Abstract
The mitogen-activated protein kinase (MAPK) cascades play diverse roles in intra- and extra-cellular signaling in plants. MAP kinases are the component of kinase modules that transfer information from sensors to responses in eukaryotes including plants. They play a pivotal role in transduction of diverse extracellular stimuli such as biotic and abiotic stresses as well as a range of developmental responses including differentiation, proliferation and death. Several cascades are induced by different biotic and abiotic stress stimuli such as pathogen infections, heavy metal, wounding, high and low temperatures, high salinity, UV radiation, ozone, reactive oxygen species, drought and high or low osmolarity. MAPK signaling has been implicated in biotic stresses and has also been associated with hormonal responses. The cascade is regulated by various mechanisms, including not only transcriptional and translational regulation but through posttranscriptional regulation such as protein-protein interactions. Recent detailed analysis of certain specific MAP kinase pathways have revealed the specificity of the kinases in the cascade, signal transduction patterns, identity of pathway targets and the complexity of the cascade. The latest insights and finding are discussed in this paper in relation to the role of MAPK pathway modules in plant stress signaling.
Key words: MAPK cascade, classification, structural conformation, function, substrate specificity, factors
Introduction
Protein kinases and phosphatases play a central role in signal transduction through the phosphorylation and de-phosphorylation of proteins. This not only leads to the activation of defense responses, but also to the activation of developmental processes like cell growth and differentiation. One of the most commonly studied mechanisms is the mitogen activated protein kinase (MAPK) cascade, comprising a class of protein kinases that plays a crucial role in eukaryotic systems often linking perception of external stimuli with changes in cellular organization or gene expression. MAPK cascades are ancient and conserved signaling cassettes that are found in unicellular and multicellular eukaryotes. Phosphorylation plays a central role in the progression of the signal through the MAP kinase cascade.
MAP Kinase was first identified by Sturgill and Ray1 as microtubule-associated protein kinase and thus, was christened microtubule-associated protein kinases. MAPK comprise a family of serine/threonine protein kinases involved in the transduction of a variety of extracellular signals and the regulation of different developmental processes. MAPK pathways transfer information from sensors to activate cellular responses in all eukaryotes. A surprisingly large number of genes encoding MAPK pathway components have been uncovered by analyzing model plant genomes, highlighting their important role in signal transduction.2 Recent investigations have confirmed major roles of defined MAPK pathways in development, cell proliferation and hormone physiology, as well as in biotic and abiotic stress signaling.
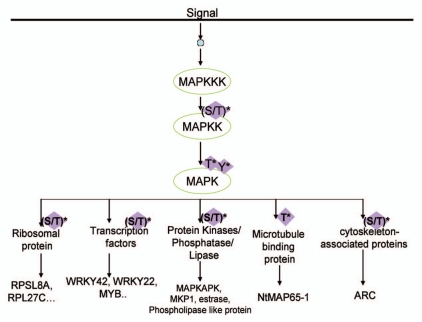
MAP Kinase cascades comprise of a series of sub-families, i.e., MAP4K, MAP3K, MAP2K, MAPK, that are sequentially activated.3,4 The sequential activation of the MAPK cascade eventually results in the activation of transcription factors, phospholipases or cytoskeletal proteins, microtubule-associated proteins and the expression of specific sets of genes in response to environmental stimuli.5–7 Plant homolog of all components of this cascade have been identified.8–14 MAPK also activates protein kinases that serve as a MAPK substrate named as MAPK activated protein kinases (MAPKAP-Kinase) found in mammalian system.15 Four classes of MAPK in Arabidopsis thaliana have been found.16,17 A generally accepted pathway of the MAPK cascade is shown in Figure 1. Animal MAPK comprises of three large families, i.e., ERK, JNK, p38 family. While plant MAPKs also constitute a large family—for example the Arabidopsis genome consists of 23 MAPKs, twelve of which are ERK type and the other are plant specific18,19—no obvious JNK or p38 MAPK homolog have been identified.
Figure 1.
A signal transduction cascade navigates the signal from MAPKKKK to MAPK by triggering a series of Threonine/Tyrosine and Serine/Threonine phosphorylation events, finally culminating in activated MAPK being transported to the nucleus where it is involved in the phosphorylation of transcription factors and reconfiguring a specific response related transcriptional reprogramming.
Different MAPK cascades are present in a single cell and often share common components. There is some crosstalk between pathways, but MAPK cassettes appear to be insulated from each other by the intrinsic specificity of the MAP2Ks and MAP3Ks and by binding interactions that are thought to organize the cassette into multi-enzyme complexes.20 Analysis of plant defense signaling cascades demonstrate the convergence of resistance gene recognition, elicitor, salicylate and also wound responses at the level of MAPK activation21 leading to changes in gene expression, cell division, cell growth and cell differentiation.22 Currently it is unclear whether these signaling pathways merge at the MAPK, upstream of MAPK or whether the same MAPK can mediate disparate responses by interacting with other proteins.23 This review attempts to address these complexities of MAPK signaling and highlight their role in diverse biological processes.
Classification of MAP Kinases
All MAPKs in plants are classified on the basis of two methods. One is phylogenetic based on analysis of the Arabidopsis genome and EST sequence data generated through sequencing projects.19,24 The other approach is based on functional analysis involving specific features and sequence signature motifs.4 The classification based on signature motif comprises of six families representing six functional groups.25 However, as phylogenetic relationships may not represent functional relationships the classification remains to be experimentally validated based on the biochemical, molecular and in silico studies.
The Arabidopsis genome contains 23 MAPKs, 10 MAP2Ks, 80 MAP3Ks and 10 MAP4K.16,19,26 Compared with MAPKs and MAP2Ks, MAP3Ks have more complex and variable primary structures and domain compositions. Average molecular mass of MAPKs ranges between 42–52 KDa.
Structure and Conformation Studies of MAPK
Crystallographic studies suggest that almost all members of MAPK family share similar three dimensional structures and function that are highly conserved.27–30 All MAPK are either two lobed structures or have two different structural subtypes comprising those containing a TEY phosphorylation motif in the activation loop (TEY subtype) and those containing a TDY motif (TDY subtype) within its active site buried at domain interface.31,32 The N-terminal domain (135 residues) is composed largely of beta-sheets and a glycine-rich loop that is termed the phosphate anchor ribbon, which acts as ATP binding pocket.33 The C-terminal domain (225 residues) contains a catalytic base, magnesium binding sites and the phosphorylation lip (activation loop).28,29 A common structural feature of MAPKs is the presence of a phosphorylation site in the activation loop between kinases domains.34
This 12 amino acid activation loop is a poorly conserved element at the C-terminal domain33,34 and forms the mouth of the active site i.e., ATP binding sites. The ATP binds between the two domains and the protein substrate is believed to be bound on the surface of the C-terminal domain. The N and C-terminals are predominantly composed of betastrand and alpha-helices respectively. The sequence of phosphorylation and activation loop influences the substrate specificity. The TXY(X-D/E/P) motif is a dual phosphorylation site and phosphorylation of both residues is a prerequisite for the activation of this cascade.37,38
Alignment of the amino acid sequences of many protein kinases reveals a common catalytic domain of 250–300 residues encoding the “two domain” structure.39 Protein kinases possess 12 conserved stretches of amino acids within their catalytic domains known as sub-domains.33,39,40 MAPKs have divergent region between sub-domain VII and VIII, located just before the TEY sequence, representing the phosphorylation site for activation by MAP2K. This domain also participates in the interaction of MAPK with its direct upstream activator MAP2K.41
MAPK Substrate Specificity
MAPKs are proline-directed serine/threonine kinases which phosphorylate the serine/threonine in the dipeptide motif S/T-P. However, ∼80% of all proteins have S/T-P sequences so it is unlikely that all these proteins are MAPK substrates.42 Notably, leucine at −1 and proline at −2 and −3 positions appear to influence whether the dipeptide acts as a phospho-acceptor.43
An important point to note is that the MAPK cascade is highly specific for its substrate i.e., MAPKK can specifically and selectively interact with a specific MAPK. Among various MAPK modules the recognition of an appropriate specific substrate by MAPK depends upon the differential interaction of two sites i.e., catalytic site and docking site. A typical MAPK consist of an active site and a common docking groove which are closely located and are implicated in recognition and binding of target proteins. MAPK signaling location, specificity and duration are regulated by scaffolding proteins and MAPK phosphatases.44 Scaffolding helps in holding the components of MAPK cascade via a single adapter together. Additionally, this arrangement also facilitate the interaction with the upstream factors that activates them, thus facilitating substrate recognition by the MAPK module.44–46
The specificity of different MAPK cascades functioning within the same cell is generated through the presence of docking domains that occur between sequence motifs in the substrate distal from the phosphorylation site and regions of the kinase outside the active site47 and that are found in various components of MAPK modules and scaffold proteins. Docking interactions enhance substrate affinity and specificity. Interaction of MAPK with partner proteins has been mapped by mutagenesis, hydrogen exchange-mass spectrometry and x-ray crystallography.48,49
Downstream phosphorylation events of MAPK cascades occur which may influence the regulation of different genes. Since, identification of first plant MAPK substrate in vivo, 1-aminoacyclopropane-1-carboxylic acid synthase, the rate-limiting enzyme in ethylene biosynthesis50—48 substrates of MAPK3 and 39 substrates of MAPK6 have been identified in Arabidopsis through the application of high-throughput proteomic screening.51,52 Additionally, MKS1 (MAP kinase substrate 1), which is required for full salicylic acid-dependent resistance, 53 has been reported to be phosphorylated by MAPK4 in Arabidopsis. Consistent with this, it has been reported that plant-specific WRKY transcription factors that contain the WRKYGQK core sequence and zinc-finger motif are phosphorylated by MAPKs, like MAPK4. This signaling cascade appears to be mediated through interaction with MKS and the target WRKY transcription factors such (WRKY25 and WRKY33).53,54 WRKY factors are associated with MAPK in the nucleus and the majority of terminal MAPKs appear to be within nucleus, associated with transcriptional complexes at target genes.55 A tobacco MAPK, NRK1/NTF6, positively regulates expansion of the phragmoplast. Phosphorylation of NtMAP65-1, a microtubule bundling protein by NRK1/NTF6 reduces its microtubule bundling activity, but not its binding ability and any single or point mutation in MAPKs phosphorylation site delays cytokinesis.7 MAP Kinase is also required to control of MAP65-1 in metaphase and telophase.56
The MAPK Cascade and its Regulation
A MAPK cascade minimally consists of a MAP3K-MAP2K-MAPK module that is linked in various configurations to upstream receptors and downstream targets. Among all components, MAP2Ks acts as a point of intersection and integration between converging signals from upstream MAP3Ks and divergent outputs through downstream MAPKs.32 Activation of a MAP3K can occur through physical interaction or phosphorylation by the receptor itself, intermediate bridging factors or interlinking MAP4Ks.16 MAP3Ks are serine/threonine kinases that activate MAP2Ks through phosphorylation on two serine/threonine residues in a conserved S/T-X3-5-S/T motif.57 Likewise, MAP2Ks are dual-specificity kinases that phosphorylate MAPKs on threonine and tyrosine residues in the T-X-Y motif.
Based on the number of MAP4K, MAP3K, MAP2K and MAPK, there are potentially thousands of combinations (10 × 80 × 10 × 23 = 184,000) that can be integrated into signaling pathways.19 The 20 MAPKs can be classified into four groups according to their sequences (TEY subtype) and structures.17 The related A, B and C groups and TDY subtype forming a more distant group D that lacks the CD domain, that is, serves as a docking interaction with MAP2K.17,58 However, remaining 3MAPKs (MAPK21–23) form a separate group-MHK group, related to both MAPKs and cyclin-dependent kinases (CDKs), but it is not clear whether they are also part of a MAPK signaling pathways32 or belong to other signaling pathways.
MAPK3 and MAPK6 of Group A and MAPK4 of Group B, have been extensively studied.59–67 MAPK4 is found to be an important negative regulator of systemic acquired resistance (SAR). These MAPKs are predominately involved in orchestrating response to stress and can be activated by a diverse set of stresses, including pathogens, osmotic stress, cold stress and oxidative stress.59,60,68–70 Downstream substrates of MAP2K3 i.e., MAPK1, MAPK2, MAPK7 and MAPK14 of Group D have been identified by yeast two-hybrid analysis.71 MAPK18, a member of group D, might target the microtubule cytoskeleton, which is proposed to be involved in integrating signaling pathways.72
The ten MAP2K members are classified into four groups. Group A includes MAP2K1, MAP2K2 and MAP2K6; Group B comprises a single member, MAP2K3; Group C MAP2K4 and MAP2K5; and Group D has MAP2K7, MAP2K8, MAP2K9 and MAP2K10. Classification of MAP2Ks is based upon protein alignment. Different members of same group are activated in the presence of different stimuli.73,74
The MAP3K family forms the largest and most complex group compared to the MAPK and MAP2K classes. 80 MAP3K are divided into three sub-families, i.e., MEKK family (21 members), ZIK like family (11 members) and the Raf-like family (48 members).58
MAP2K1 is involved in defense responses including flg22-induced activation of MAPK4.60,75 MAP2K2 is involved in cold and stress signaling. MAP2K659 is involved in cell division. MAP2K3 participates in jasmonate-mediated developmental signaling and in pathogen defense responses through activation of MAPK6.76 MAP2K4 and MAP2K5 are implicated in stomatal developmental responses.59,60,71,77 Function of the Group D MAP2Ks is not well understood. MAP2K7 has shown to be a negative regulator of polar auxin transport78 and a positive regulator of SAR.79 MAP2K9 may act as a negative regulator of abiotic stress responses.80 It was hypothesized that MAP2K7/MAP2K9 regulate cell death during pathogen defense.81 It has been reported recently that the MAP2K9-MAPK3/MAPK6 cascades promote ethylene-insensitive3 (EIN3)-mediated transcription in ethylene signaling.82 By contrast to this MAP3K8 (MEKK1) was also shown to act upstream of MAPK3,60 therefore it may be concluded that components involved in different functions might also form the same cascade.
The first complete MAPK cascade in regulating plant defense against bacterial pathogen, MAP3K1-MAP2K4/MAP2K5-MAPK3/MAPK6-WRKY22/WRKY29, was proposed as being downstream of the flagellin receptor kinase (FLS2 LRR), which potentially activates the MAP3K1 by phosphorylating the Ser/Thr residues.60 Beside this, the MAPK cascade comprising YODA-MAP2K4/MAP2K5-MAPK3/MAPK6, plays an important role in regulating stomatal development.83–86 Another MAPK cascade, MAP3K1-MAPKK1/MAPKK2-MAPK4-MKS1 mediate jasmonate- and salicylate-dependent defense responses.66 MAP3K1 acts as a negative regulator of plant immune responses and along with MAPK4 it interacts with two MAP2Ks i.e., MAP2K1 and MAP2K2.87
The MAP3K1-MAP2K1/2-MAPK4/6 module was activated by various stress treatments.59,68,75,88 The MAP2K3-MAPK6 module was activated by JA.76 Similarly, tobacco stress-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK) share a common upstream MAP2K, NtMEK2.14 One of the best studied MAP kinases, MAPK 6, was found to be activated after ethylene treatment. MAPK6 is also activated in the constitutive triple response1 (ctr1) mutant and is implicated in stomatal development, biotic and abiotic stress responses and embryo and floral organ development. The MAPK6 along with MAPK3, are required for complete “priming” of plants. The pre-stress deposition of these kinases is a critical step for full induction of defense responses during induced resistance.89
When the transcriptional activation of MAPK cascades are studied on the basis of Pearson correlation coefficients for all MAPK components of Arabidopsis genes, different co-regulation of MAPK components were evident. MAP2K1, MAP2K2, MAP2K4, MAP2K5 and MAPK3 are all strongly co-regulated with a number of WRKY transcription factors. MAP2K9, EDR1 and MAP3K8 (MEKK1) and MAPK3 show global correlations in their expression with each other. Similarly, a strong correlation was also seen in between MAP2K6 and MAP3K12, which are implicated in the regulation of cell proliferation or cytokinesis.90 Slightly weaker, but still significant correlations were seen between MAP2K6 and MAPK13, components that have been shown to interact and constitute a signaling pathway together.91 Likewise, MAP3K12, MAP2K6, MAPK13 all have a clear mitosis-specific expression pattern in cell synchronization experiments.32
MAPK Function
In plants, MAPK signaling appears to involve cross-talk with a variety of stress responses and developmental processes forming complex interconnected networks within cells.92 Traditional genetic and biochemical methods have identified MAP3K/MAP2K/MAPK signaling modules with overlapping roles in controlling diverse cellular functions. These include cell division, development, hormone signaling (abscisic acid-ABA, auxin and ethylene) and synthesis and response to abiotic stress (wounding, high and low temperature, high salinity, UV radiation, ozone, ROS, drought and high and low osmolarity, heavy metals) and pathogens.4,93–98 The responses to pathogen attack may include changes in redox status, the hypersensitive response-HR, cell death, generation of reactive oxygen species (ROS), systemic acquired resistance (SAR), activation of pathogenesis related-PR genes and other protective genes.25,99
Role of MAPK Under Abiotic Stress
Environmental stresses, such as cold, drought, salinity and heavy metals are important factors that affect growth and metabolism of plants. The MAP kinase pathways are intracellular signal modules that mediate signal transduction from the cell surface to the nucleus. Several cascades are induced by different abiotic stresses.4,100
Heavy metal toxicity is one of the current environmental health problems which result in the potentially dangerous bioaccumulation of toxic levels of heavy metals in the ecosystem. It has been reported that As3+, V4+, Cr3+, Cu6+ and Zn2+ activate MAP kinases in mammalian systems.101,102 Recently it was shown that heavy metals can activate MAPKs in higher plants.4,103 CdCl2 application results in the transcriptional activation of Arabidopsis MAP3K Arabidopsis MAP3K.103,104 Likewise, exposure of Medicago seedlings to excess copper or cadmium ions results in the activation of four distinct MAPKs: SIMK (stress-induced MAPK), MMK2 (MAPK2), MMK3 (MAPK3) and SAMK (stress-activated MAPK).4,105 MAPK3 and MAPK6 are activated in response to cadmium through the accumulation of ROS level produced by oxidative stress in Arabidopsis.93
In plants cold, drought and salt stresses all stimulate the accumulation of compatible osmolytes and antioxidants.106 Different MAPKs are activated by salt stress at different times after the onset of stress and the activities of these MAPKs are activated within different time periods.107 MAPK4 and MAPK6 are activated by cold, salt and drought.68,108 MAP2K1 is transcriptionally induced by salt stress, drought and cold,8 but it also mediates flagellin signaling via activation of MAP2K4 and MAP2K5.60 In contrast, a MAPK module consisting of MAP3K1-MAP2K1/MAP2K2-MAPK4/MAPK6 has now been confirmed in cold and salt stress by yeast two hybrid analyses and yeast complementation.59 Yeast two-hybrid analysis has recently been used to screen protein-protein interaction between all the Arabidopsis MAPKs. The outcome of these studies will generate an extensive pattern of interactions.109 Besides this, MAP2K1 is also get activated by wounding and drought stress and can phosphorylate MAPK4.59,77
Many stress-responsive genes have been identified and altered gene expression plays an important role in plant drought resistance.110–112 Nine genes have been identified that play an important role in mediating drought tolerance. Recently a novel nuclear protein kinase i.e., Drought-sensitive mutant 1 (DSM1) have been identified with sequence similarity to Raf-like MAP3Ks that play critical roles in drought and oxidative stress resistance in rice either by direct or indirect regulation.113
MAPKs are known to be activated by osmotic stresses in Medicago and Tobacco.4 During osmolarity signaling MAP kinases module seem to be widely involved.114 Understanding the in vivo function of Arabidopsis histidine kinase1 (AtHK) and other putative histidine kinases that function as osmo-sensors and show relation between osmotic stress-activated MAPK pathways will provide insight into osmotic stress signal transduction.115 To survive under high osmotic stress condition the two component signal regulates High Osmolarity Glycerol (HOG), which result in production of high glycerol.116 Yeast cells also respond to this hypertonic shock by activation of a MAP kinase cascade i.e., the HOG response pathway.117 Likewise, in alfalfa SIMKK-SIMK and in tobacco NtMEK2 (MAP2K)-SIPK/WIPK (SA-Induced Protein Kinase/Wounding-Induced Protein Kinase) are reported to be involved in osmotic stress.14,118 MAPK3 can also be activated by osmotic stress119 whereas; MAPK4 and MAPK6 are activated by wounding and touch.68 An interlink between osmotic stress and oxidative stress has been seen in Arabidopsis where it has been shown that many MAPK components have been activated or their gene expression is induced by salt and other stresses.120,121
Hydrogen peroxide (H2O2) is produced by various environmental and developmental stimulants and can act as a signaling molecule that regulates plant development and stress tolerance and programmed cell death (PCD) including aleurone cell death, the hypersensitive response to pathogens and allelopathic plantplant interactions.122 H2O2-induced PCD itself is essential for a number of developmental processes and environmental responses. H2O2 elicits the activation of a MAPK in Arabidopsis123 and it activates two MAPKs in Arabidopsis plants, one of which is also activated independently via SA, JA and ethylene signaling pathways.124 H2O2 activates, through the MAP3K ANP1, AtMPK6 and the related AtMPK3.125–127 As alluded to above, AtMPK6 is activated in response to elicitor challenge and cold stress.68,127,128 In addition, ozone and H2O2 treatment induced the activation of the tobacco ortholog of AtMPK, SIPK1.129 It was shown that transgenic tobacco plants overexpressing a tobacco MAP3K orthologue of ANP1 had increased tolerance to heat shock, freezing and salt stress, thus indicating that manipulation of a key signaling component responsive to H2O2 can protect plants against various environmental stresses.130 MAP2K is an important regulator of stomatal movement, which is believed to mediate the H2O2 generation induced by ABA in guard cells of Vicia faba.131 H2O2 may have an autocatalytic function to facilitate its own generation, e.g., once an H2O2-generation system was triggered, a small amount of H2O2 could accelerate MAP2K or CDPK activation due to the formation of an H2O2 feedback-loop. This crosstalk of H2O2 and MAPKKs or CDPKs may lead to the formation of a self-amplification loop.132 A rapid and very complex response are seen in nitric oxide (NO) and H2O2 in hypersensitive cell death.133 NO is involved in the activation of MAPK activity during the plant defense responses against pathogen infections in tobacco (Nicotiana tabacum)134 and Arabidopsis thaliana,135 and the adventitious root formation induced by indole acetic acid in cucumber (Cucumis sativus).136 An NO-activated protein kinase in tobacco with characteristics of a MAPK was identified as SIPK, for which SA is necessary for activation.135 NO synthesis and signaling also are implicated in the regulation of protein phosphates.131
Role of MAPK under Biotic Stress
The plants respond to pathogen attack by activating multi-step defense responses, including rapid production of reactive oxygen species (ROS), strengthening of cell walls and induction of the fHR leading to localized cell death at the sites of infection. Plant defense responses also include synthesis of pathogen-related proteins and phytoalexins.60,110 It has been firmly established that MAPKs play a central role in pathogen defense in Arabidopsis, tobacco, tomato, parsley, brassica and rice.
The role of MAPKs has been seen during symbiotic association between plant and pathogenic organism. A putative role of the MAPKs i.e., SIMK and SAMK in rhizobia-legume symbiosis has been suggested in Lupinus albus. MAPKs are involved in the infection and nodulation of L. albus by Bradyrhizobium sp. (Lupinus). Inoculation of L. albus with B. lupinus leads to activation of SIMK and SAMK while inoculation with dead B. lupinus along with Sinorhizobium meliloti did not induce SIMK and SAMK.137 This suggests that activation of these MAPK pathways is a specific response of the host cells to live bacteria which may lead to a successful symbiotic interaction, suggesting that MAPKs may take part in the recognition of compatible partners. Beside this symbiotic association, host MAPK cascades are also activated by fungal pathogens.138 Infection by plant fungal pathogen Phytophthora infestans led to the rapid transcriptional induction of MAP3K19, MAP2K9 and MAP2K4, while with that of Botrytis cinerea infection led to the rapid transcriptional induction of MAP3K18, MAP3K19 and MAP3K20, Raf43, ZIK2, ZIK8, suggesting that signaling to bacterial and fungal pathogen attack is distinct.32 The alfalfa MAPKs, SIMK and SAMK, which were originally identified as inducible by osmotic stress139 and wounding,140 respectively, were later also found to be activated by various fungal elicitors.141 Two other alfalfa MAPKs, MMK2 and MMK3 (Medicago MAPK2 and MAPK3, respectively), are involved in cell growth and division142 but are also activated by elicitors. Despite all this, a fungal biocontrol agent Trichoderma asperellum has recently been shown to induce systemic resistance in plants through a mechanism that employs jasmonic acid and ethylene signal-transduction pathways leading to induction of a Trichoderma-induced MAPK (TIPK) gene in cucumber (Cucumis sativus).143
Role of MAPKs in Stress-Induced Plant Growth and Development
MAPKs signaling act as a positive regulators for environmental stress-induced, stomatal closure and negative regulators for stomatal development.83,84,144 Stomatal development in Arabidopsis is regulated by a MAPK cascade involving the MAP3K-YODA, the MAP2Ks-MAP2K4 and MAP2K5, the MAPKs MAPK3 and MAPK6 and the bHLH transcription factor SPEECHLESS (SPCH).62,145 This cascade regulates asymmetric cell division that provides cell fate specification in stomata. Arabidopsis thaliana MAP3K-YODA plays an important role in early embryo development and its mutant loses the property to differentiate an extra embryonic suspensor. Absence of this protein compromises stomatal cell specification.83,86 It has been demonstrated that MAP2K7 and MAP2K9 also play role as a positive and negative regulators respectively, at different stages of stomatal development.147 A role for MAPK6 in early root development in Arabidopsis thaliana has been reported. MAPK6 expression is high in most apical parts of the root meristem and in the root transition zone.146
Crosstalk of MAPKs in Different Stress-Induced Pathways
Emerging evidence suggests that hormone signaling pathways (abscisic acid, salicylic acid, jasmonic acid, auxin and ethylene) as well as ROS signalling pathways, play key roles in the crosstalk between biotic and abiotic stress signaling.148 These hormones may interact with one another in regulating stress signaling and plant stress tolerance. MAPKs may have role in ABA signaling, which mediates responses to environmental stress, chiefly water stress. MAPK9 and MAPK12, which are preferentially activated in guard cells, share functional redundancy and serve as positive regulators acting downstream of reactive oxygen species and calcium signaling, but upstream of anion channels in guard cell ABA signaling.149 Besides this, MAPK are activated by low concentration application of ABA to barley aleurone protoplasts.150 The role of ABA in osmotic stress signal transduction was previously addressed by studying the stress induction of several genes in Arabidopsis.151 A calcium-independent protein kinase in faba bean (Vicia faba) has been isolated, and which was activated upon ABA treatment but unexpectedly could not be immunoprecipitated with an anti-phosphotyrosine antibody. Thus, the role of MAPK in ABA signaling has not yet been directly established but cannot yet be ruled out.
The role of MAPK signaling cascades and protein phosphorylation in auxin signaling has been documented in numerous studies. For example, auxin was shown to activate an unknown MAPK in Arabidopsis seedling roots.152 A tobacco MAP3K activated in response to oxidative stress was found to negatively regulate auxin induced gene expression.153 Recently, the Arabidopsis MAP2K7 was shown to control polar auxin transport.78 However, there are no MAPK substrates known to affect gene expression in response to auxin. Some evidence shows that H2O2 induced MAPK cascade leads to specific stress-responsive gene regulation but it inhibits the action of auxin,154 revealing a molecular link between oxidative stress response and auxin signal transduction.
Conclusion
Significant progress on the description of MAPK signaling in plants has been made in recent years using a combination of physiological, biochemical and genetic approaches. Over the past few years researchers have identified a multitude of signaling responses that involve MAPKs. These studies are now focused on some of the complete MAPK cascades that have been identified for signaling in biotic and abiotic stresses but a few are still missing. So a need still exists to design novel approaches and strategies to define specific functions and elucidate the underlying mechanisms of signal transduction through identification and validation of functional plant MAPK components. Potentially useful approaches to dissect the stress stimulated MAP kinase pathways in response to various stimuli will come from integrating publically available transcriptomics datasets and development of targeted phospho/proteomics approaches in combination with knockout and knock in transgenic approaches to perturb MAPK signaling networks. Substrates and signaling components in different stress induced signaling pathways will be identified through the application of protein-protein interaction, approaches (yeast two hybrid system, SPR, Immuno-chips).
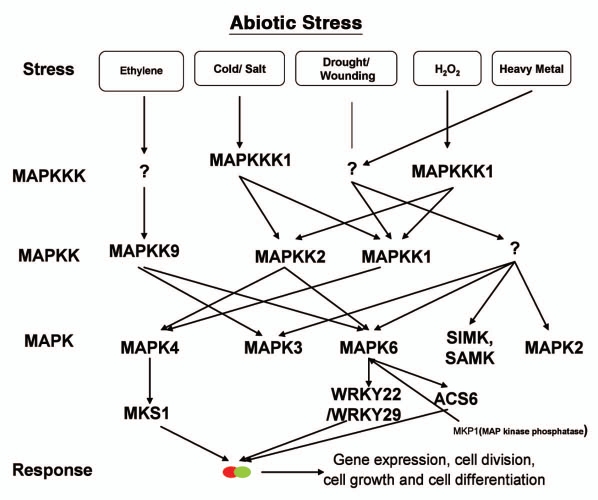
Figure 2.
Model describing the interconnectivity between different members of kinases cascades involve in abiotic stress responses.
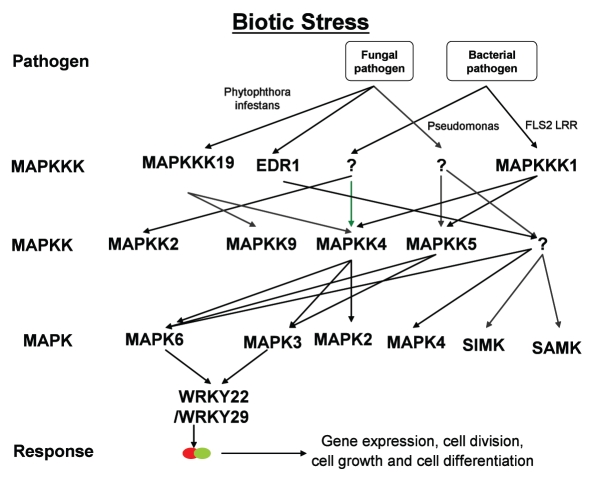
Figure 3.
Model describing the interconnectivity between different members of kinases cascades involved in biotic stress responses.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13020
References
- 1.Sturgill TW, Ray LB. Muscle proteins related to microtubule associated protein-2 are substrates for an insulinstimulatable kinase. Biochem Biophys Res Commun. 1986;134:565–571. doi: 10.1016/s0006-291x(86)80457-0. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG. Plant protein serine/threonine kinases: classification and functional. Ann rev Plant physiol Plant Mol Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- 3.Tatebayashi K, Takekawa M, Saito H. A docking site determining specificity of Pbs2 MAPKK for Ssk2/Ssk22 MAPKKKs in the yeast HOG pathway. EMBO J. 2003;22:3624–3634. doi: 10.1093/emboj/cdg353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonak C, Okresz L, Bogrw L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5:415–424. doi: 10.1016/s1369-5266(02)00285-6. [DOI] [PubMed] [Google Scholar]
- 5.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 6.Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, et al. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003;132:1961–1972. doi: 10.1104/pp.103.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, et al. Phosphorylation of NtMAP65-1 by a MAP kinase downregulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006;20:1004–1014. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizoguchi T, Hayashida N, Yamaguchi-shinozaki K, Kamada H, Shinozaki K. AtMPKs: a gene family of MAPK in Arabidopsis thaliana. FEBS Let. 1993;336:440–444. doi: 10.1016/0014-5793(93)80852-l. [DOI] [PubMed] [Google Scholar]
- 9.Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, et al. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi T, Ichimura K, Irei K. Identification of a possible MAPK cascade in Arabidopsis thaliana based on pairwise yeast two hybrid analysis and functional test of yeast mutants. FEBS Let. 1998;437:56–60. doi: 10.1016/s0014-5793(98)01197-1. [DOI] [PubMed] [Google Scholar]
- 11.Seo S, Okamoto N, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP Kinase- a possible mediator in wound signal transduction pathways. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 12.Ligterink W, Kroj T, Zurnieden U, Hirt H, Scheel D. Receptor mediated activation of MAP Kinase in pathogen defense of plants. Science. 1997;276:2054–2057. doi: 10.1126/science.276.5321.2054. [DOI] [PubMed] [Google Scholar]
- 13.Zhang SQ, Klessig DF. Salicyclic acid activates 48 KDa MAP kinase in tobacco. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang KY, Liu Y, Zhang S. Activation of a Mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA. 2001;98:741–746. doi: 10.1073/pnas.98.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerits N, Shiryaev A, Kostenko S, Klenow H, Shiryaeva O, Johannessen M, et al. The transcriptional regulation and cell-specific expression of the mapk-activated protein kinase mk5. Cell Mol Biol Letter. 2008;14:548–574. doi: 10.2478/s11658-009-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swarbreck D, Wilks C, Lamesch P, Berardini TZ, Garcia-Hernandez M, Foerster H, et al. The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res. 2008;36:1009–1014. doi: 10.1093/nar/gkm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MAPK group, author. Mitogen activated protein kinases cascades in plants: A new nomenclature. Trends Plants Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- 18.Katuo S, Karita E, et al. Catalytic activation of the plant MAPK phosphatase NtMKP1 by its physiological substrate salicylcic acid-induced protein kinase but not by Calmodulins. J Biol Chem. 2005;280:59569–59581. doi: 10.1074/jbc.M508115200. [DOI] [PubMed] [Google Scholar]
- 19.Cvetkovska M, Ramptitsch C, Bykova N, Xing T. Genomic analysis of MAPK Cascade in Arabidopsis defense responses. Plant Mol Biol Rep. 2005;23:331–343. [Google Scholar]
- 20.Zaitsevskaya-Carter T, Cooper JA. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. EMBO J. 1997;16:1318–1331. doi: 10.1093/emboj/16.6.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romies T, Peidras P, Zhang S, Klessig DF, Hirt H, Jones JDG. Rapid Avr9 and Cf9 dependent activation of MAPK in tobacco cell cultures and leaves: convergence of resistance gene.licitor, wound and salicylate response. Plant Cell. 1999;11:273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Gibson TB, Robinson R, Silvestrol PG, Xu B, Wright A, et al. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 23.Cobb M, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 24.MAPK Project Functional analysis of plant MAPK cascades in stress and hormone signaling. 2005. http://genetics.mgh.harvard.edu/sheenweb/mapk_project.html.
- 25.Zhang SQ, Klessig DF. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001;6:520–527. doi: 10.1016/s1360-1385(01)02103-3. [DOI] [PubMed] [Google Scholar]
- 26.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signaling network involved in multiple biological processes. Biochem J. 2008;413:217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Strand A, Robbin D, Cobb MH, Goldsmith EJ. Structure of the MAP kinase ERK2 at 2.3 Å resolutions. Nature. 1994;367:704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- 28.Wilson KP, Fitzgibbon MJ, Caron PR, Griffith JP, Chen W, MccCaffrey PG, et al. Crystal structure of p38 mitogen-activated protein kinase. J Biol Chem. 1996;271:27696–27700. doi: 10.1074/jbc.271.44.27696. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Harkins PC, Ulevitch RJ, Han J, Cobb MH. Goldsmith EJ, The structure of MAPK p38 at 2.1-A resolution. Proc Natl Acad Sci USA. 1997;94:2327–2332. doi: 10.1073/pnas.94.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie H, Pallero MA, Gupta K, Chang P, Ware MF, Witke W, et al. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCgamma signaling pathway. J Cell Sci. 1998;111:615–624. doi: 10.1242/jcs.111.5.615. [DOI] [PubMed] [Google Scholar]
- 31.Goldsmith EJ, Cobb MH. Protein kinases. Curr Opin Struct Biol. 1994;4:833–840. doi: 10.1016/0959-440x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 32.Menges M, Doczi R, Okresz L, Morandini P, Mizzi L, Soloviev M, et al. Comprehensive gene expression atlas for the Arabidopsis MAP kinase signalling pathways. New Phytol. 2008;179:643–662. doi: 10.1111/j.1469-8137.2008.02552.x. [DOI] [PubMed] [Google Scholar]
- 33.Knigton DR, Zheng J, Ten Eyck LF, Xuong HH, Taylor SS, Sowadski JM. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:414–429. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- 34.Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 35.Robinson MJ, Cheng M, Khokhlatchev A, Ebert D, Ahn N, Guan KL, et al. Contributions of the mitogen-activated protein (MAP) kinase backbone and phosphorylation loop to MEK specificity. J Biol Chem. 1996;271:29734–29739. doi: 10.1074/jbc.271.47.29734. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Li Z, Schwarz EM, Lin A, Guan K, Ulevitch RJ, et al. Structure-function studies of p38 mitogen-activated protein kinase. Loop 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J Biol Chem. 1997;272:11096–11102. doi: 10.1074/jbc.272.17.11096. [DOI] [PubMed] [Google Scholar]
- 37.Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Oshima Y. Identification of the regulatory phosphorylation sites in pp42/mitogen activated protein kinase (MAP Kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gartner A, Nasmyth K, Ammerer G. Signal transduction in Saccharamyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes Dev. 1992;6:1280–1292. doi: 10.1101/gad.6.7.1280. [DOI] [PubMed] [Google Scholar]
- 39.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1998;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 40.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 41.Mizoguchi T, Hayashida N, Shinozaki K. MAP Kinasis in plants. Riken Rev. 1994;6:25–26. [Google Scholar]
- 42.Bardwell L. Mechanisms of MAPK signaling specificity. Biochemical Society Transactions. 2006;34:837–841. doi: 10.1042/BST0340837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshioka K. Scaffold proteins in mammalian MAP kinase cascades. J Biochem. 2004;135:657–661. doi: 10.1093/jb/mvh079. [DOI] [PubMed] [Google Scholar]
- 45.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 46.Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 47.Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J Biol Chem. 2008;283:19511–19520. doi: 10.1074/jbc.M801074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanoue T, Adachi M, Moriguchi T, Nishida EA. conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 49.Chang CI, Xu B, Akella R, Cobb M, Goldsmith EJ. Crystal structure of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol Cell. 2002;9:1241–1249. doi: 10.1016/s1097-2765(02)00525-7. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Zhang S. Phosphorylation of 1-aminocyclo-propane-1-Carboxylic acid synthase by MAPK6, a stress-responsive MAPK, induce ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peck SC. Early phosphorylation events in biotic stress. Curr Opin Plant Biol. 2003;6:334–338. doi: 10.1016/s1369-5266(03)00056-6. [DOI] [PubMed] [Google Scholar]
- 52.Feilner T, Hultschig C, Lee J, et al. High throughput identification of potential Arabidopsis mitogen-activated protein kinases substrates. Mol Cell Proteom. 2005;4:1558–1568. doi: 10.1074/mcp.M500007-MCP200. [DOI] [PubMed] [Google Scholar]
- 53.Andreasson E, Jenkins T, Brodersen P, et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005;24:2579–2589. doi: 10.1038/sj.emboj.7600737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menke FL, Pelt JA, Pieterse CM, Klessig DF. Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell. 2004;16:897–907. doi: 10.1105/tpc.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- 56.Smertenko AP, Chang HY, Sonobe S, Fenyk SI, Weingartner M, Bogre L, et al. Control of the AtMAP65-1 interaction with microtubules through the cell cycle. J Cell Sci. 2006;119:3227–3237. doi: 10.1242/jcs.03051. [DOI] [PubMed] [Google Scholar]
- 57.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci. 2005;10:1360–1385. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Shun-Wu Y, Ke-Xaun T. MAP kinase cascades responding to environment stress in plants. Acta Botanica Sinica. 2004;46:127–136. [Google Scholar]
- 59.Teige M, Scheik E, Eulgem T, Do czi R, Ichimura K, Shinozaki K, et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 60.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, et al. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 61.Ecker JR. Re-entry of the ethylene MAPK6 module. Plant Cell. 2004;16:3164–3174. [Google Scholar]
- 62.Lampard GR, Macalister CA, Bergmann DC. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science. 2008;322:1113–1116. doi: 10.1126/science.1162263. [DOI] [PubMed] [Google Scholar]
- 63.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/s0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 64.Hord LH, Sun YJ, Pillitteri LJ, Torri KV, Wang H, Zhang S, et al. Regulation of Arabidopsis early anther development by the Mitogen activated protein kinases, MPK3 and MPk6 and the ERECTA and related receptor like kinases. Mol Plant. 2008;4:645–658. doi: 10.1093/mp/ssn029. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Liu Y, Bruffett K, Lee J, Hause G, Walker JC, et al. Haplo insufficiency of MAPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell. 2008;20:602–613. doi: 10.1105/tpc.108.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu JL, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, Thorgrimsen S, et al. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 2008;27:2214–2221. doi: 10.1038/emboj.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bethke G, Unthan T, Uhrig JF, Poschi Y, Gust AA, Scheel D, et al. Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis via ethylene signaling. Proc Natl Acad Sci USA. 2009;106:8067–8072. doi: 10.1073/pnas.0810206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ichimura K, et al. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 69.Yuasa T, Ichimura K, Mizoguchi T, Shinozaki K. Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol. 2001;42:1012–1016. doi: 10.1093/pcp/pce123. [DOI] [PubMed] [Google Scholar]
- 70.Mishra NS, Tuteja R, Tuteja N. Signaling through MAP kinase networks in plants. Arch Biochem Biophys. 2006;452:55–68. doi: 10.1016/j.abb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Doczi R, Brader G, Pettko-Szandtner A, Rajh I, Djamei A, Pitzschke A, et al. The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell. 2007;19:3266–3279. doi: 10.1105/tpc.106.050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walia A, Lee JS, Wasteneys G, Ellis B. Arabidopsis mitogen-activated protein kinase MPK18 mediates cortical microtubule functions in plan cells. Plant J. 2009;59:565–575. doi: 10.1111/j.1365-313X.2009.03895.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhou C, Cai Z, Guo Y, Gan S. An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 2009;150:167–177. doi: 10.1104/pp.108.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, et al. Activation of MAPK Kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem. 2008;283:26996–27006. doi: 10.1074/jbc.M801392200. [DOI] [PubMed] [Google Scholar]
- 75.Meszaros T, Helfer A, Hatzimasoura E, Magyar Z, Serazetdinova L, Rios G, et al. The Arabidopsis MAP kinase kinase MKK1 participates in defense responses to the bacterial elicitor flagellin. Plant J. 2006;48:485–498. doi: 10.1111/j.1365-313X.2006.02888.x. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, et al. The Mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell. 2007;19:805–818. doi: 10.1105/tpc.106.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuoka D, et al. Activation of AtMEK1, an Arabidopsis mitogen-activated protein kinase kinase, in vitro and in vivo: analysis of active mutants expressed in E. coli and generation of the active form in stress response in seedlings. Plant J. 2002;29:637–647. doi: 10.1046/j.0960-7412.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 78.Dai Y, Wang H, Li B, Huang J, Liu X, Zhou Y, et al. Increased expression of MAP Kinase Kinase 7 causes deficiency in polar auxin transport and leads to plant architectural abnormality in Arabidopsis. Plant Cell. 2006;18:308–320. doi: 10.1105/tpc.105.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, Dai Y, Xiong Y, Defraia C, Li J, Dong X, et al. Overexpression of Arabidopsis MAP kinase kinase 7 leads to activation of plant basal and systemic acquired resistance. Plant J. 2007;52:1066–1079. doi: 10.1111/j.1365-313X.2007.03294.x. [DOI] [PubMed] [Google Scholar]
- 80.Alzwiya IA, Morris PC. A mutation in the Arabidopsis MAP kinase kinase 9 gene results in enhanced seedling stress tolerance. Plant Sci. 2007;173:302–308. [Google Scholar]
- 81.Popescu SC, Popescu GV, Snyder M, Dinesh SP. Integrated analysis of co-expressed MAP kinase substrates in Arabidopsis thaliana. Plant Signal & Behav. 2009;4:524–527. doi: 10.4161/psb.4.6.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J. Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signaling. Nature. 2008;451:789–795. doi: 10.1038/nature06543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- 84.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by Arabidopsis MAPK network environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, et al. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bayer M, Nawy T, Giglione C, Galli M, Meinnel T, Lukowitz W. Paternal Control of Embryonic Patterning in Arabidopsis thaliana. Science. 2009;323:1485–1488. doi: 10.1126/science.1167784. [DOI] [PubMed] [Google Scholar]
- 87.Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, et al. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008;18:1190–1198. doi: 10.1038/cr.2008.300. [DOI] [PubMed] [Google Scholar]
- 88.Brader G, Djamei A, Teige M, Palva ET, Hirt H. The MAP kinase kinase MKK2 affects disease resistance in Arabidopsis. Mol Plant Microbe Interact. 2007;20:589–596. doi: 10.1094/MPMI-20-5-0589. [DOI] [PubMed] [Google Scholar]
- 89.Beckers GJM, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, et al. Mitogen-Activated Protein Kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell. 2009;21:944–953. doi: 10.1105/tpc.108.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soyano T, Nishihama R, Morikiyo K, Ishikawa M, Machida Y. NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK mediated MAPK cascade and is required for plant cytokinesis. Genes Dev. 2003;17:1055–1067. doi: 10.1101/gad.1071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melikant B, Giuliani C, Halbmayer-Watzina S, Limmongkon A, Heberle-Bors E, Wilson C. The Arabidopsis thaliana MEK AtMKK6 activates the MAP kinase AtMPK13. FEBS Let. 2004;576:5–8. doi: 10.1016/j.febslet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 92.Pedley KF, Martin GB. Role of mitogen-activated protein kinases in plant immunity. Curr Opin Plant Biol. 2005;8:541–547. doi: 10.1016/j.pbi.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Liu XM, Kim KE, Kim KC, Nguyen XC, Han HJ, Jung MS, et al. Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry. 2010;71:614–618. doi: 10.1016/j.phytochem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Jonak C, Nakahami H, Hirt H. Heavy metal stress activation of distinct mitogen activated protein kinase pathway by copper and cadmium. Plant Physiol. 2004;136:3276–3283. doi: 10.1104/pp.104.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirt H. Connecting oxidative stress, auxin and cell cycle regulation through a plant MAPK pathway. Proc Natl Acad Sci USA. 2000;97:2405–2407. doi: 10.1073/pnas.97.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin CW, Chang HB, Huang HJ. Zinc induces mitogen-activated protein kinase activation mediated by reactive oxygen species in rice roots. Plant Physiol Biochem. 2005;43:963–968. doi: 10.1016/j.plaphy.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Yeh CM, Hsiao LJ, Huang HJ. Cadmium activates a mitogen activated protein kinase gene and MBP kinases in rice. Plant Cell Physiol. 2004;45:1306–3283. doi: 10.1093/pcp/pch135. [DOI] [PubMed] [Google Scholar]
- 98.Usami S, Bannon H, Itoy Y, Nishama R, Machida Y. Cutting activates a 46 KDa protein kinases in plants. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xing T, Ouellet T, Miki BL. Towards genomic and proteomic studies of protein phosphorylation in plant-pathogen interaction. Trends Plant Sci. 2002;7:224–231. doi: 10.1016/s1360-1385(02)02255-0. [DOI] [PubMed] [Google Scholar]
- 100.Mizoguchi T, Ichimura K, Shinozaki K. Environmental stress response in plants: The role of mitogen-activated protein kinases. Trends Biotechnol. 1997;15:15–19. doi: 10.1016/S0167-7799(96)10074-3. [DOI] [PubMed] [Google Scholar]
- 101.Tessier DM, Pascal LE. Activation of MAPK by hexavalent chromium, magnese and nickel in human epithelial cell. Toxicol Let. 2006;167:114–121. doi: 10.1016/j.toxlet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 102.Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, et al. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am J Physiol-Lung Cell Mol Physiol. 1998;275:551–558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 103.Yeh CM, Chien PS, Huang HJ. Distinct signaling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J Exp Bot. 2007;58:659–671. doi: 10.1093/jxb/erl240. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki N, Koizumi N, Sano H. Screening of cadmium-responsive genes in Arabidopsis thaliana reveals protein denaturation and oxidative stresses to be critical components of cadmium toxicity. Plant Cell Environ. 2001;24:1177–1188. [Google Scholar]
- 105.Cardinale F, Jonak C, Ligtering W, Niehaus K, Boller T, Hirt H. Differential activation of four specific MAPK pathways by distinct elicitors. J Biol Chem. 2000;275:36734–36740. doi: 10.1074/jbc.M007418200. [DOI] [PubMed] [Google Scholar]
- 106.Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Mol Plant Physiol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 107.Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell. 2000;12:165–178. [PMC free article] [PubMed] [Google Scholar]
- 108.Droillard MJ, et al. Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Let. 2004;574:42–48. doi: 10.1016/j.febslet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Lee JS, Huh KW, Bhargava A, Ellis BE. Comprehensive analysis of protein-protein interactions between Arabidopsis MAPKs and MAPK kinases helps define potential MAPK signalling modules. Plant Signal Behav. 2008;3:1037–1041. doi: 10.4161/psb.3.12.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee HK, Cho SK, Son O, Xu Z, Hwang I, Kim WT. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell. 2009;21:622–641. doi: 10.1105/tpc.108.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, et al. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and post transcriptionally to promote drought resistance. Plant Cell. 2008;20:2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ning J, Li X, Hicks LM, Xiong L. A Raf-Like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010;152:876–890. doi: 10.1104/pp.109.149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nuccio ML, Rhodes D, McNeil SD, Hanson AD. Metabolic engineering of plants for osmotic stress resistance. Curr Opin Plant Biol. 1999;2:128–134. doi: 10.1016/s1369-5266(99)80026-0. [DOI] [PubMed] [Google Scholar]
- 115.Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, et al. Transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaur N, Gupta AK. Signal transduction pathways under abiotic stresses in plants. Curr Sci. 2005;88:1771–1780. [Google Scholar]
- 117.Brewster JL, Valoir TD, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 118.Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, Liwosz A, et al. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress induced MAPK, SIMK. Plant Cell. 2000;12:2247–2258. doi: 10.1105/tpc.12.11.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Droillard M, et al. Different protein kinase families are activated by osmotic stresses in Arabidopsis thaliana cell suspensions. Involvement of the MAP kinases AtMPK3 and AtMPK6. FEBS Lett. 2002;527:43–50. doi: 10.1016/s0014-5793(02)03162-9. [DOI] [PubMed] [Google Scholar]
- 120.Meskiene I, Hirt H. MAP kinase pathways. Molecular plug- and-play chips for the cell. Plant Mol Biol. 2000;42:791–806. doi: 10.1023/a:1006405929082. [DOI] [PubMed] [Google Scholar]
- 121.Xiong L, Ishitani M, Zhu JK. Interaction of osmotic stress, ABA and low temperature in the regulation of stress gene expression in Arabidopsis thaliana. Plant Physiol. 1999;119:205–211. doi: 10.1104/pp.119.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 123.Desikan R, Clarke A, Hancock JT, Neill SJ. H2O2 activates a MAP kinase-like enzyme in Arabidopsis thaliana suspension cultures. J Exp Bot. 1999;50:1863–1866. [Google Scholar]
- 124.Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000;23:441–450. doi: 10.1046/j.1365-313x.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- 125.Palavan-Unsal N, Arisan D. Nitric oxide signaling in plants. Springer. 2009;75:203–229. [Google Scholar]
- 126.Neill SJ, Radhika D, John TH, Kazuya I, Kazuo S. Harpin induces activation of the Arabidopsis MAP Kinases AtMPK4 and AtMPK6. Plant Physiol. 2001;126:1579–1587. doi: 10.1104/pp.126.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ. Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 2001;26:1579–1587. doi: 10.1104/pp.126.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nuhse TS, Peck SC, Hirt H, Boller T. Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK6. J Biol Chem. 2000;275:7521–7526. doi: 10.1074/jbc.275.11.7521. [DOI] [PubMed] [Google Scholar]
- 129.Samuel MA, Miles GP, Ellis BE. Ozone treatment rapidly activates MAP kinase signaling in plants. Plant J. 2000;22:367–376. doi: 10.1046/j.1365-313x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 130.Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, et al. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytologist. 2007;175:36–50. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 132.Song XG, She XP, Guo IY, Meng ZN, Huang AX. MAPK kinase and CDP kinase modulate hydrogen peroxide levels during dark-induced stomatal closure in guard cells of Vicia faba. Botan Stud. 2008;49:323–334. [Google Scholar]
- 133.De Gara L, de Pinto MC, Moliterni VM, D'Egidio MG. Redox regulation and storage processes during maturation in kernels of Triticum durum. J Exp Bot. 2003;54:249–258. doi: 10.1093/jxb/erg021. [DOI] [PubMed] [Google Scholar]
- 134.Kumar D, Klessig DF. Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene and jasmonic acid. Mol Plant Microbe Interact. 2000;13:347–351. doi: 10.1094/MPMI.2000.13.3.347. [DOI] [PubMed] [Google Scholar]
- 135.Clark D, Durner J, Navarre DA, Klessig DF. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact. 2000;13:1380–1384. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- 136.Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 2004;135:279–286. doi: 10.1104/pp.103.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernandez-Pascual M, Lucas MM, Felipe MR, Bosca L, Hirt H, Golvano MP. Involvement of mitogen-activated protein kinases in the symbiosis Bradyrhizobium-Lupinus. J Exp Bot. 2006;57:2735–2742. doi: 10.1093/jxb/erl038. [DOI] [PubMed] [Google Scholar]
- 138.Izumitsu K, Yoshimi A, Kubo D, Morita A, Saitoh Y, Tanaka C. The MAPKK kinase ChSte11 regulates sexual/asexual development, melanization, pathogenicity and adaptation to oxidative stress in Cochliobolus heterostrophus. Curr Genet. 2009;55:439–448. doi: 10.1007/s00294-009-0257-7. [DOI] [PubMed] [Google Scholar]
- 139.Munnik T, Ligterink W, Meskiene I, Calderini O, Beyerly J, Musgrave A, et al. Distinct osmo-sensing protein kinase pathways are involved in signalling moderate and severe hyperosmotic stress. Plant J. 1999;20:381–388. doi: 10.1046/j.1365-313x.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 140.Bogre L, Ligterink W, Meskien I, Barker PJ, Heberle-Bors E, Huskisson NS, et al. Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cardinale F, Meskiene I, Ouaked F, Hirt H. Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases. Plant Cell. 2002;14:703–711. [PMC free article] [PubMed] [Google Scholar]
- 142.Bögre L, Calderini O, Binarova P, Mattauch M, Till S, Kiegerl S, et al. A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell. 1999;11:101–113. [PMC free article] [PubMed] [Google Scholar]
- 143.Shoresh M, Gal-On A, Leibman D, Chet I. Characterization of a MAPK gene from cucumber required for Trichoderma conferred plant resistance. Plant Physiol. 2006;142:1169–1179. doi: 10.1104/pp.106.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, et al. Nitric oxide, stomatal closure and abiotic stress. J Exp Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 145.Rodriguez MCS, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. An Rev Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 146.Muller J, Beck M, Mettbach U, Komis G, Hause G, Menzel D, et al. Arabidopsis MPK6 is involved in cell division plane control during early root development and localizes to the pre-prophase band, phragmoplast, trans-Golgi network and plasma membrane. Plant J. 2010;61:234–248. doi: 10.1111/j.1365-313X.2009.04046.x. [DOI] [PubMed] [Google Scholar]
- 147.Eckardt NA. Unraveling the MAPK signaling network in stomatal development. Plant Cell. 2009;21:3413. [Google Scholar]
- 148.Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin in Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 149.Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA. 2009;106:20520–20525. doi: 10.1073/pnas.0907205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heimovaara-Dijkstra S, Testerink C, Wang M. Mitogen-activated protein kinase and abscisic acid signal transduction. Fonte: Results Probl Cell Differ. 2000;27:131–144. doi: 10.1007/978-3-540-49166-8_10. [DOI] [PubMed] [Google Scholar]
- 151.Xiong L, Zhu JK. Abiotic stress signal transduction in plants: Molecular and genetic perspectives. Physiol Plant. 2001;112:152–166. doi: 10.1034/j.1399-3054.2001.1120202.x. [DOI] [PubMed] [Google Scholar]
- 152.Mockaitis K, Howell SH. Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J. 2000;24:785–796. doi: 10.1046/j.1365-313x.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 153.Kovtun Y, Chiu WL, Zeng W, Sheen J. Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature. 1998;395:716–720. doi: 10.1038/27240. [DOI] [PubMed] [Google Scholar]
- 154.Mizoguchi T, Gotoh Y, Nishida E, Yamaguchi-Shinozaki K, Hayashida N, Iwasaki T, et al. Characterization of two cDNAs that encode MAP kinase homologues in Arabidopsis thaliana and analysis of the possible role of auxin in activating such kinase activities in cultured cells. Plant J. 1994;5:111–122. doi: 10.1046/j.1365-313x.1994.5010111.x. [DOI] [PubMed] [Google Scholar]