Abstract
Callose in polypodiaceous ferns performs multiple roles during stomatal development and function. This highly dynamic (1→3)-β-D-glucan, in cooperation with the cytoskeleton, is involved in: (a) stomatal pore formation, (b) deposition of local GC wall thickenings and (c) the mechanism of stomatal pore opening and closure. This behavior of callose, among others, probably relies on the particular mechanical properties as well as on the ability to form and degrade rapidly, to create a scaffold or to serve as a matrix for deposition of other cell wall materials and to produce fibrillar deposits in the periclinal GC walls, radially arranged around the stomatal pore. The local callose deposition in closing stomata is an immediate response of the external periclinal GC walls experiencing strong mechanical forces induced by the neighboring cells. The radial callose fibrils transiently co-exist with radial cellulose microfibrils and, like the latter, seem to be oriented via cortical MTs.
Key words: callose, cytoskeleton, fern stomata, guard cell wall thickening, stomatal function, stomatal pore formation
Callose represents a hemicellulosic matrix cell wall component, usually of temporal appearance, which is synthesized by callose synthases, enzymes localized in the plasmalemma and degraded by (1→3)-β-glucanases.1–4 It consists of triple helices of a linear homopolymer of (1→3)-β-glucose residues.5–7 The plant cell is able to form and degrade callose in a short time. On the surface of the plasmolyzed protoplast a thin callose surface film may arise within seconds.8 Callose is the only cell wall component that is implicated in a great variety of developmental plant processes, like cell plate formation,9–11 microspore development,12–14 trafficking through plasmodesmata,15,16 formation and closure of sieve pores,16 response of the plant cells to multiple biotic and abiotic stresses,4,5 establishment of distinct “cell cortex domains”,17 etc.
Despite the widespread occurrence of callose, its general function(s) is (are) not well understood (reviewed in refs. 4 and 5). It may serve as: a matrix for deposition of other cell wall materials, as in developing cell plates;9 a cell wall-strengthening material, as in cotton seed hairs and growing pollen tubes;18 a sealing or plugging material at the plasma membrane of pit fields, plasmodesmata and sieve plate pores;16 a mechanical obstruction to growth of fungal hyphae or a special permeability barrier, as in pollen mother cell walls and muskmelon endosperm envelopes.4,19,20 The degree of polymerization, age and thickness of callose deposits may cause variation in its physical properties.5
Evidence accumulated so far showed that a significant number of ferns belonging to Polypodiales and some other fern classes forms intense callose deposits in the developing GC wall thickenings.21–28 This phenomenon has not been observed in angiosperm stomata, although callose is deposited along the whole surface of the young VW and in the VW ends of differentiating and mature stomata (our unpublished data; reviewed in refs 29 and 30).
Stomata are specialized epidermal bicellular structures (Fig. 1A) regulating gas exchange between the aerial plant organs and the external environment. Their appearance in the first land plants was crucial for their adaptation and survival in the terrestrial environment. The constituent GCs have the ability to undergo reversible changes in shape, leading to opening and closure of the stomatal pore (stomatal movement). The mechanism by which GCs change shape is based on: (a) the particular mechanical properties of GC walls owed to their particular shape, thickening, fine structure and chemical composition and (b) the reversible changes in vacuole volume, in response to environmental factors, through fairly complicated biochemical pathways.30–33
Figure 1.
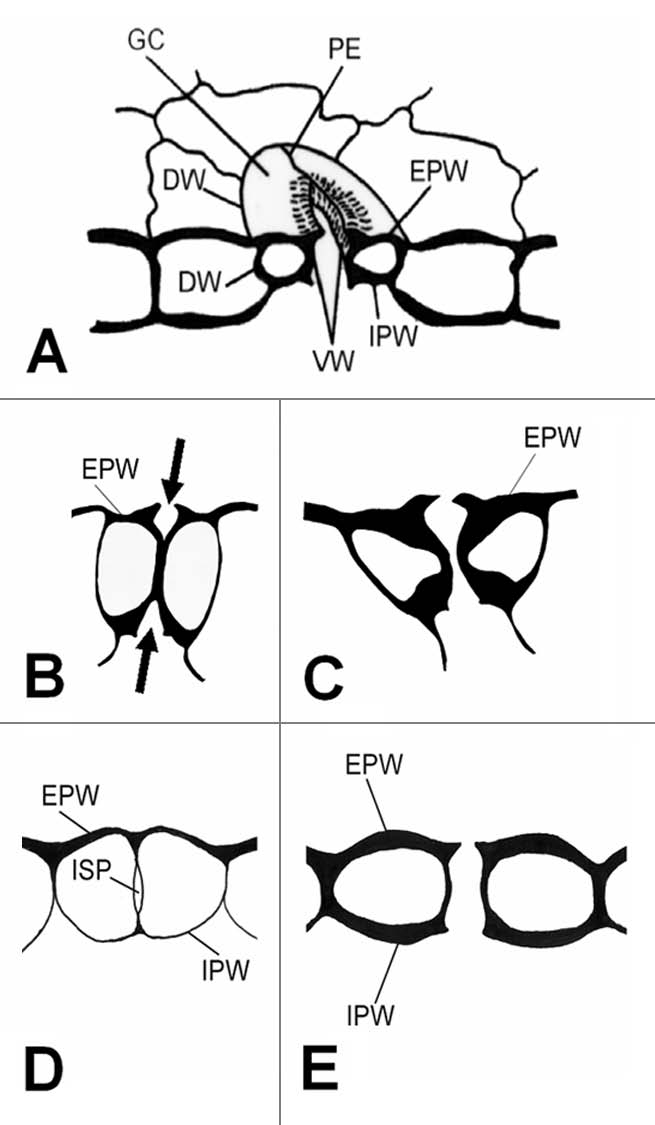
(A) Diagrammatic representation of an elliptical stoma. (B–E) Diagram to show the process of stomatal pore formation in angiosperms (B and C) and Polypodiales ferns (D and E). The arrows in (B) indicate the forming stomatal pore. DW, dorsal wall; EPW, external periclinal wall; GC, guard cell; IPW, internal periclinal wall; ISP, internal stomatal pore; PE polar ventral wall end; VW, ventral wall.
The present review is focused on the multiple-role of callose in differentiating and functioning fern stomata, as they are substantiated by the available information, including some unpublished data, and in particular in: stomatal pore formation, deposition of GC wall thickenings and opening and closure of the stomatal pore. The mode of deposition of fibrillar callose deposits in GC walls and the mechanism of their alignment are also considered.
Callose and Stomatal Pore Formation
The main tasks of GC differentiation are the formation of the stomatal pore and the development of the mechanism regulating its opening and closure.30,34 Stomatal pore is an intercellular space forming schizogeneously in the median region of the adjacent VWs separating the differentiating GCs, which brings into communication the labyrinth of the mesophyll intercellular spaces with the external environment (Fig. 1A). In most plants, this process begins from the external and internal periclinal GC walls and proceeds inwards (Fig. 1B and C; reviewed in ref. 30 and 31). In angiosperms, stomatal pore formation keeps pace with GC morphogenesis, a process largely controlled by the cortical MTs.35,36
In ferns Asplenium nidus, Adiantum capillus-veneris and two Anemia species, an extraordinary mechanism of stomatal pore formation functions.24,25,37,38 Stomatal pore appears as an intercellular space at the centre of the adjacent post-cytokinetic VWs (“internal stomatal pore”; Figs. 1D and 2A), gradually broadening towards the external and internal periclinal GC walls. Finally, the periclinal walls over the “internal stomatal pore” are disrupted and the stomatal pore is completed (Fig. 1E). The “internal stomatal pore” formation starts before the deposition of any detectable cell wall material in the VW by the local apart movement of the adjacent plasmalemmata (Fig. 2A). MT and AF bundles, lining anticlinally the mid-region of the VW, seem to be implicated in this process.24,26,37
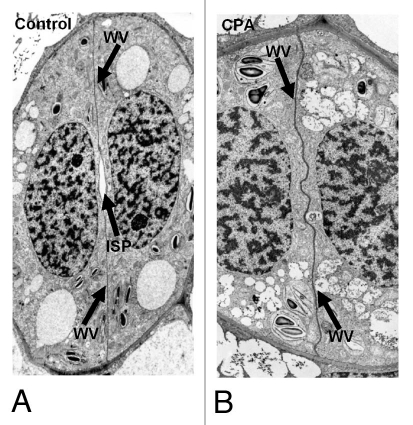
Figure 2.
(A and B) TEM micrographs illustrating median paradermal views of post-cytokinetic A. nidus stomata. (A) Control stoma. (B) Stoma affected by 25 µM CPA for 24 h, which lacks an “internal stomatal pore” (B; cf. A). ISP, internal stomatal pore; VW ventral wall. A: x1,900; (B: x2,300.)
In A. nidus, the “internal stomatal pore” formation is also related to the pattern of callose degradation in the nascent VW that accumulates considerable quantities of this glucan along its whole surface.26 Callose degradation commences at the centre of the nascent VW and proceeds towards its periphery. This is coupled with the initiation of the “internal stomatal pore” at the mid-depth of the VW (Fig. 2A), which further broadens centrifugally towards the periclinal GC walls. Callose was also detected along the whole surface of the nascent daughter walls of the dividing ordinary protodermal cells, but its degradation is centripetal, i.e., it commences from the periphery and proceeds inwards. Increased ER quantities were found by TEM and immunolabeling of the HDEL proteins in the cytoplasm lining the median regions of the nascent VW of A. nidus and Adiantum capillus-veneris stomata (reviewed in ref. 26 and 37 and our unpublished data). Among others, they may be involved in synthesis of proteins implicated in the local callose formation and/or degradation and/or establishment of local Ca2+ gradients controlling the above processes.
In A. nidus stomata treated with 2-deoxy-D-glucose (2-DDG) and tunicamycin, substances inhibiting callose synthesis,39,40 the newly formed VWs lacked callose as well as an “internal stomatal pore”.26 Gradually, they become abnormally thickened, appeared electron-transparent and included membranous elements, probably because of the uncontrolled growth and the extensive out folding of the plasmalemma into the apoplast. Moreover, treatment with cyclopiazonic acid (CPA) that disturbs cytoplasmic Ca2+ homeostasis,41 inhibited both callose deposition and “internal stomatal pore” formation of A. nidus stomata (reviewed in ref. 26; also Fig. 2B; cf. 2A). The VW of the 2-DDG-, tunicamycinand CPA-affected stomata displayed polysaccharides, other than callose, positive to PAS staining and fluorescing intensely after calcofluor staining. Inhibition of cellulose synthesis by coumarin and dichlobenil that promote callose synthesis42–44 also blocked callose degradation in the nascent VWs of A. nidus stomata. The affected stomata retained for a relatively long time large callose quantities in the nascent VWs, a phenomenon accompanied by the absence of “internal stomatal pore”.26 Therefore, both the absence and prolonged presence of callose in the nascent VW of the affected stomata inhibit “internal stomatal pore” formation.
The existence of considerable callose quantities in the cell plate and the early post-cytokinetic daughter walls probably offers mechanical support to the daughter plasmalemmata.9,11,45 In A. nidus stomata, the presence of callose in nascent VWs probably makes the adjacent plasmalemmata more rigid and difficult to be separated for the “internal stomatal pore” formation, while the possibility that callose, forming a gel “sticking” the partner VW plasmalemata to one another cannot be excluded. This difficulty is overcome by the rapid local callose removal that seems to allow the anticlinal MT and/or AF bundles lining the adjacent plasmalemmata at the middle of the VW24,25 to mediate their movement apart from each other and thus to initiate the “internal stomatal pore”. The temporal and spatial coincidence between callose degradation and “internal stomatal pore” formation supports the above view.
In addition, the maintenance of large callose quantities in the aberrant VWs formed in the dichlobenil- and coumarin-affected stomata, which is possibly accompanied by elevated deposition of pectic materials in them,43 probably keeps the plasmalemmata together preventing the “internal stomatal pore” formation. Moreover, the inhibition of callose synthesis possibly results in the earlier deposition of wall materials in the affected stomata,26 allowing the development of connections between the partner VWs and the adjacent plasmalemma that makes the “internal stomatal pore” formation impossible.
Callose and Deposition of Local GC Wall Thickenings
The differentiating GCs of fern stomata,21,22,24,25,37,46 like all the kidney-like GCs,30–32 form local wall thickenings at the sites of junction of the mid-region of the VW with the periclinal ones. Gradually, they expand covering the median region of the periclinal walls around the stomatal pore. In mature stomata, these thickenings disappear, mainly contributing to the asymmetrical expansion of the external and internal VW areas at the stomatal pore region. The cellulose microfibrils in the periclinal GC wall thickenings as well as in the rest of the periclinal walls are radially arranged around the stomatal pore, playing an essential role in GC morphogenesis-stomatal pore formation and especially in stomatal movement.30
The GC wall thickenings in species of the fern genera Asplenium, Angiopteris, Blechnum, Dryopteris, Marratia, Nephrolepis, Ophioglossum, Polypodium, Pteris and Pteridium accumulate large quantities of callose localized at the periphery and the interior of the rising wall thickenings (reviewed in ref. 21–23 and 25–27 and our unpublished data). At the margins of the developing GC wall thickenings of A. nidus large ER quantities are accumulated (Fig. 3C), which among others, may participate in the local callose deposition. Surprisingly, in the periclinal GC walls of A. nidus stomata callose is deposited in the form of fibrils radially arranged around the stomatal pore (reviewed in ref. 27; see also Fig. 3A). Examination of the figures included in the articles of Peterson et al.21 and Waterkeyn and Bienfait23 revealed the existence of radial callose fibrillar arrays in the external periclinal GC walls of the ferns Ophioglossum lusitanicum, Ophioglossum crotalophoroides, Angiopteris teysmanniana and Marattia fraxinea. The differentiating stomata of the ferns Nephrolepis exaltata and Blechnum sp. also display radial callose arrays (our unpublished data).
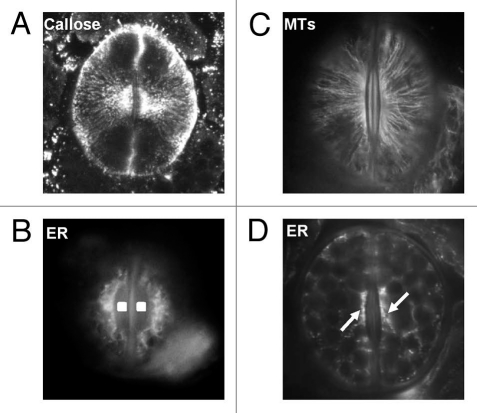
Figure 3.
(A) Callose immunolabeling in a differentiating stoma of A. nidus. Confocal-laser-scanning-microscope image constructed from seven consecutive optical paradermal sections. The callose fibrils are radially arranged around the stomatal pore. x850 (B) MT immunolabeling in a closed A. nidus stoma. The MTs underneath the periclinal walls are radially arranged around the stomatal pore (cf. A). x1,000. (C and D) Immunolabeling of HDEL ER proteins in a differentiating (C) and a mature closed stoma (D) of A. nidus. In the stoma shown in (C) distinct ER aggregations are localized at the margins of the wall thickenings (squares in C) deposited at the stomatal pore region. The arrows in (D) point to local ER aggregations in the cytoplasm adjacent to the walls at the stomatal pore region. C: x1,000; D: x750.
Therefore, in the periclinal GC walls of above ferns radial fibrillar arrays of two glucans (cellulose and callose) are organized. The radial callose fibrils probably reinforce the radial cellulose microfibrils to force differentiating GCs to assume a kidney-like form. The deposition of shapeless callose deposits in periclinal GC wall thickenings might interfere with the tangential periclinal GC wall expansion, thus worsening GC morphogenesis. Fibrillar callose has been also found in differentiating cotton fibers47 and in protoplasts regenerating cell wall.48–50 Micelle-like and fibrillar callose structures are also produced during in vitro callose synthesis.51–53
The radial pattern of fibrillar callose arrays in the periclinal walls of A. nidus GCs copies that of the underlying cortical MTs (reviewed in reference 27, also Fig. 3A; cf. 3B). This mutual alignment closely resembles that between cellulose microfibrils and cortical MTs in the kidney-shaped GCs.30 The oryzalin induced MT disintegration in A. nidus stomata disturbs the pattern of callose deposition, which becomes shapeless.27 Therefore, similarly to cellulose microfibrils, callose fibrils are probably aligned via cortical MTs. Alternatively, the radial pattern of callose fibril deposition could be controlled by the preexisting radial cellulose microfibrils in the periclinal GC walls. However, the findings that: (a) in dichlobenil- or coumarin-affected A. nidus stomata lacking radial cellulose microfibrils, radial callose arrays were deposited and (b) in some oryzalin-affected stomata the deposition of callose was disturbed, despite the presence of intact radial cellulose microfibril systems,27 show clearly that the latter are not implicated in callose fibril orientation. The cortical AFs underlying the periclinal GC walls25 do not participate in this process. Experimental AF disintegration in A. nidus stomata did not affect the pattern of radial callose fibrils.27
The mechanism by which the cortical MTs control the oriented deposition of callose fibrils must be similar to that involved in the cellulose microfibril deposition. In the latter case, the cortical MTs, acting as molecular rails, directly guide the cellulose synthases as they synthesize cellulose microfibrils,54,55 or they serve as passive constraints creating channels that determine the movement of cellulose synthases in the plasmalemma (reviewed in references 56–58). The former hypothesis seems now more probable. In that case, both callose and cellulose synthases must be guided via cortical MTs. In the absence of MTs, shapeless callose masses were deposited in the periclinal GC walls of A. nidus. Therefore, the MTs control callose deposition in the form of fibrils as well as their alignment.
Apart from the fern stomata, callose in the developing cell wall thickenings has been found in tracheary elements,5,59 transfer cells,60 cotton fibers,47,61 pollen grains,62 moss spores63 and bordered pits.64 In these cases, as well as in fern stomata, callose may: (a) create a scaffold or a suitable microenvironment for the deposition of cellulose microfibrils and other cell wall polysaccharides,47,48,60,61,64 (b) when degraded provide glucose residues for the synthesis of other cell wall polysaccharides43,47 and (c) offer support to plasmalemma to resist to mechanical stresses exerted to it as the wall thickenings grow into the cytoplasm.26,60
The extensive plasmalemma out folding into the apoplastic space of the atypical GC wall thickenings in A. nidus stomata affected by callose synthesis inhibitors,26 suggests that the absence of callose leads to destabilization of the mechanism of plasmalemma growth and cycling via endocytosis of the excessive membrane material.
Callose Implication in Stomatal Pore Opening and Closure
In functioning A. nidus stomata, callose presents a periodic synthesis/degradation cycle during stomatal pore opening/closure. Callose is definitely absent from the open stomata, but rapidly appears, in the form of fibrils, in the external periclinal cell walls of the closed ones,28 a phenomenon also confirmed in the ferns Blechnum sp. and Nephrolepis exaltata (our unpublished data). Snap-freezing of A. nidus leaf portions in liquid nitrogen, followed by tubulin immunolabeling, as well as MT imunolocalization after chemical fixation, revealed the existence in both open and closed stomata of well-organized radial MT systems below the periclinal GC walls (Fig. 3B; our unpublished data). This is in contrast to the elliptical stomata of the dicotyledon Vicia faba, where the open stomata display highly organized radial MT systems that break down in the closed ones.65,66 The persistence of radial MT arrays in A. nidus stomata is probably related to the control radial callose deposition in the closing stomata.
In closing stomata of A. nidus, callose is mainly deposited in particular external periclinal GC wall regions that bend intensely.28 They are thinner than the rest wall as the result of particular thickening/expansion mode of the periclinal GC walls,25,67 a phenomenon commencing in GC mother cells. The central region of the external periclinal wall of these cells is curved, thin and impregnated by callose, probably as a response to local stretching, while its margins appear thickened.67
During stomatal closure in A. nidus the surrounding epidermal cells seem to exert strong mechanical stresses on the external periclinal GC walls, leading to their local deformation. The plasmalemma lining the deforming regions probably senses this mechanical stress that induces the local increase of Ca2+ uptake in the GCs, which in turn triggers local callose synthesis.68 Mechanical forces have been shown to alter plasmalemma ion channel permeability, which is associated with Ca2+ and other ion fluxes.69,70 Immunolabeling of the HDEL ER proteins, after chemical fixation or snap-freezing in liquid nitrogen, revealed that in both opened and closed stomata of A. nidus increased quantities of ER line the GC wall thickenings (Fig. 3D; our unpublished data). Among others, they may be involved in the local callose cycling and/or cellulose synthesis.
These stresses are relieved in the opened stomata, the external periclinal walls of which recover. These walls did not display any local deformation as well as callose.28 During recovery, new polysaccharidic material, including cellulose microfibrils might be incorporated in them, a hypothesis that should be investigated. If it is true, during stomatal movement callose synthesis may alternate with that of cellulose microfibrils. Matrix cell wall material and cellulose, but not callose, are synthesized in the opening stomata of the dicotyledon Vicia faba.71
Regardless of the above, in A. nidus, GC wall composition definitely changes during stomatal movement, an observation posing the question whether or not the cycling callose that is deposited in response to a signal generated by mechanical stress, is involved in the mechanism of opening and closure of the stomatal pore. A series of experimental data substantiated callose implication in stomatal movement of A. nidus28 and in particular:
The worsening of the ability of stomata to open in white light and to close in darkness after the enzymatic degradation of callose by β-1,3-D-glucanase.
The decrease in the ability of stomata to open in white light and close in darkness after inhibition of callose synthesis by 2-DDG. The presence of well-organized radial cellulose microfibrils arrays in 2-DDG-affected stomata supports that this substance disturbs stomatal movement via inhibition of callose synthesis and not by inhibition of cellulose microfibril synthesis.
The improvement of the ability of stomata to open in white light and the prevention of their complete closure in darkness, after induction of callose synthesis by coumarin or dichlobenil. Since coumarin- and dichlobenil-affected functioning stomata possessed well-organized cellulose microfibrils that have been deposited before the onset of the treatment, these drugs probably interfere with stomatal opening and closure by induction of excessive callose synthesis.
Regarding the particular role(s) of callose in the mechanism of stomatal pore opening and closure it can be suggested that this glucan, intercalating between preexisting cell wall polysaccharides may:
Create a proper microenvironment or a scaffold in periclinal GC wall for the deposition of cell wall polysaccharides during stomatal opening, hypothesis previously made for other cell types.47,60
Loosen or disrupt the chemical bonds between the matrix cell wall polysaccharides or even develop chemical bonds with them, in all cases modifying the elastic properties of the periclinal GC walls.28 However, there is not information about the type and the extent of chemical bonds that callose may develop with the matrix cell wall polysaccharides and/or cellulose microfibrils. In angiosperms, the elastic properties of the GC walls seem to change during stomatal movement, probably by compositional changes of the wall matrix polysaccharides. Enzymatic disruption of arabinans, feruloyl esters of pectins and homogalacturonans affects stomatal pore opening and closing.72,73
Facilitate or more probably reinforce the ability of the periclinal GC walls to expand tangentially, promoting stomatal pore opening. In case of shapeless callose deposition, the expansion of the periclinal walls could be deteriorated.
It should be mentioned here that the chemical composition of the primary cell walls of ferns differs from those of other plant divisions.74 Our preliminary data derived by examination of GC wall autofluorescence and chemical GC wall composition using JIM5, JIM7, LM6 and LM13 antibodies, showed that the chemical composition of A. nidus GC walls differ from those of the elliptical angiosperm stomata investigated so far. They are rich in phenolic esters of pectins, esterified homogalacturonan and linear arabinans and seem to lack unesterified homogalacturonans and pectic arabinans. Comparison of the chemical composition of the A. nidus GC walls to those of elliptical angiosperm stomata29,72,73 revealed that the intense presence of the phenolic esters of pectins and the absence of unesterified homogalacturonans and pectic arabinans constitute particular characteristics of the GCs walls of A. nidus. Considering the above mentioned, it may be speculated that the implication of callose in the function of the fern stomata is related to their particular GC wall chemical composition, and that it plays a role analogous to that of arabinans in the functioning angiosperm stomata.72
Future Perspectives
Accumulated information on fern stomata revealed that callose participates in stomatal differentiation and function. However, further studies are needed in order to: (a) elucidate the exact function(s) of callose in developing and functioning fern stomata. In this direction, the study of GC wall chemistry as well as the nature and extent of chemical bonds that callose may develop with the other cell wall polysaccharides is essential. (b) Find out whether callose plays any role in stomatal development and function in other plant divisions. (c) Examine the structure of callose fibrils and whether all callose synthases or some of them are able to interact with MTs to create the callose fibrils. Twelve genes encoding putative callose synthases have been found in Arabidopsis.1
Acknowledgements
We are grateful to Dr. E. Giannoutsou and Mr. P. Livanos (Department of Botany, University of Athens) for their help in ER immunolabeling and preparation of figures and Dr. H. Quader (Biocentre Klein Flottbeck, University of Hambourg) for the kind offer of the anti-HDEL antibody. The present study was financed by the University of Athens (Project “Kapodistrias”).
Abbreviations
- AF
actin filament
- A. nidus
Asplenium nidus
- 2-DDG
2-deoxy-D glucose
- ER
endoplasmic reticulum
- GC
guard cell
- MT
microtubule
- TEM
transmission electron microscopy
- VW
ventral wall
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12959
References
- 1.Verma DPS, Hong Z. Plant callose synthase complexes. Plant Mol Biol. 2001;47:693–701. doi: 10.1023/a:1013679111111. [DOI] [PubMed] [Google Scholar]
- 2.Bulone V. In vitro synthesis and analysis of plant (1→3)-β-D-glucans and cellulose: a key step towards the characterization of glucan synthases. In: Brown RM Jr, Saxena IM, editors. Cellulose: Molecular and Structural Biology. Heidelberg: Springer; 2007. pp. 123–145. [Google Scholar]
- 3.Chen X-Y, Kim J-Y. Callose synthesis in higher plants. Plant Signal Behav. 2009;6:489–492. doi: 10.4161/psb.4.6.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacic A, Fincher GB, Stone BA. Chemistry, Biochemistry and Biology of (1→3)-β-Glucans and Related Polysaccharides. Amsterdam: Academic Press; 2009. [Google Scholar]
- 5.Stone BA, Clarke AE. Chemistry and Biology of (1,3)-β-Glucans. Bundora Australia: La Trobe University Press; 1992. [Google Scholar]
- 6.Stone BA. Chemistry of β-glucans. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, Biochemistry and Biology of (1→3)-β-glucans and Related Polysaccharides. Amsterdam: Academic Press; 2009. pp. 5–46. [Google Scholar]
- 7.Sletmoen M, Stokke BT. Higher order structure of (1,3)-β-D-glucans and its influence on their biological activities and complexation abilities. Biopolymers. 2008;89:310–321. doi: 10.1002/bip.20920. [DOI] [PubMed] [Google Scholar]
- 8.Eschrich W. Physiologie der Siebrohrencallose. Planta. 1965;65:280–300. (Ger). [Google Scholar]
- 9.Samuels AL, Giddings TH, Staehelin LA. Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol. 1995;30:1345–1357. doi: 10.1083/jcb.130.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiele K, Wanner G, Kindzierski V, Jurgens G, Mayer U, Pachl F, Assaad FF. The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J. 2009;58:13–26. doi: 10.1111/j.1365-313X.2008.03760.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown RC, Lemmon BE. Callose in cell division. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, Biochemistry and Biology of (1→3)-β-Glucans and Related Polysaccharides. Amsterdam: Academic Press; 2009. pp. 425–437. [Google Scholar]
- 12.Taylor LP, Hepler PK. Pollen germination anf tube growth. Ann Rev Plant Physiol Plant Mol Biol. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Prenss D. Callose (β-1,3glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol. 2005;5:22–30. doi: 10.1186/1471-2229-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newbigin E, Bacic E, Read S. Callose and its role in pollen and embryo sac development in flowering plants. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, Biochemistry and Biology of (1→3)-β-Glucans and Related Polysaccharides. Amsterdam: Academic Press; 2009. pp. 465–498. [Google Scholar]
- 15.Oparka KJ, Roberts AG. Plasmodesmata. A not so open-and-shut case. Plant Physiol. 2001;125:123–126. doi: 10.1104/pp.125.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy A, Epel BL. Cytology of the (1→3)-β-glucan (callose) in plasmodesmata and sieve pores. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, Biochemistry and Biology of (1→3)-β-Glucans and Related Polysaccharides. Amsterdam: Academic Press; 2009. pp. 439–463. [Google Scholar]
- 17.Baluška F, Volkmann D, Barlow PW. Actin-based domains of the “cell periphery complex” and their associations with polarized “cell bodies” in higher plants. Plant Biol. 2000;2:253–267. [Google Scholar]
- 18.Parre E, Geitmann A. More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol. 2005;37:274–286. doi: 10.1104/pp.104.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heslop-Harrison J. Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. In: Linskens HF, editor. Pollen Physiology and Ferilization. North-Holland: Amsterdam; 1964. pp. 29–47. [Google Scholar]
- 20.Yim KO, Bradford JK. Callose deposition is responsible for apoplastic semipermeability of the endosperm envelope of muskmelon seeds. Plant Physiol. 1998;118:83–90. doi: 10.1104/pp.118.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson RL, Firminger MS, Dobrindt LA. Nature of the guard cell wall in leaf stomata of three Ophioglossum species. Can J Bot. 1975;53:1698–1711. [Google Scholar]
- 22.Peterson RL, Hambleton S. Guard cell ontogeny in leaf stomata of the fern Ophioglossum petiolatum. Can J Bot. 1978;56:2836–2852. [Google Scholar]
- 23.Waterkeyn L, Bienfait A. Production et dégradation de callose dans les stomates des fougères. La Cellule. 1979;73:83–97. (Fre). [Google Scholar]
- 24.Apostolakos P, Galatis B. Probable cytoskeleton involvement in stomatal pore formation in Asplenium nidus L. Protoplasma. 1998;203:48–57. [Google Scholar]
- 25.Apostolakos P, Galatis B. Microtubule and actin filament organization during stomatal morphogenesis in the fern Asplenium nidus. II. Guard cells. New Phytol. 1999;141:209–223. doi: 10.1046/j.1469-8137.1999.00348.x. [DOI] [PubMed] [Google Scholar]
- 26.Apostolakos P, Livanos P, Nikolakopoulou TL, Galatis B. The role of callose in guard cell wall differentiation and stomatal pore formation in the fern Asplenium nidus. Ann Bot. 2009;104:1373–1387. doi: 10.1093/aob/mcp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apostolakos P, Livanos P, Galatis B. Microtubule involvement in the deposition of radial fibrillar callose arrays in stomata of the fern Asplenium nidus L. Cell Motil Cyt. 2009;66:342–349. doi: 10.1002/cm.20366. [DOI] [PubMed] [Google Scholar]
- 28.Apostolakos P, Livanos P, Nicolakopoulou TL, Galatis B. Callose implication in stomatal opening and closure in the fern Asplenium nidus. New Phytol. 2010;186:623–635. doi: 10.1111/j.1469-8137.2010.03206.x. [DOI] [PubMed] [Google Scholar]
- 29.Majewska-Sawka A, Münster A, Rodríguez-García MI. Guard cell wall: immunocytochemical detection of polysaccharide components. J Exp Bot. 2002;53:1067–1079. doi: 10.1093/jexbot/53.371.1067. [DOI] [PubMed] [Google Scholar]
- 30.Galatis B, Apostolakos P. The role of the cytoskeleton in the morphogenesis and function of stomatal complexes. New Phytol. 2004;161:613–639. doi: 10.1046/j.1469-8137.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- 31.Sack FD. The development and structure of stomata. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal function. Stanford CA, USA: Stanford University Press; 1987. pp. 59–89. [Google Scholar]
- 32.Wilmer C, Fricker M. Stomata. 2nd Edn. London: Chapman and Hall; 1996. [Google Scholar]
- 33.Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. doi: 10.1016/s0092-8674(01)00606-7. [DOI] [PubMed] [Google Scholar]
- 34.Galatis B, Apostolakos P. Microtubule organization and morphogenesis of stomata in caffeine-affected seedlings of Zea mays. Protoplasma. 1991;165:11–26. [Google Scholar]
- 35.Galatis B. Microtubules and guard cell morphogenesis in Zea mays L. J Cell Sci. 1980;45:211–244. doi: 10.1242/jcs.45.1.211. [DOI] [PubMed] [Google Scholar]
- 36.Galatis B, Mitrakos K. The ultrastructural cytology of the differentiating guard cells of Vigna sinensis. Am J Bot. 1980;67:1243–1261. [Google Scholar]
- 37.Galatis B, Apostolakos P, Katsaros Ch. Microtubules and their organizing centres in differentiating guard cells of Adiantum capillus-veneris. Protoplasma. 1983;115:176–192. [Google Scholar]
- 38.Zachariadis M, Apostolakos P, Galatis B. Morphogenesis of ‘floating’ stomata in the fern Anemia mandioccana. Stomatal pore formation. In: Tsekos I, Moustakas M, editors. Progress in Botanical Research. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 615–618. [Google Scholar]
- 39.Jaffe MJ, Leopold AC. Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-D-glucose. Planta. 1984;161:20–26. doi: 10.1007/BF00951455. [DOI] [PubMed] [Google Scholar]
- 40.Škalamera D, Heath MC. Cellular mechanisms of callose deposition in response to fangal infection or chemical damage. Can J Bot. 1996;74:1236–1242. [Google Scholar]
- 41.Quader H, Bechtler Ch. The calcium transport inhibitor cyclopiazonic acid reversibly affects the shape of the endoplasmic reticulum in onion epidermal cells. Mitt Inst Allg Bot Hamburg. 1996;26:191–199. [Google Scholar]
- 42.Vaughn KC, Hoffman JC, Hahn MG, Staehelin LA. The herbicide dichlobenil disrupts cell plate formation: immunogold characterization. Protoplasma. 1996;194:117–132. [Google Scholar]
- 43.Sabba RP, Durso NA, Vaughn KC. Structural and immunocytochemical characterization of the walls of dichlobenil-habituated BY-2 cells. Plant Sci. 1999;160:275–290. [Google Scholar]
- 44.DeBolt S, Gutierrez R, Ehrhardt DW, et al. Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proc Nat Acad Sci USA. 2007;104:5855–5859. doi: 10.1073/pnas.0700789104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong Z, Delauney AJ, Verma DPS. A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell. 2001;13:755–768. doi: 10.1105/tpc.13.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens RA, Martin ES. Structural and functional aspects of stomata: I. Developmental studies in Polypodium vulgare. Planta. 1978;142:307–316. doi: 10.1007/BF00385082. [DOI] [PubMed] [Google Scholar]
- 47.Waterkeyn L. Cytochemical localization and function of the 3-linked glucan callose in the developing cotton fibre cell wall. Protoplasma. 1981;106:49–67. [Google Scholar]
- 48.Van Amstel TNM, Kengen HMP. Callose deposition in the primary wall of suspension cells and regenerating protoplasts, and its relationship to patterned cellulose synthesis. Can J Bot. 1996;74:1040–1049. [Google Scholar]
- 49.Hirai N, Sonobe S, Hayashi T. In situ synthesis of β-glucan microfibrils on tobacco plasma membrane sheets. Proc Natl Acad Sci USA. 1998;95:15102–15106. doi: 10.1073/pnas.95.25.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukumoto T, Hayashi N, Sasamoto H. Atomic force microscopy and laser confocal scanning microscopy analysis of callose fibers developed from protoplasts of embryogenic cells of a conifer. Planta. 2005;223:40–45. doi: 10.1007/s00425-005-0065-3. [DOI] [PubMed] [Google Scholar]
- 51.Bulone V, Fincher GB, Stone BA. In vitro synthesis of a microfibrillar (1→3)-β-D-glucan by a rye grass (Lolium multiflorum) endosperm. (1→3)-β-D-glucan synthase enriched by product entrapment. Plant J. 1995;8:213–225. [Google Scholar]
- 52.Him JLK, Pelosi L, Chanzy H, Putaux J-L, Bulone V. Biosynthesis of (1→3)-β-D-glucan (callose) by detergent extracts of a microsomal fraction from Arabidopsis thaliana. Eur J Biochem. 2001;268:4628–4638. doi: 10.1046/j.1432-1327.2001.02382.x. [DOI] [PubMed] [Google Scholar]
- 53.Cifuentes C, Bulone V, Emons AMC. Biosynthesis of callose and cellulose by detergent extracts of tobacco cell membranes and quantification of the polymers synthesized in vitro. J Int Plant Biol. 2010;52:221–233. doi: 10.1111/j.1744-7909.2010.00919.x. [DOI] [PubMed] [Google Scholar]
- 54.Heath IB. A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J Theor Biol. 1974;48:445–449. doi: 10.1016/s0022-5193(74)80011-1. [DOI] [PubMed] [Google Scholar]
- 55.Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- 56.Wasteneys GO, Fujita M. Establishing and maintaining axial growth: wall mechanical deposition and the cytoskeleton. J Plant Res. 2006;119:5–10. doi: 10.1007/s10265-005-0233-3. [DOI] [PubMed] [Google Scholar]
- 57.Lloyd CW, Chan J. The parallel lines of microtubules and cellulose microfibrils. Curr Opin Plant Biol. 2008;11:641–646. doi: 10.1016/j.pbi.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Nick P. Control of cell axis. In: Nick P, editor. Plant Microtubules: Development and Flexibility. Vol. 17. Berlin: Springer-Verlag; 2008. pp. 3–46. Plant Cell Monographs. [Google Scholar]
- 59.Gregory ACE, Smith C, Kerry ME, Wheatley ER, Bolwell PG. Comparative subcellular immunolocalization of polypeptides associated with xylan and callose synthases in french bean (Phaseolus vulgaris) during secondary wall formation. Phytochemistry. 2002;59:249–259. doi: 10.1016/s0031-9422(01)00440-x. [DOI] [PubMed] [Google Scholar]
- 60.Vaughn KC, Talbot MJ, Offler CE, McCurdy DW. Wall ingrowths in epidermal transfer cells of Vicia faba cotyledons are modified primary walls marked by localized accumulations of arabinogalactan proteins. Plant & Cell Physiol. 2007;48:159–168. doi: 10.1093/pcp/pcl047. [DOI] [PubMed] [Google Scholar]
- 61.Salnikov V, Grimson MJ, Seagull RW, Haigler CH. Localization of sucrose synthase and callose in freeze-substituted secondary-wall-stage cotton fibers. Protoplasma. 2003;221:175–184. doi: 10.1007/s00709-002-0079-7. [DOI] [PubMed] [Google Scholar]
- 62.Ferguson C, Teezi TT, Siika-aho M, Read SM, Bacic A. Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta. 1998;206:452–460. [Google Scholar]
- 63.Schuette S, Wood AJ, Geisler M, Geisler-Lee J, Ligrone R, Renzaglia KS. Novel localization of callose in spores of Physcomitrella patens and phylogenomics of the callose synthase gene family. Ann Bot. 2009;103:749–756. doi: 10.1093/aob/mcn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaffey NJ, Barlow PW. Myosin, microtubules and microfilaments: co-operation between cytoskeletal components during cambial cell division and secondary vascular differentiation in trees. Planta. 2002;214:526–536. doi: 10.1007/s004250100652. [DOI] [PubMed] [Google Scholar]
- 65.Fukuda M, Hasezawa S, Asai N, Nakajima N, Kondo N. Dynamic organization of microtubules in guard cells of Vicia faba L. with diurnal cycle. Plant & Cell Physiol. 1998;39:80–86. doi: 10.1093/oxfordjournals.pcp.a029293. [DOI] [PubMed] [Google Scholar]
- 66.Yu R, Huang RF, Wang XC, Yuam M. Microtubule dynamics are involved in stomatal movement of Vicia faba L. Protoplasma. 2001;216:113–118. doi: 10.1007/BF02680138. [DOI] [PubMed] [Google Scholar]
- 67.Apostolakos P, Panteris E, Galatis B. Microtubule and actin filament organization during stomatal morphogenesis in the fern Asplenium nidus. I. Guard cell mother cell. Protoplasma. 1997;198:93–106. doi: 10.1046/j.1469-8137.1999.00348.x. [DOI] [PubMed] [Google Scholar]
- 68.Kauss H. Some aspects of calcium-dependent regulation in plant metabolism. Ann Rev Plant Physiol. 1987;38:47–72. [Google Scholar]
- 69.Haley A, Russel AJ, Wood N, Allan AC, Knight A, et al. Effects of mechanical signaling on plant cell cytosolic calcium. Proc Nat Acad Sci USA. 1995;92:4124–4128. doi: 10.1073/pnas.92.10.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silver FH, Siperko LM. Mechanosensing and mechanochemical transduction: How is mechanical energy sensed and converted into chemical energy in an extracellular matrix. Crit Rev Biomed Engin. 2003;31:255–331. doi: 10.1615/critrevbiomedeng.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi Y, Kondo N. Effect of abscisic acid on cellwall metabolism in guard cells of Vicia faba L. Plant Cell Physiol. 1988;29:573–580. [Google Scholar]
- 72.Jones L, Milne JL, Ashford D, McQueen-Mason SJ. Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci USA. 2003;100:11783–11788. doi: 10.1073/pnas.1832434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones L, Milne JL, Ashford D, McCann MC, McQueen-Mason SJ. A conserved functional role of pectic polymers in stomatal guard cells from a range of plant species. Planta. 2005;221:255–264. doi: 10.1007/s00425-004-1432-1. [DOI] [PubMed] [Google Scholar]
- 74.Popper ZA, Fry SG. Primary cell wall composition of pteridophytes and spermatophytes. New Phytol. 2004;164:165–174. doi: 10.1111/j.1469-8137.2004.01146.x. [DOI] [PubMed] [Google Scholar]