Abstract
The Medicago truncatula LATD/NIP gene is essential for the development of lateral and primary root and nitrogen-fixing nodule meristems as well as for rhizobial invasion of nodules. LATD/NIP encodes a member of the NRT1(PTR1) nitrate and di-and tri-peptide transporter family, suggesting that its function is to transport one of these or another compound(s). Because latd/nip mutants can have their lateral and primary root defects rescued by ABA, ABA is a potential substrate for transport. LATD/NIP expression in the root meristem was demonstrated to be regulated by auxin, cytokinin and abscisic acid, but not by nitrate. LATD/NIP's potential function and its role in coordinating root architecture and nodule formation are discussed.
Key words: nodule development, lateral root development, root architecture, symbiotic nitrogen fixation, Medicago truncatula, NRT1(PTR) gene family
Unlike most other plants, legumes form two kinds of lateral root organs: lateral roots and nitrogen-fixing root nodules that form in conjunction with compatible symbiotic rhizobium bacteria. Although the morphology and function of these two root organs is distinct, both require the function of the LATD/NIP gene, indicating shared genetic components for these two developmental processes and providing support for a model in which legume nodules evolved from a lateral root blueprint. Both lateral roots and nodules initiate in previously differentiated root cells in response to environmental and developmental cues mediated by hormones. Interestingly, regulation of nodules and lateral roots by hormones is often opposite, allowing formation of one organ or another depending on the conditions.
The LATD/NIP Gene is Required for Root and Nodule Meristem Function as well as Rhizobium Invasion
The LATD/NIP gene plays a key role in the formation and maintenance of lateral root and nodule meristems. In mutants that lack LATD/NIP function, lateral roots and nodules initiate, but are unable to form functional meristems at their apex. In the most severely affected latd allele, lateral roots arrest immediately after emergence from the primary root, nodules arrest as small white half-domes and the primary root growth gradually slows until it stops at approximately 20 days. The weaker nip-1 mutant has as many arrested lateral roots as the more severe latd mutant, but the phenotype is leaky and some lateral roots are able to extend.1,2 Nodules in nip-1 mutants, like those in latd roots, have no obvious apical meristem.1 The nip-3 mutant appears to have fairly normal meristem function in nodules and lateral roots: only the somewhat reduced length of primary and lateral roots hint at a possible defect in meristem activity.3
In addition to its role in meristem function, LATD/NIP is required for rhizobial infection during nodulation at multiple levels: infection thread growth, bacterial release from the infection thread and nitrogen fixation. Analysis of the allelic series of LATD/NIP mutants revealed defects at different stages of infection. The strong latd allele causes the earliest block to infection: infection threads form in root hairs, but there is little branching in the cortex and few infection threads reach the nodule primordium.4 The intermediate allele, nip-1, causes the formation of excessive swollen infection threads, which ramify through the nodule, but fail to release bacteria into symbiosomes.1 The weakest allele, nip-3, has no apparent effect on infection thread formation or growth, but may affect bacterial release or proliferation, since fewer bacteria are seen within infected cells and nitrogen fixation rates are reduced.3 Thus, phenotypic analysis of different alleles reveals successive requirements for LATD/NIP function throughout rhizobial infection. In addition, the nodules of all three allelic latd/nip mutants show the accumulation of brown autofluorescent pigments. This has been interpreted as an inappropriate activation of a defense-like response during nodulation.1
LATD/NIP Encodes a Putative Transporter of the NRT1(PTR) Family
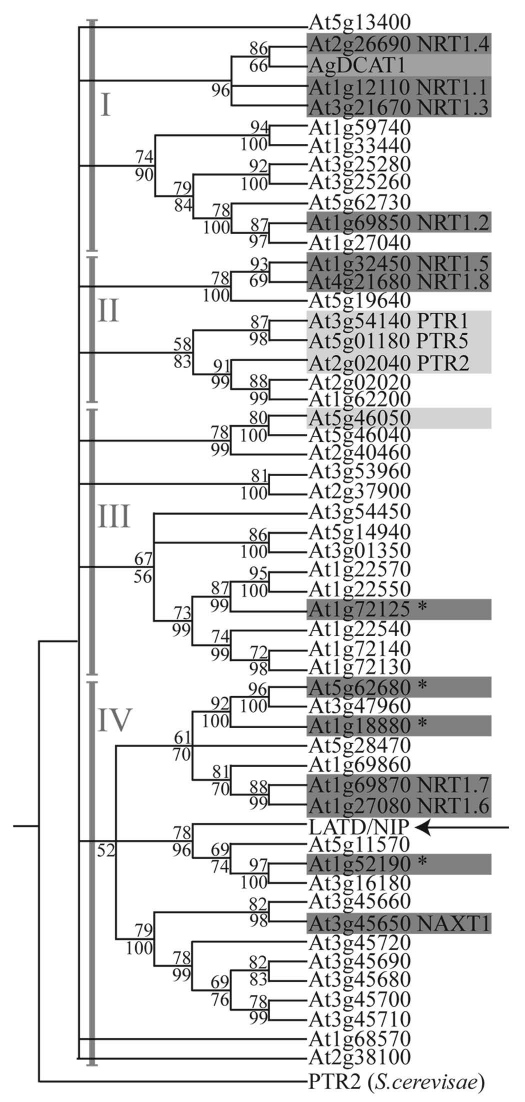
We recently cloned the LATD/NIP gene and found that it encodes a member of the NRT1(PTR) family of transporters.2 These transporters are represented in S. cerevisiae by a single family member.5 The NRT1(PTR) family has expanded greatly in plants, with 53 family members in Arabidopsis and 80 in rice.6 PTR family members transport di- or tri-peptides like the yeast PTR gene,7 while many NRT family members transport nitrate.6 An exception is the Alnus AgDCAT1 transporter, which has been demonstrated to transport dicarboxylates. AgDCAT1 is expressed in actinorhizal nitrogen-fixing nodules formed on Alnus roots. The available data indicate that the dicarboxylate transport is from the host cell cytosol outward and may supply the intracellular bacteria with dicarboxylates as carbon sources.8 AgDCAT1 is found in clade I of the NRT1(PTR) family (Fig. 1); the other tested transporters in this branch transport nitrate. This clade contains Arabidopsis NRT1.1 (CHL1), a high- and low-affinity nitrate transporter, that acts as a nitrate sensor and also transports auxin in a nitrate-regulated fashion.9–13 Thus, while phylogenetic proximity may give hints about function, it is not a reliable indicator of substrate in the NRT1(PTR) family.
Figure 1.
Maximum parsimony tree for LATD/NIP, Arabidopsis members of the NRT1 (PTR) family and AgDCAT1, modified from Figure 5S in Yendrek et al. (2010).2 Numbers below branches are maximum parsimony bootstrap values (MEGA4); numbers above branches are posterior probabilities from the Bayesian analysis (MrBayes 3.1.2). Four clades, as determined by Tsay et al. (2007),6 are represented by gray roman numerals. Genes highlighted in dark gray are nitrate transporters; those with an asterisk have only been reported as nitrate transporters in a review in reference 6. Genes boxed in light gray are known peptide transporters and the gene boxed in medium gray is a dicarboxylate transporter. The tree is rooted to S. cerevisiae PTR2.
LATD/NIP is found in clade IV (Fig. 1). Of the 19 Arabidopsis NRT1(PTR) members that fall into this clade, only 3, NRT1.6, NRT1.7 and NAXT1, have a biochemically demonstrated function: all are low-affinity nitrate transporters.14–16 Clade IV includes one of the most closely related Arabidopsis NRT1(PTR) members to LATD/NIP, encoded by At1g52190, which has been reported in a review to be a low-affinity nitrate transporter,6 but the data are unpublished. The physiological roles of these proteins are likely to be quite diverse as they each have a distinct tissue-specific expression pattern. NRT1.7 is expressed in the phloem of mature leaves and most likely plays a role in nitrate remobilization from old leaves.14 NRT1.6 is expressed in siliques and is required for early embryo development.15 NAXT1 is expressed in mature regions of the root, predominantly within the cortex, where it mediates passive nitrate efflux in response to acidification of the rhizosphere.16 Thus, the physiological role of LATD/NIP, which is required for meristem function and rhizobium infection, is likely to be quite different from that of the characterized members of clade IV.
Regulation of Root and Nodule Meristems by Nitrate and LATD/NIP
LATD/NIP is expressed at low levels in all tissues examined, but expression is enriched in root and nodule meristems, consistent with a function for LATD/NIP in modulating meristem activity.2 Although both roots and nodules play important roles in nutrient acquisition, different conditions favor the formation of one organ over the other. For example, access to sufficient concentrations of nitrate or other bioavailable nitrogen suppresses the formation of nodules. While nodules are efficient sources of fixed nitrogen, they are also energetically quite expensive for the plant; nitrogenase requires high amounts of ATP, thus nodules consume large amounts of photosynthate. Once nodules initiate, autoregulation of nodulation (AON) assures that over-nodulation does not occur—AON regulation occurs before nitrogen fixation commences, demonstrating that AON is independent of the availability of a new nitrogen source. The AON regulatory system shares common elements with nodulation suppression by nitrate.17,18 AON and nitrate-suppression of nodulation are mediated, at least in part, by leucine-rich repeat receptor-like kinases (LRR-RLKs), SUNN in Medicago19 and orthologous LRR-RLKs in other legumes.20,21 These AON LRR-RLKs are evolutionarily related to Arabidopsis CLAVATA1 (CLV1), which binds to CLE peptides (12–13 amino acids) as part of its regulatory mechanism. Specific CLE peptides have been shown to act in AON and nitrate suppression of nodulation.18,22 Although LATD/NIP is in a transporter family known to transport nitrate and peptides, it is unlikely to be involved in AON; see below.
In Arabidopsis, local patches of nitrate stimulate root branching within the patch, whereas globally high levels of nitrate inhibit lateral root elongation.23 Thus, growth and development of nodules and lateral roots is oppositely regulated by nitrate, when considering the effects of nitrate patches and similarly when considering global nitrate provision.
Nitrate regulates lateral root growth at the level of meristem activation in the developing primordium, and is mediated by the hormone, abscisic acid (ABA).24,25 The LATD/NIP gene regulates root meristem activity, and ABA can restore meristem function lost in latd/nip mutants.26 Although the substrate of LATD/NIP is unknown, most NRT1(PTR) family members are involved in the acquisition of bioavailable nitrogen, either as nitrate or as di- or tri-peptides.6 The homology of LATD/NIP to nitrate/peptide transporters and its role in ABA signaling suggest that LATD/NIP may be a component of the nitrate-ABA signaling pathway that regulates activation of the meristem in primary and lateral roots. It further suggests that this role is common to a similar transition in the formation of nodules.
A Role for LATD/NIP Coordinating Root Architecture and Nodule Formation?
Because of its function regulating nodule meristems as well as root meristems, LATD/NIP is poised to participate in the intricate process of coordinating root architecture and nodule formation in response to nutrient and hormonal signals. Legume nodules exist within the context of plant development; whether to build a new nodule or not depends on resource allocation of the plant as a whole. Thus, the formation of new nodules and lateral roots in legumes is dependent not only on environmental signals and available resources, but also on the number of existing or developing nodules and lateral roots.27 In other words, the formation of new nodules is partially dependent on the suppression of lateral root emergence.28 Since LATD/NIP is expressed in both lateral root and nodule meristems and is required for their function, regulation of this gene could play a key role in the balance between development of lateral roots and nodules.
Auxin, Cytokinin and Abscisic Acid Regulate Lateral Root and Nodule Development
Hormones coordinate the responses to the external environment, regulating the initiation and development of both nodules and lateral roots. Auxin, cytokinin and ABA play major roles both in the development of lateral roots, as well as in nodule formation.12,17,29–31 Auxin and cytokinin exert opposite effects on nodules and lateral roots. An auxin maximum specifies the fate of the lateral root founder cell, which can be counteracted by cytokinin.24,29 In contrast, auxin transport in indeterminate nodulators is transiently blocked just above the site of nodule initiation, while cytokinin appears to promote cell divisions and activate the nodule developmental program in the inner cortex.28,32
The role of ABA during lateral root and nodule development is more complex. In lateral roots, ABA appears to function later than auxin and cytokinin, regulating meristem activity during emergence from the primary root, but the effect of exogenous ABA is opposite in legumes and non-legumes: promoting lateral root elongation in legumes, but inhibiting it in non-legumes.33,34 In nodulation, the role of ABA is similarly complex: it exerts an inhibitory effect on nodule initiation, and a possible stimulatory effect on later nodule development. The inhibitory effect of ABA modulates the frequency of calcium spiking, such that high levels of ABA can counteract the effect of Nod factor, inhibiting nodule initiation.35 The role of ABA in nodule initiation was further confirmed by overexpressing the dominant abi1-1 allele of the Arabidopsis (ABI1) ABSCISIC ACID INSENSITIVE1 gene in Medicago, which interferes with ABA signaling and confers a hypernodulation phenotype.35 The phenotype of the latd mutant, in which nodules arrest shortly after emerging from the root, suggests a second role for ABA in nodule development at a later stage than initiation, probably via a stimulatory effect on meristem function.26
Phenotypes of other legume ABA signaling mutants suggest a complex pathway with at least one branch regulating nodulation, and with a second branch not affecting nodulation. Like the Arabidopsis abi1-1 allele, the Lotus japonicus ENF1 (ENHANCED NITROGEN FIXATION 1) gene functions in the ABA signaling branch that regulates nodule initiation. enf1 mutants have increased nodule number and higher nitrogen fixation.36,37 The Medicago STA (SENSITIVITY TO ABA) gene regulates both nodule initiation as well as cytokinin responses in the cortex.35 Evidence for a separate branch of the ABA signaling pathway that does not regulate nodulation comes from the L. japonicus beyma mutant, which is ABA-insensitive for several classic ABA-responsive phenotypes, but has no effect on nodule number.38 Together, analysis of these mutants suggests a complex role for ABA in coordinating multiple aspects of nodule development. Characterization of additional legume mutants will extend our understanding of the ABA signaling pathway and the way in which it intersects with nodulation.
Expression of LATD/NIP in the Root Meristem is Regulated by Auxin, Cytokinin and ABA
Expression of the LATD/NIP gene in root tips is strongly regulated by hormones: we showed that it is stimulated by cytokinin and inhibited by auxin and ABA2 (Fig. 2). The effect of these hormones on LATD/NIP expression in nodule meristems or primordia is unknown, but its strong stimulation by cytokinin in roots indicates a potential role for LATD/NIP in formation of the nodule primordium. LATD/NIP's activation by cytokinin also suggests that LATD/NIP may not act in the epidermal pathway, but rather in the cortical pathway or subsequent to primordium formation, during meristem activation. Although nodule development is defective in latd and nip mutants, nodule numbers are measured to be wild-type in the case of latd26 and two-fold higher than wild-type in the case of nip-1,1 suggesting that LATD/NIP may not function in AON. Curiously, LATD/NIP expression is not regulated by nitrate,2 which distinguishes it from many NRT members in the NRT1(PTR) family.39
Figure 2.
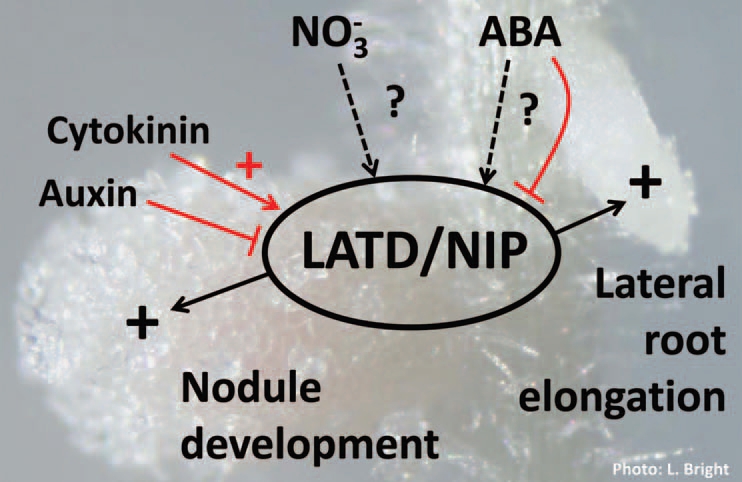
LATD/NIP function, regulation and potential biochemical activity. LATD/NIP is required for lateral root and nodule meristem activation (black arrows). LATD/NIP gene expression is upregulated in root tips by cytokinin and downregulated by auxin and ABA (red arrows and lines). We hypothesize that LATD/NIP could transport nitrate or ABA or another substance, or could possibly have another activity (dashed arrows).
Towards LATD/NIP Function
Is LATD/NIP a transporter? And if so, what does it transport? Most NRT1(PTR) members are still uncharacterized and phylogenetically close NRT1(PTR) family members have different functions (viz. AgDCAT1 and Arabidopsis NRT1.1) so it is risky to use phylogenetic position to assume function. Because of their large size, the CLE peptides that are induced by nitrate and rhizobia,18,22 and function in nitrate suppression of nodulation and AON are unlikely candidates. The CLE peptides are generally longer than 10 amino acids long, and thus significantly larger than the di- or tri-peptides transported by PTR family members. Also, there is no evidence that either LATD/NIP specifically or ABA signaling in general is involved in AON.2,38 If LATD/NIP is a transporter, more likely candidates for transport are nitrate and ABA (Fig. 2).
ABA would be a logical substrate for transport by LATD/NIP, since it is similar in size to a dipeptide, and is charged at neutral pH, thus requiring a transporter. A role for LATD/NIP as an ABA transporter would help explain the conundrum that latd mutants are ABA insensitive, yet can be rescued by adding ABA. If the defect in latd mutants were in getting ABA to the right place, then flooding the root system with ABA could be sufficient to rescue a transport defect. However, even though no member of the NRT1(PTR) family has been demonstrated to transport ABA, the recent observation that NRT1.1 can transport auxin in addition to nitrate, makes transport of ABA by LATD/NIP an intriguing possibility.12
Based on the position of LATD/NIP in clade IV of the NRT1(PTR) family, several of which have been shown to function as low-affinity nitrate transporters6,14–16 makes nitrate a strong candidate as the substrate for LATD/NIP. However it is difficult to rationalize a role for a low-affinity nitrate transporter as essential for nodulation since low-affinity (high concentration) nitrate transport would more likely be associated with nitrate suppression of nodulation. The only characterized dual-affinity (low- and high-affinity) nitrate transporter in the NRT1(PTR) family is NRT1.1 in clade I, not close phylogenetically to LATD/NIP. However, high- or dual-affinity nitrate transport should also be considered as a potential function for LATD/NIP, because of observations that low concentrations of nitrate do not suppress nodulation and promote root growth of legumes.40 With NRT1(PTR) member AgDCAT1 characterized as a dicarboxylate transporter,8 compounds such as dicarboxylates also become candidates.
An intriguing possibility is that LATD/NIP could have a different function from or in addition to that of a transporter. We note that dual-affinity NRT1.1 has been shown to have an additional role: that of nitrate sensor.9–11,13 LATD/NIP may function as a sensor or participate in a sensing mechanism, possibly of nitrate, or perhaps of a hormone such as ABA. Future biochemical studies should help pin down LATD/NIP's function.
Conclusions
The positioning of LATD/NIP expression in active meristems of both nodules and lateral roots, its homology to nitrate/peptide transporters and its regulation by hormones that direct lateral root and nodule development implicate LATD/NIP action at a crucial step in the process of coordinating lateral root and nodule development. An understanding of the biochemical mechanism(s) of LATD/NIP function and a more detailed knowledge of its role in meristem activation should clarify the molecular processes that function to regulate root architecture and symbiotic nodule formation in legumes.
Acknowledgements
Work in the Harris lab was supported by National Science Foundation grants IOB-0615822, IOS-0920096 and work in the Dickstein lab by NSF grants IOB-0520728 and IOS-0923756.
Abbreviations
- ABA
abscisic acid
- ABI1
abscisic acid insensitive 1
- AgDCAT1
Alnus glutinosa DICARBOXYLIC ACID TRANSPORTER 1
- AON
autoregulation of nodulation
- ATP
adenosine triphosphate
- CLE
CLAVATA3/ESR-related
- CLV1
CLAVATA 1
- ENF1
enhanced nitrogen fixation 1
- LATD
lateral root defective
- LRR-RLKs
leucine-rich repeat receptor-like kinases
- NAXT1
nitrate excretion transporter1
- NIP
numerous infection threads, polyphenolics
- NRT
nitrate transporter
- PTR
peptide transporter
- STA
sensitivity to ABA
- SUNN
super numeric nodules
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13165
References
- 1.Veereshlingam H, Haynes JG, Sherrier DJ, Penmetsa RV, Cook DR, Dickstein R. nip, a symbiotic Medicago truncatula mutant that forms root nodules with aberrant infection threads and plant defense-like response. Plant Physiol. 2004;136:3692–3702. doi: 10.1104/pp.104.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yendrek CR, Lee YC, Morris V, Liang Y, Pislariu CI, Burkart G, et al. A putative transporter is essential for integrating nutrient and hormone signaling with lateral root growth and nodule development in Medicago truncatula. Plant J. 2010;62:100–112. doi: 10.1111/j.1365-313X.2010.04134.x. [DOI] [PubMed] [Google Scholar]
- 3.Teillet A, Garcia J, de Billy F, Gherardi M, Huguet T, Barker DG, et al. api, a novel Medicago truncatula symbiotic mutant impaired in nodule primordium invasion. Mol Plant Microb Interact. 2008;21:535–546. doi: 10.1094/MPMI-21-5-0535. [DOI] [PubMed] [Google Scholar]
- 4.Bright L, Liang Y, Mitchell DM, Harris JM. LATD, a gene required for both nodule and root development. Mol Plant Microbe Interact. 2005;18:521–532. doi: 10.1094/MPMI-18-0521. [DOI] [PubMed] [Google Scholar]
- 5.Hauser M, Narita V, Donhardt AM, Naider F, Becker JM. Multiplicity and regulation of genes encoding peptide transporters in Saccharomyces cerevisiae. Mol Membr Biol. 2001;18:105–112. [PubMed] [Google Scholar]
- 6.Tsay YF, Chiu CC, Tsai CB, Ho CH, Hsu PK. Nitrate transporters and peptide transporters. FEBS Letts. 2007;581:2290–2300. doi: 10.1016/j.febslet.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Stacey G, Koh S, Granger C, Becker JM. Peptide transport in plants. Trends Plant Sci. 2002;7:257–263. doi: 10.1016/s1360-1385(02)02249-5. [DOI] [PubMed] [Google Scholar]
- 8.Jeong J, Suh S, Guan C, Tsay YF, Moran N, Jae Oh C, et al. A nodule-specific dicarboxylate transporter from alder is a member of the peptide transporter family. Plant Physiol. 2004;134:969–978. doi: 10.1104/pp.103.032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, et al. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci USA. 2006;103:19206–19211. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Xing X, Wang Y, Tran A, Crawford NM. A genetic screen for nitrate regulatory mutants captures the nitrate transporter gene NRT1.1. Plant Physiol. 2009;151:472–478. doi: 10.1104/pp.109.140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Krouk G, Crawford NM, Coruzzi GM, Tsay YF. Nitrate signaling: adaptation to fluctuating environments. Curr Opin Plant Biol. 2010;13:266–273. doi: 10.1016/j.pbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Fan SC, Lin CS, Hsua PK, Lin SH, Tsay YF. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell. 2009;21:2750–2761. doi: 10.1105/tpc.109.067603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almagro A, Lin SH, Tsay YF. Characterization of the Arabidopsis nitrate transporter NRT1.6 reveals a role of nitrate in early embryo development. Plant Cell. 2008;20:3289–3299. doi: 10.1105/tpc.107.056788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segonzac C, Boyer JC, Ipotesi E, Szponarski W, Tillard P, Touraine B, et al. Nitrate efflux at the root plasma membrane: Identification of an Arabidopsis excretion transporter. Plant Cell. 2007;19:3760–3777. doi: 10.1105/tpc.106.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson BJ, Indrasumunar A, Hayashi S, Lin MH, Lin YH, Reid DE, et al. Molecular analysis of legume nodule development and autoregulation. Journal of Integrative Plant Biol. 2010;52:61–76. doi: 10.1111/j.1744-7909.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, et al. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 2009;50:67–77. doi: 10.1093/pcp/pcn194. [DOI] [PubMed] [Google Scholar]
- 19.Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol. 2005;58:809–822. doi: 10.1007/s11103-005-8102-y. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 21.Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, et al. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science. 2003;299:109–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- 22.Mortier V, Den Herder G, Whitford R, Van de Velde W, Rombauts S, D'haeseleer K, et al. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 2010;153:222–237. doi: 10.1104/pp.110.153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology. 2002;53:203–224. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- 24.Nibau C, Gibbs DJ, Coates JC. Branching out in new directions: the control of root architecture by lateral root formation. New Phytol. 2008;179:595–614. doi: 10.1111/j.1469-8137.2008.02472.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Rong H, Pilbeam D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J Exp Bot. 2007;58:2329–2338. doi: 10.1093/jxb/erm114. [DOI] [PubMed] [Google Scholar]
- 26.Liang Y, Mitchell DM, Harris JM. Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Dev Biol. 2007;304:297–307. doi: 10.1016/j.ydbio.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 27.Nutman PS. Physiological studies on nodule formation. I. The relation between nodulation and lateral root formation in red clover. Ann Bot. 1948;112:81–96. [Google Scholar]
- 28.Ding Y, Oldroyd GED. Positioning the nodule, the hormone dictum. Plant Signal Behav. 2009;4:89–93. doi: 10.4161/psb.4.2.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Mol Biol. 2009;69:437–449. doi: 10.1007/s11103-008-9417-2. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Rizzo S, Laporte P, Crespi M, Frugier F. Legume Root Architecture: A Peculiar Root System. In: Beeckman T, editor. Annual Plant Reviews, Root Development. Wiley-Blackwell; 2010. pp. 239–287. [Google Scholar]
- 31.Malamy JE. Lateral Root Formation. In: Beeckman T, editor. Annual Plant Reviews, Root Development. Wiley-Blackwell; 2010. pp. 83–126. [Google Scholar]
- 32.Mathesius U. Auxin, at the root of nodule development? Funct Plant Biol. 2008;35:651–668. doi: 10.1071/FP08177. [DOI] [PubMed] [Google Scholar]
- 33.De Smet I, Signora L, Beeckman T, Inzé D, CH F, Zhang H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003;33:543–555. doi: 10.1046/j.1365-313x.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 34.Liang Y, Harris JM. Response of root branching to abscisic acid is correlated with nodule formation both in legumes and non-legumes. Am J Bot. 2005;92:1675–1683. doi: 10.3732/ajb.92.10.1675. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Kalo P, Yendrek C, Sun J, Liang Y, Marsh JF, et al. Abscisic acid coordinates Nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell. 2008;20:2681–2695. doi: 10.1105/tpc.108.061739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tominaga A, Nagata M, Futsuki K, Abe H, Uchiumi T, Abe M, et al. Effect of abscisic acid on symbiotic nitrogen fixation activity in the root nodules of Lotus japonicus. Plant Signal Behav. 2010;5:1–4. doi: 10.4161/psb.5.4.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tominaga A, Nagata M, Futsuki K, Abe H, Uchiumi T, Abe M, et al. Enhanced nodulation and nitrogen fixation in the abscisic acid low-sensitive mutant enhanced nitrogen fixation1 of Lotus japonicus. Plant Physiol. 2009;151:1965–1976. doi: 10.1104/pp.109.142638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas B, Chan PK, Gresshoff PM. A novel ABA insensitive mutant of Lotus japonicus with a wilty phenotype displays unaltered nodulation regulation. Mol Plant. 2009;2:487–499. doi: 10.1093/mp/ssp009. [DOI] [PubMed] [Google Scholar]
- 39.Gojon A. Faculty of 1000. 2010.
- 40.Fei H, Vessey JK. Stimulation of nodulation in Medicago truncatula by low concentrations of ammonium: quantitative reverse transcription PCR analysis of selected genes. Physiol Plant. 2008;135:317–330. doi: 10.1111/j.1399-3054.2008.01192.x. [DOI] [PubMed] [Google Scholar]