Abstract
The cytokinins regulate a broad range of plant developmental events. We recently reported that the home-odomain transcription factor STIMPY (STIP) positively mediates the cytokinin signals in maintaining proliferative and pluoripotent properties of the shoot apical meristem in Arabidopsis. In line with our proposed model, light-grown stip seedlings are less sensitive to the growth inhibition effect of the exogenously applied cytokinins than wild type. Here we investigate STIP's role in cytokinin signaling in dark-grown seedlings, in which elevated cytokinin levels promote photomorphogenesis. We found that stip mutants show enhanced de-etiolation phenotype in response to cytokinin treatment in the dark, suggesting that STIP may be a negative regulator of cytokinin signaling under this condition. We discuss possible explanations for this observed developmental stage-specific function of STIP.
Key words: arabidopsis, STIMPY, de-etiolation, cytokinin, dark germination
The cytokinins have been implicated in a wide variety of developmental processes in plants, from cell division to nutrient response.1 An interesting aspect of the cytokinin function is its role in promoting photomorphogenesis in dark-grown plants. Arabidopsis seedlings with elevated endogenous cytokinin levels develop key features of photomorphogenesis, including short hypocotyls and open cotyledons, when grown in the dark.2,3 This effect can be phenocopied in wildtype plants grown on medium supplied with high levels of cytokinins.4 In the search for genes that mediate cytokinin responses in regulating seedling development in Arabidopsis, we identified the homeodomain transcription factor STIMPY (STIP/WOX9)5,6 as a new member of the cytokinin signaling pathway in maintaining pluripotency and cell proliferation of the seedling shoot apical meristem. Light-grown stip mutant seedlings show reduced sensitivity to the growth inhibition effect by exogenous cytokinins.7 Given that STIP acts within a newly identified branch of the cytokinin signaling pathway, we investigated effects of cytokinin on dark-grown stip seedlings.
Dark-Grown stip Mutants Have Increased Cytokinin Sensitivity
To assay for cytokinin sensitivity in dark-grown seedlings, seeds were incubated on 1/2 Murashige Minimal Organics Medium (MS) with various concentrations of cytokinin N6-(D2-isopentenyl)-adenine (2-iP) at 22°C in the dark for four days after a three-day stratification at 4°C. A light treatment of approximately eight hours was applied prior to germination. In addition to wild type (Col-0) and stip-1,6 amp1-1, which disrupts a putative glutamate carboxypeptidase and has higher amounts of endogenous cytokinins,2,3,8 was included as a positive control.
Without exogenous cytokinin, both the wildtype and stip seedlings germinated in the dark developed the typical etiolation phenotype, with elongated hypocotyls, apical hooks and closed cotyledons (Fig. 1A and D). Consistent with the previous report,2 the amp1-1 mutants had shorter hypocotyls and open cotyledons (Fig. 1G), both of which are signs of de-repressed photomorphogenesis, under the same conditions. The added 2-iP in the growth medium significantly inhibited hypocotyl elongation in all three genotypes tested, but with different effects on the apical hook and cotyledon opening. As expected, the cotyledons of the amp1-1 remained open on 2-iP-containing medium (Fig. 1H and I). In contrast, while enhanced apical hooks, which indicate elevated ethylene signaling due to the cytokinin treatment,9 could be occasionally found in Col-0 samples (Fig. 1B), we did not observe the straightening of the apical hook or open cotyledons in the wildtype seedlings at the highest 2-iP concentration used in our study (Fig. 1C). When compared to wild type, stip seedlings had a more profound de-etiolation phenotype when germinated on cytokinin in the dark. On 20 µM 2-iP, nearly all stip seedlings developed enhanced apical hooks (Fig. 1E) and the treatment with 50 µM 2-iP resulted in fully opened cotyledons and hypocotyls shorter than those of Col-0 and amp1-1 (Fig. 1F), indicating that the stip mutants have enhanced cytokinin sensitivity under these conditions.
Figure 1.
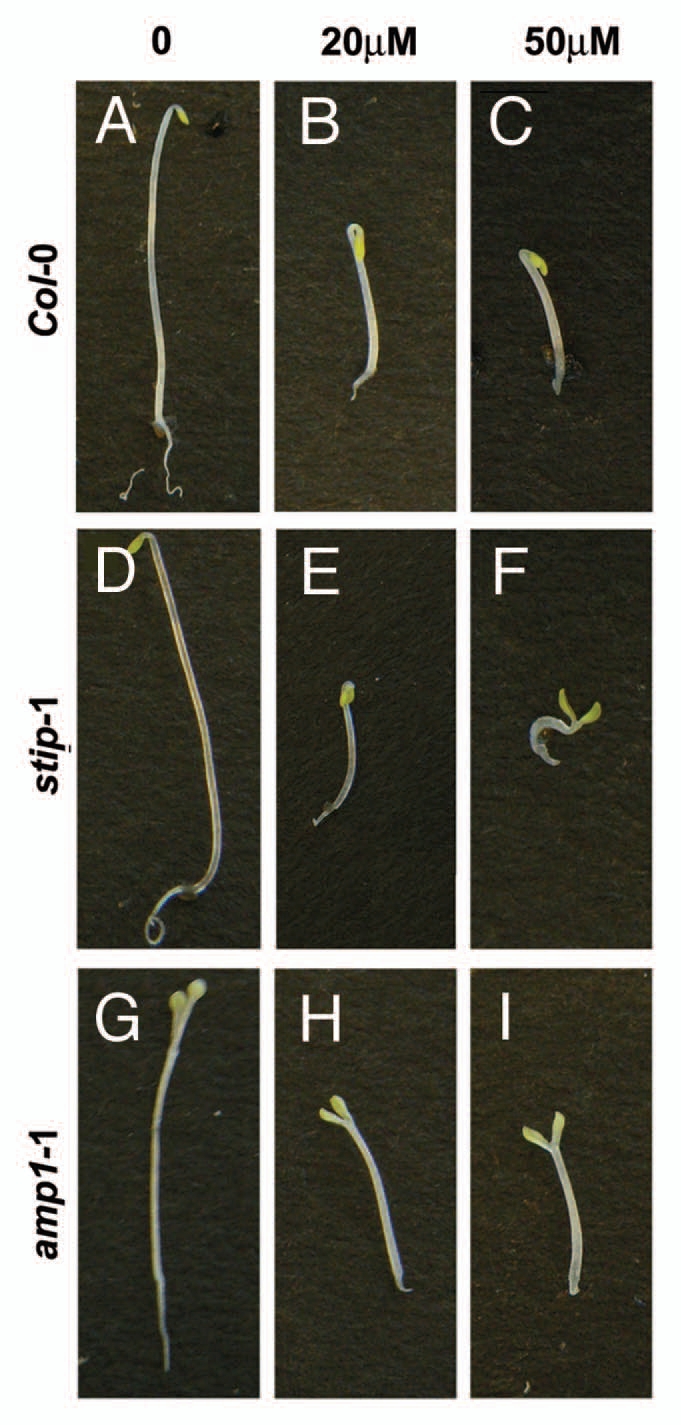
Dark-grown stip mutants have increase cytokinin sensitivity. After being grown in the dark for four days, Col-0 (A-C), stip-1 (D-F) and amp1-1 (G and H) seedlings display different response to the exogenous 2-iP.
How Can STIP Have Developmental Stage-Dependent Responses to Cytokinin Stimuli?
We recently reported that STIP positively regulates cytokinin responses in the meristematic regions of light-grown seedlings.7 However, the enhanced de-etiolation phenotype of the dark-germinated stip mutants in response to exogenous cytokinin points to a negative role of STIP in the cytokinin pathway at this stage. There are many possible explanations for this developmental stage-dependent effect. For example, STIP may interact with different branches of the cytokinin pathway during dark germination and vegetative development. Alternatively, STIP has no direct role in regulating etiolation. Instead, stip mutants could have slightly higher levels of endogenous cytokinin at germination due to its reduced cytokinin sensitivity, thus exaggerating the effects of the exogenously added hormone. A more plausible scenario is that STIP interacts with other pathways known to control de-etiolation. Cytokinin inhibits hypocotyl elongation in dark-grown seedlings by promoting ethylene biosynthesis.9 Although stip mutants do not have the phenotype typical of ethylene signaling mutants, the higher frequency of enhanced apical hook seen in stip mutants treated with 20 µM 2-iP (Fig. 1E) could be explained by increased ethylene biosynthesis or sensitivity when compared to the wild type. More convincingly, the strongly curved hypocotyls of stip mutants grown on 50 µM 2-iP (Fig. 1F) is visibly different from that of the amp1 seedlings (Fig. 1I) and could be the result of the combination of a highly enhanced apical hook and open cotyledons. In addition to ethylene, another major regulator of the de-etiolation process is the light-sensing pathway, which converges with the cytokinin network during germination.3,10 Higher order mutants of the phytochrome-interacting factors (PIFs) undergo cotyledon opening during dark growth.11,12 Although light-germinated stip mutants have normal response to light (M Chen, personal communication), we cannot exclude the possibility that STIP interacts with the light sensing mechanisms during germination and the effects of which is detected under cytokinin treatment.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13356
References
- 1.To JP, Kieber JJ. Cytokinin signaling: two-components and more. Trends Plant Sci. 2008;13:85–92. doi: 10.1016/j.tplants.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhury AM, Letham S, Craig S, Dennis ES. amp1—a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993;4:907–916. [Google Scholar]
- 3.Chin-Atkins A, Craig S, Hocart CH, Dennis ES, Chaudhury AM. Increased endogenous cytokinin in the Arabidopsis amp1 mutant corresponds with de-etiolation responses. Planta. 1996;198:549. doi: 10.1007/BF00262641. [DOI] [PubMed] [Google Scholar]
- 4.Chory J, Reinecke D, Sim S, Washburn T, Brenner M. A role for cytokinins in de-etiolation in Arabidopsis (det mutants have an altered response to cytokinins) Plant Physiol. 1994;104:339–347. doi: 10.1104/pp.104.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, et al. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Dabi T, Weigel D. Requirement of homeo-box gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr Biol. 2005;15:436–440. doi: 10.1016/j.cub.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 7.Skylar A, Hong F, Chory J, Weigel D, Wu X. STIMPY mediates cytokinin signaling during shoot meristem establishment in Arabidopsis seedlings. Development. 2010;137:541–549. doi: 10.1242/dev.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helliwell CA, Chin-Atkins AN, Wilson IW, Chapple R, Dennis ES, Chaudhury A. The Arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. Plant Cell. 2001;13:2115–2125. doi: 10.1105/TPC.010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cary AJ, Liu W, Howell SH. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995;107:1075–1082. doi: 10.1104/pp.107.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su W, Howell SH. The Effects of cytokinin and light on hypocotyl elongation in Arabidopsis seedlings are independent and additive. Plant Physiol. 1995;108:1423–1430. doi: 10.1104/pp.108.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol. 2008;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin J, Kim K, Kang H, Zulfugarov IS, Bae G, Lee CH, et al. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci USA. 2009;106:7660–7665. doi: 10.1073/pnas.0812219106. [DOI] [PMC free article] [PubMed] [Google Scholar]


