Abstract
Many cool-season grasses (Poaceae, subfam. Pooideae) possess seed-borne fungal symbionts, the epichloae, known for their bioprotective properties and especially for production of anti-insect alkaloids such as lolines. Asexual epichloae (Neotyphodium species) are primarily or entirely transmitted vertically, whereas the sexual structures (stromata) of the related Epichloä species give rise to horizontally transmissible spores (ascospores). In certain grass-Neotyphodium species symbiota, levels of lolines are extremely high and apparently limited by availability of precursor amino acids, whereas sexual epichloae generally produce much lower levels. This may reflect the inherent conflict between the vertical and horizontal transmission; although the plant and seeds may be protected by the alkaloids, the sexual cycle depends on anthomyiid flies for cross-fertilization. Given this insect role, we predicted that loline biosynthesis would be down-regulated in the stromata relative to the corresponding asymptomatic tissues (inflorescences) of the same symbiota. This prediction was substantiated, and RNA-seq and RT-qPCR analysis indicated that the loline biosynthesis genes are dramatically upregulated in asymptomatic inflorescences compared to stromata. The fundamental difference between asexual and sexual epichloae in regulation of loline alkaloid levels is in keeping with evolutionary trends for greater host control on metabolism of their vertically transmitted symbionts compared to contagious symbionts.
Key words: grass endophyte, symbiont, Neotyphodium, Epichloä, stroma, loline alkaloids, insecticidal, RT-qPCR, RNA-seq
The epichloae are fungal symbionts (endophytes) of cool-season grasses (Poaceae, subfamily Pooideae), and represent a good model to study the evolutionary continuum from antagonism to mutualism.1 The sexual species (generally classified as Epichloä species) can suppress (“choke”) maturation of host inflorescences, whereas asexual Neotyphodium species cause no adverse symptoms, permit normal inflorescence development and, in fact, vertically transmit by infecting host seeds and embryos. Many of the Epichloä species, such as Epichloä festucae, are capable of vertical transmission and choke only some tillers. The stromata on choked tillers attract anthomyiid flies (Botanophila species), which cross-fertilize them.2 The resulting sexual spores (ascospores) mediate horizontal transmission.3,4 Among the advantages conferred by many epichloae to their grass hosts is protection from insects by production of fungal alkaloids including lolines, lolitrems, ergot alkaloids and peramine.5 Such protective capabilities are much more typical of asexual Neotyphodium species than sexual Epichloä species.6 Nevertheless, lolines and peramine produced by E. festucae have been shown to provide significant protection from insects.7,8
In certain grass-epichloae symbiota, loline alkaloids are produced at very high levels, especially in young tissues. We previously investigated the relationship between loline alkaloid levels, loline biosynthesis gene (LOL) expression and substrate availability in young vegetative tissues of meadow fescue (MF)-Neotyphodium uncinatum and MF-Neotyphodium siegelii symbiota.9 Our evidence indicated that substrate availability determined loline alkaloid levels in those systems. However, this finding raises the question of how levels of lolines are controlled in symbiota of MF with the sexual species, Epichloä festucae, where lolines are much less abundant than in the former two symbiota.7 Given the role of Botanophila flies in the Epichloä sexual cycle, we predicted that loline alkaloid biosynthesis would be downregulated in the stromata relative to asymptomatic inflorescences.
We collected stromata (S) and asymptomatic inflorescences (I) from two symbiota, each consisting of a plant symbiotic with a different single-ascospore isolate of Epichloä festucae from the Wilkinson et al.7 study. In keeping with our prediction, both plants had over 2.5-fold higher loline alkaloid levels in I than in S (Table 1). We then conducted an RNA-seq experiment to compare expression levels for genes in the LOL cluster in E. festucae growing in I compared to S for plants with a third E. festucae ascospore isolate. RNA was isolated with the RNeasy Plant Minikit (Qiagen, Valencia, CA USA), and DNase I-treated with the DNA-free kit (Ambion, Austin, TX USA), then poly(A) RNA was enriched and fragmented and cDNA was synthesized by random priming as specified in the mRNA Sequencing Sample Preparation Guide (Illumina, Inc.). The cDNA fragments were ligated to adapters, size-selected and PCR-amplified (15 cycles), as specified in the Guide, then subjected to Solexa sequencing (Illumina) of 82 bases from one end of each fragment. Sequences were aligned to the E. festucae gene models using the program ELAND (Efficient Local Alignment of Nucleotide Data), in the Pipeline v.1.5 package. As expected, the RNA-seq results indicated much more living fungus in S than in I. In S, 45% of the reads (7,544,841 out of 16,776,937) mapped to the E. festucae genome, whereas in I, only 1.8% (292,917 out of 16,430,529) mapped to the fungal genome. The higher loline alkaloid levels in I contrasted with the much lower fungal biomass, strongly suggesting that loline alkaloid biosynthesis was highly regulated.
Table 1.
Concentrations of lolines in asymptomatic inflorescences and stromata of two MF-E. festucae symbiota
| Lolines (µg/g dry mass) | Total lolines (µmol/kg dry mass) | |||||
| Symbiotum | Tissue | Loline | NFL | NAL | Total | |
| 2048-1 | Stromata | 0 | 39.5 | 20.0 | 59.5 | 313 |
| 2048-1 | Inflorescences | 10.2 | 77.5 | 65.2 | 152.9 | 814 |
| 2102-4 | Stromata | 0 | 42.5 | 23.5 | 65.9 | 348 |
| 2102-4 | Inflorescences | 14.0 | 90.7 | 83.3 | 188.0 | 1002 |
Abbreviations: NAL, N-acetylloline; NFL, N-formylloline.
The RNA-seq data were analyzed by Fischer's exact test, using a contingency table of hits and non-hits to each gene from the total number of sequences that mapped to E. festucae gene models. Results are shown (Table 2) for LOL cluster genes, for an adjacent gene (lteA, encoding an Lte1-like GDP/GTP exchange factor protein) on the centrameric side of LOL and for the gene, tefA, encoding translation elongation factor 1α. LOL included the nine genes described in Spiering et al.10 plus two more genes (tentatively, lolM and lolN) that were found in the E. festucae genome sequence assembly to be linked to the cluster. The additional LOL genes were predicted to encode an acetamidase (lolN) and an N-methyltransferase (lolM), enzymes in the proposed loline alkaloid biosynthetic pathway.11 Levels of LOL gene expression estimated by RNA-seq were dramatically higher in I than in S, in keeping with the higher loline alkaloid levels in I. The differences in gene expression were highly significant, except in the case of lolA, which appeared to be very poorly expressed (more on this, later).
Table 2.
Results of RNA-seq analysis of MF-.Epichloë festucae symbiotum 2194, comparing LOL gene expression with expression of a housekeeping gene (lteA) located adjacent to the LOL cluster, and the translation elongation factor 1-α gene (tefA)
| Reads (Total mapped to genes) | |||||||
| Gene | mRNA length (bp) | S reads (5,128,582) | S reads/kb | I reads (197,933) | I reads/kb | i/s† | p value‡ |
| lolF | 1843 | 228 | 124 | 79 | 43 | 9.0 | 1.2E-42 |
| lolC | 1707 | 101 | 59 | 409 | 240 | 104.9 | <1E-300 |
| lolD | 1413 | 9 | 6 | 102 | 72 | 293.7 | 4.9E-134 |
| lolO | 1291 | 37 | 29 | 824 | 638 | 577 | <1E-300 |
| lolA | 570 | 4 | 7 | 2 | 4 | 13 | 1.9E-02 |
| lolU | 1491 | 44 | 30 | 98 | 66 | 57.7 | 1.5E-104 |
| lolP | 1503 | 269 | 179 | 326 | 217 | 31.4 | 5.9E-295 |
| lolT | 1365 | 512 | 375 | 48 | 35 | 2.4 | 1.3E-07 |
| lolE | 1078 | 658 | 610 | 95 | 88 | 3.7 | 1.1E-24 |
| lolN | 750 | 47 | 63 | 132 | 176 | 72.8 | 1.1E-146 |
| lolM | 1600 | 150 | 94 | 348 | 218 | 60.1 | <1E-300 |
| lteA | 6099 | 632 | 104 | 19 | 3 | 0.8 | 3.5E-01 |
| tefA | 1565 | 49659 | 31731 | 1747 | 1116 | 0.9 | 1.1E-04 |
Abbreviations: S, stromata; I, asymptomatic inflorescences.
i/s = fold-difference of expression in I versus S, where i = the number of reads mapped to the gene in I divided by the total number of reads in I that mapped to E. festucae genes, and s is similarly calculated for mapped reads in S.
Fischer's exact test.
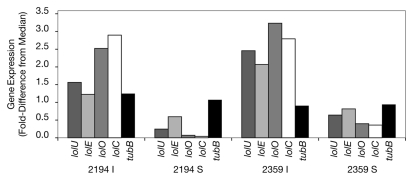
The differences in expression of four LOL genes was confirmed by reverse-transcription quantitative PCR (RT-qPCR), conducted on total RNA from additional stromata and inflorescences. The TaqMan primers and procedure were as described earlier.9 The results (Fig. 1) confirmed two-fold to more than 30-fold higher expression levels in I compared to S and were consistent with the RNA-seq results.
Figure 1.
Relative expression of several LOL genes in E. festucae in inflorescence (I) and stroma (S) samples of two MF-E. festucae symbiota, 2194 and 2359. Taqman RT -qPCR primers for lolU, lolE, lolO and lolC mRNA were specifically designed for E. festucae based on its genome sequence. Gene expression is indicated as fold-difference from the median for each gene in the experiment. The tefA gene was used as the reference gene to normalize across samples.
The results in this study contrasted with our previous results with MF-N. uncinatum and MF-N. siegelii symbiota,9 which indicated that substrate availability controlled loline alkaloid levels, with little or no involvement of transcriptional control. However, MF-N. uncinatum and MF-N. siegelii generally have much higher loline alkaloid levels than MF-E. festucae.7 We consistently observed approximately 4,000–18,000 µmol/kg dry mass in mature vegetative tissues (and much higher in younger tissues) of MF-N. uncinatum and MF-N. siegelii, which were 4–18-fold higher levels than the maximum observed in asymptomatic inflorescences of MF-E. festucae (see Table 1). We note, however, that we do not have a direct comparison of inflorescence tissues, but we do not expect much difference from vegetative tissues because the levels of lolines in MF-E. festucae inflorescences were comparable to those previously observed in mature vegetative tissues of the same symbiota.7
If levels of lolines in MF-E. festucae are typically much lower than in MF-N. uncinatum or MF-N. siegelii, we expect less influence of substrate levels on loline alkaloid levels in MF-E. festucae than we previously observed in the other two symbiota.9 Thus, it stands to reason that LOL gene regulation would be a more important factor in regulating loline alkaloid levels in MF-E. festucae. This is in keeping with the dramatic differences in LOL gene expression corresponding with differences in loline alkaloid levels between I and S. This difference suggests selection to minimize levels of these anti-insect alkaloids in young E. festucae stromata, which must attract the Botanophila flies that cross-fertilize the stromata.
Expression of lolA was consistently much lower than expression of other LOL genes (Table 2). This gene and lolC were the first two LOL genes to be identified in N. uncinatum cultures by use of the SSH-PCR technique and the two genes appeared to be expressed at comparable levels in that fungus.12 This raises the possibility that lower lolA expression is primarily responsible for the much lower levels of lolines in MF-E. festucae compared to MF-N. uncinatum. The predicted LolA protein is closely related to the C-terminal third of aspartyl kinase and lacks the kinase active site, but includes the allosteric regulatory domain.10 We speculate that LolA might interfere with the normally tight regulation of aspartyl kinase and thereby increase homoserine production. Homoserine, in turn, is a loline alkaloid precursor (along with proline). A planned experiment is to increase expression of lolA in E. festucae and determine if loline production increases as a consequence. If so, additional studies will be required to determine whether the effect is due to an enzymatic or regulatory role.
The evolutionary conversion of an infectious symbiont to a non-infectious, vertically transmissible symbiont causes a shift in selection toward mutualism, because the fate of the symbiont becomes more intimately tied to the fate of its host. Typically, after such a shift the symbiont relinquishes control of some metabolic capabilities. This is how evolution of mitochondria and plastids is thought to have occurred, whereby genes were gradually transferred from intracellular bacterial symbionts to host nuclear control. Likewise, the Buchnera species (bacteria) in homopteran insects have limited metabolic capabilities.13 Contrasting vertically transmissible Neotyphodium species with their ancestral Epichloä species provides an excellent study of this evolutionary trend early in this symbiogenesis process as the host gains greater control over symbiont metabolism. Our previous findings9 suggest that production of protective loline alkaloids by the symbiont is under host control based on substrate availability. In contrast, the related infectious symbiont, E. festucae, directly regulates levels of lolines by modulating gene expression, providing higher levels to asymptomatic host tissues, but lower levels to its fruiting body. However, lolines are mobile in the plant,14 limiting the degree to which levels can be controlled on a tissue-specific basis. Perhaps for this reason, overall production of lolines by E. festucae is much lower than is the case for many asexual Neotyphodium species. In balance, E. festucae can use lolines to protect its food source from some herbivorous insects, but would not benefit if it deterred its symbiotic Botanophila flies. Thus, the high levels of lolines produced by symbiotic N. uncinatum and N. siegelii compared to lower levels produced by E. festucae is emblematic of a shift to control by the host of a symbiont metabolic process.
Acknowledgements
We thank Jolanta Jaromczyk for statistical analysis and Walter Hollin and J. Douglas Brown for technical support. This work was funded by United States Department of Agriculture Grant 200911131030.
Abbreviations
- LOL
loline biosynthesis gene locus
- MF
meadow fescue
- S
stromata
- I
inflorescences
- SSH-PCR
subtractive suppression hybridization PCR
- N
Neotyphodium
- E
Epichloë
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13395
References
- 1.Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat. 2002;160:99–127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- 2.Bultman TL, Bell G, Martin WD. A fungal endophyte mediates reversal of wound-induced resistance and constrains tolerance in a grass. Ecology. 2004;85:679–685. [Google Scholar]
- 3.Chung KR, Schardl CL. Sexual cycle and horizontal transmission of the grass symbiont, Epichloä typhina. Mycol Res. 1997;101:295–301. [Google Scholar]
- 4.Arroyo García R, Martínez Zapater JM, García Criado B, Zabalgogeazcoa I. Genetic structure of natural populations of the grass endophyte Epichloä festucae in semiarid grasslands. Mol Ecol. 2002;11:355–364. doi: 10.1046/j.0962-1083.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 5.Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Ann Rev Plant Biol. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. [DOI] [PubMed] [Google Scholar]
- 6.Leuchtmann A, Schmidt D, Bush LP. Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloä/Neotyphodium endophytes. J Chem Ecol. 2000;26:1025–1036. [Google Scholar]
- 7.Wilkinson HH, Siegel MR, Blankenship JD, Mallory AC, Bush LP, Schardl CL. Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. Mol Plant Microbe Interact. 2000;13:1027–1033. doi: 10.1094/MPMI.2000.13.10.1027. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Tapper BA, Popay A, Parker EJ, Scott B. A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol Microbiol. 2005;57:1036–1050. doi: 10.1111/j.1365-2958.2005.04747.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang DX, Nagabhyru P, Schardl CL. Regulation of a chemical defense against herbivory produced by symbiotic fungi in grass plants. Plant Physiol. 2009;150:1072–1082. doi: 10.1104/pp.109.138222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiering MJ, Moon CD, Wilkinson HH, Schardl CL. Gene clusters for insecticidal loline alkaloids in the grass-endophytic fungus Neotyphodium uncinatum. Genetics. 2005;169:1403–1414. doi: 10.1534/genetics.104.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang DX, Stromberg AJ, Spiering MJ, Schardl CL. Coregulated expression of loline alkaloid-biosynthesis genes in Neotyphodium uncinatum cultures. Fung Genet Biol. 2009;46:517–530. doi: 10.1016/j.fgb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Spiering MJ, Wilkinson HH, Blankenship JD, Schardl CL. Expressed sequence tags and genes associated with loline alkaloid expression by the fungal endophyte Neotyphodium uncinatum. Fung Genet Biol. 2002;36:242–254. doi: 10.1016/s1087-1845(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 13.Ochman H, Moran NA. Genes lost and genes found: Evolution of bacterial pathogenesis and symbiosis. Science. 2001;292:1096–1098. doi: 10.1126/science.1058543. [DOI] [PubMed] [Google Scholar]
- 14.Schardl CL, Grossman RB, Nagabhyru P, Faulkner JR, Mallik UP. Loline alkaloids: currencies of mutualism. Phytochemistry. 2007;68:980–996. doi: 10.1016/j.phytochem.2007.01.010. [DOI] [PubMed] [Google Scholar]