excitable plant cells. Therefore, the R-type channel is a good candidate to be involved in the depolarizing phase of the AP carried by an anion efflux in plants. Such a hypothesis could be surely verified by the study of AP on mutant deleted in R-type channel, after the molecular identification of the channel.
Looking for Genes Involved in R-Type Channel Conductance
The identification of the genes coding for the R-type channel and corresponding mutants will help us to clarify the channel function in plant cells. Sadly, despite being the first anion channel to be described in plants, the molecular identity of R-type channel is still an enigma. In 2001, the complete genome of Arabidopsis has been published giving the opportunity to scan for gene families coding for proteins with homology with anion channels of animals, fungi or bacteria. Such a targeted approach provides several interesting families like VDACs, ABC transporters or CLCs40 but has been validated by our laboratory for only one channel: AtCLCa.6 Additionally none of the CLC members were reported to be targeted at the plasma membrane. More recently, other candidate families were identified in untargeted approaches. A functional expression screen in xenopus oocytes allowed the identification of the 13 member ALMT family.7 A forward genetic screen based on ozone sensitivity identified SLAC1 as the guard cell Arabidopsis S-type channel.9 SLAC1, with a low homology to dicarboxylate transporter rather than other anion channels, belongs to a five member family.40 Despite the fact that the electrophysiological characteristics of SLAC1 and ALMT1 activities differ significantly of the R-type channel, the R-type channel could be possibly encoded by different members of those two families.
On the other hand, R-type channel may be also encoded by a different family of genes. An alternative could consist therefore in building functional screens in heterologous systems. Classical approaches based on complementation by a cDNA library of yeast mutant lacking a related transporter were successfully applied 20 years ago for potassium channel and led to the discovery of the first AKT1.4,5 For anions, the situation is much different due firstly to the interchangeability of anions, and secondly to possible compensation of inorganic anion by organic acids. The challenge is thus to develop new screening methods in order to identify other channels including the R-type anion channel. Screens have to be based either on the peculiarity of the channel such as the sulphate selectivity of the R-type channel or on performing direct measurements based on channel activity on selected candidate genes.
Screening directly on channel activity is now possible by the use of automated patch machine allowing mid to high throughput. These robots can accurately analyze only standard currents, for this reason, up to now these machines have been validated only for screening new drugs able to modify channel activity.41 In order to overcome this weak point of the patch approach we have designed in our laboratory a screen based on the co-expression in mammalian cells of a ratiometric fluorescent probe which is sensitive to anions42 together with a plant membrane protein. This screen is performed in 96 well plates allowing mid high throughput screen. The candidate genes were selected from an Arabidopsis plasma membrane proteome (www.grenoble.prabi.fr/data/PlantProteomics07/). To date, we have not been able to discover new anion plasma membrane channels/transporters.
R-type anion channel is still keeping its identity secret. With the progress in technology (patch robot), the progress in the knowledge of Arabidopsis, the genetic approaches developed in several laboratories, this rapid-type anion channel might be unpredictably discovered. It is a matter of time!
Acknowledgements
The authors thank the Agence National de la Recherche Genoplante Grant ANR_06_GPLA_012, Julian Schroeder for support of S.T. at UCSD when data in Figure 3 were obtained.
Appendix
In a very recent report Mumm et al (Plant J. 2010; 60:1054–62) showed for the first time that a member of the aluminium activated malate transporter family (AtALMT) is a component of the R-type channel. In plants lacking AtALMT12 R-type anion current in guard cell is reduced when malate is present in the bath medium. Following expression of AtALMT12 in Xenopus oocyte, voltage-dependent anion current reminiscent to R-type channel is activated.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12921
References
- 1.Barbier-Brygoo H, Vinauger M, Colcombet J, Ephritikhine G, Frachisse J, Maurel C. Anion channels in higher plants: functional characterization, molecular structure and physiological role. Biochim Biophys Acta. 2000;1465:199–218. doi: 10.1016/s0005-2736(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Ward JM, Pei ZM, Schroeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourie JI. Transient Cl− and K+ Currents during the action potential in Chara inflata (effects of external sorbitol, cations and ion channel blockers) Plant Physiol. 1994;106:651–660. doi: 10.1104/pp.106.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, et al. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 6.De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 8.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–734. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZH, Hills A, Lim CK, Blatt MR. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J. 2010;61:816–825. doi: 10.1111/j.1365-313X.2009.04108.x. [DOI] [PubMed] [Google Scholar]
- 11.Frachisse JM, Thomine S, Colcombet J, Guern J, Barbier-Brygoo H. Sulfate is both a substrate and an activator of the voltage-dependent anion channel of Arabidopsis hypocotyl cells. Plant Physiol. 1999;121:253–262. doi: 10.1104/pp.121.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt C, Schelle I, Liao YJ, Schroeder JI. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colcombet J, Thomine S, Guern J, Frachisse JM, Barbier-Brygoo H. Nucleotides provide a voltage-sensitive gate for the rapid anion channel of arabidopsis hypocotyl cells. J Biol Chem. 2001;276:36139–36145. doi: 10.1074/jbc.M103126200. [DOI] [PubMed] [Google Scholar]
- 17.Hedrich R, Marten I. Malate-induced feedback regulation of plasma membrane anion channels could provide a CO2 sensor to guard cells. EMBO J. 1993;12:897–901. doi: 10.1002/j.1460-2075.1993.tb05730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colcombet J, Lelievre F, Thomine S, Barbier-Brygoo H, Frachisse JM. Distinct pH regulation of slow and rapid anion channels at the plasma membrane of Arabidopsis thaliana hypocotyl cells. J Exp Bot. 2005;56:1897–1903. doi: 10.1093/jxb/eri184. [DOI] [PubMed] [Google Scholar]
- 19.Frachisse JM, Colcombet J, Guern J, Barbier-Brygoo H. Characterization of a nitrate-permeable channel able to mediate sustained anion efflux in hypocotyl cells from Arabidopsis thaliana. Plant J. 2000;21:361–371. doi: 10.1046/j.1365-313x.2000.00689.x. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder JI, Keller BU. Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA. 1992;89:5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich P, Hedrich R. Interconversion of fast and slow gating modes of GCAC1, a Guard Cell Anion Channel. Planta. 1994;195:301–304. [Google Scholar]
- 22.Keller B, Hedrich R, Raschke K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature. 1989;341:450–453. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiegle E, Gilliham M, Haseloff J, Tester M. Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J. 2000;21:225–229. doi: 10.1046/j.1365-313x.2000.00659.x. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann S, Thomine S, Guern J, Barbier-Brygoo H. An anion current at the plasma membrane of tobacco protoplasts shows ATP-dependent voltage regulation and is modulated by auxin. Plant J. 1994;6:707–716. [Google Scholar]
- 25.Thomine S, Zimmermann S, Guern J, Barbier-Brygoo H. ATP-Dependent regulation of an anion channel at the plasma membrane of protoplasts from epidermal cells of Arabidopsis hypocotyls. Plant Cell. 1995;7:2091–2100. doi: 10.1105/tpc.7.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diatloff E, Roberts M, Sanders D, Roberts SK. Characterization of anion channels in the plasma membrane of Arabidopsis epidermal root cells and the identification of a citrate-permeable channel induced by phosphate starvation. Plant Physiol. 2004;136:4136–4149. doi: 10.1104/pp.104.046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colcombet J, Mathieu Y, Peyronnet R, Agier N, Lelievre F, Barbier-Brygoo H, et al. R-type anion channel activation is an essential step for ROS-dependent innate immune response in Arabidopsis suspension cells. Functional Plant Biology. 2009;36:832–843. doi: 10.1071/FP09096. [DOI] [PubMed] [Google Scholar]
- 28.Raschke K. Alternation of the slow with the quick anion conductance in whole guard cells effected by external malate. Planta. 2003;217:651–657. doi: 10.1007/s00425-003-1034-3. [DOI] [PubMed] [Google Scholar]
- 29.Levchenko V, Konrad KR, Dietrich P, Roelfsema MR, Hedrich R. Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proc Natl Acad Sci USA. 2005;102:4203–4208. doi: 10.1073/pnas.0500146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol. 2006;9:654–663. doi: 10.1016/j.pbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt C, Schroeder JI. Anion Selectivity of slow anion channels in the plasma membrane of guard cells (large nitrate permeability) Plant Physiol. 1994;106:383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nurnberger T, Colling C, Hahlbrock K, Jabs T, Renelt A, Sacks WR, et al. Perception and transduction of an elicitor signal in cultured parsley cells. Biochem Soc Symp. 1994;60:173–182. [PubMed] [Google Scholar]
- 33.Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, et al. Early events induced by the elicitor cryptogein in tobacco cells: Involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendehenne D, Lamotte O, Frachisse JM, Barbier-Brygoo H, Pugin A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell. 2002;14:1937–1951. doi: 10.1105/tpc.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz-Lessdorf B, Lohse G, Hedrich R. GCAC1 recognizes the pH gradient across the plasma membrane: a pH-sensitive and ATP-dependent anion channel links guard cell membrane potential to acid and energy metabolism. Plant J. 1996;10:993–1004. [Google Scholar]
- 36.Thomine S, Guern J, Barbier-Brygoo H. Voltage-dependent anion channel of Arabidopsis hypocotyls: nucleotide regulation and pharmacological properties. J Membr Biol. 1997;159:71–82. doi: 10.1007/s002329900270. [DOI] [PubMed] [Google Scholar]
- 37.Saint-Ges V, Roby C, Bligny R, Pradet A, Douce R. Kinetic studies of the variations of cytoplasmic pH, nucleotide triphosphates (31P-NMR) and lactate during normoxic and anoxic transitions in maize root tips. Eur J Biochem. 1991;200:477–482. doi: 10.1111/j.1432-1033.1991.tb16207.x. [DOI] [PubMed] [Google Scholar]
- 38.Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007;30:249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 39.Favre P, Greppin H, Degli Agosti R. Repetitive action potentials induced in Arabidopsis thaliana leaf by wounding and potassium chloride application. Plant Physiol Biochem. 2001;39:961–969. [Google Scholar]
- 40.Ward JM, Maser P, Schroeder JI. Plant ion channels: gene families, physiology and functional genomics analyses. Annu Rev Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Priest B, Swensen A, McManus O. Automated electrophysiology in drug discovery. Current Pharmaceutical Design. 2007;13:2325–2337. doi: 10.2174/138161207781368701. [DOI] [PubMed] [Google Scholar]
- 42.Markova O, Mukhtarov M, Real E, Jacob Y, Bregestovski P. Genetically encoded chloride indicator with improved sensitivity. J Neurosci Methods. 2008;170:67–76. doi: 10.1016/j.jneumeth.2007.12.016. [DOI] [PubMed] [Google Scholar]