Abstract
We recently showed that the efficacy of an entomopathogenic nematode (EPN) as a biological control agent against a root pest could be enhanced through artificial selection. The EPN Heterorhabditis bacteriophora was selected for higher responsiveness towards (E)-β-caryophyllene (EβC), a sesquiterpene that is emitted by maize roots in response to feeding damage by the western corn rootworm (WCR). EβC is normally only weakly attractive to H. bacteriophora, which is one of the most infectious nematodes against WCR. By selecting H. bacteriophora to move more readily along a EβC gradient we obtained a strain that was almost twice more efficient in controlling WCR population in fields planted with an EβC-producing maize variety. However, artificial selection for one trait may come at a cost for other important traits such as infectiousness, establishment and/or persistence in the field. Indeed, infectiousness was slightly but significantly reduced in the selected strain. Yet, this apparent cost was largely compensated for by the higher responsiveness to the root signal. Here we show that the selection process had no negative effect on establishment and persistence of field-released EPN. This knowledge, combined with the previously reported results, attest to the feasibility of manipulating key traits to improve the efficacy of beneficial organisms.
Key words: entomopathogenic nematodes, tritrophic interactions, artificial selection, biological control, Diabrotica virgifera virgifera, western corn rootworm, persistence, establishment
Diabrotica virgifera virgifera LeConte (Chrysomelidae: Coleptera, western corn rootworm, WCR) is a major well established pest of maize in the American Corn Belt and more recently also in Europe.1 The larval stages of this beetle can cause significant damages to maize roots, leading to reduction of plant growth, deficiencies in nutrient and water uptake, lodging, increased susceptibility to water stress and reduced grain yield.2 This combination of factors result in an estimated loss of one billion US dollars per year in the USA.3 The pest has been introduced in Europe in the early '90s,4 and it is expected that at full establishment the costs resulting from WCR damages will be half a billion Euros.5 Several strategies are available to control this soil-dwelling pest, including crop rotation, pesticides and transgenic Bt maize, but WCR can readily evolve resistance to each of these methods.6–8 This is why efforts have been invested in biological control alternatives.
Entomopathogenic nematodes (EPN) show great promise as biological agents against WCR.9 Root-produced volatiles appear to play an important role in the recruitment of EPN10–13 and one such volatile, (E)-β-caryophyllene (EβC), has recently been identified for maize roots14 and was found to be an ideal below-ground alarm signal.15 EPN efficacy can be improved by exploiting the ability of WCR-damaged maize roots to emit the attractant.14 Further studies have shown the importance of choosing the right species of nematodes.16 Among the EPN species tested against WCR, Heterorhabditis bacteriophora has proven to be one of the most virulent nematodes,17 but it barely responds to EβC.16 We therefore recently selected H. bacteriophora for higher responsiveness to EβC.18 In the field, the selected strain exhibited better abilities to control WCR larvae, but logically only in maize plots with plants that emitted EβC. However, previous studies have shown that enhancing beneficial traits through selective breeding can incur costs and negatively alter other traits in the selected strain.19 For EPN such trade-offs after selective breeding have also been reported, for instance resulting in reduced storage stability20 or a lower capacity to kill their hosts.21 After selection for enhanced responsiveness to EβC response, we observed a small, but significant negative effect on infectiousness of the selected strains. However, this drawback was readily outweighed by the improved ability to locate hosts in the field.18
Not only infectiousness is a crucial trait for the successful use of EPN in biological control: establishment and persistence in the field are of decisive importance as well. These traits vary with EPN species and are determined by biotic factors such as pathogens and predators22 or abiotic factors such as soil type,23 humidity,24 temperature25 or pH.24 But the main factor that is thought to determine long-term persistence in the field is the presence of available host insects.25 In field trials in Hungary, three EPN species, H. bacteriophora, H. megidis and Steinernema feltiae, were released to test their control potential against WCR. They all persisted at least as long WCR were present in soil, during the same year.26 There was no significant difference between the three species in the establishment or persistence. Yet, independent of timing of application, EPN populations dramatically decreased within five months after application. The authors26 propose that this short persistence is due to the absence of suitable alternative hosts in intensively cultivated crop fields in Europe.
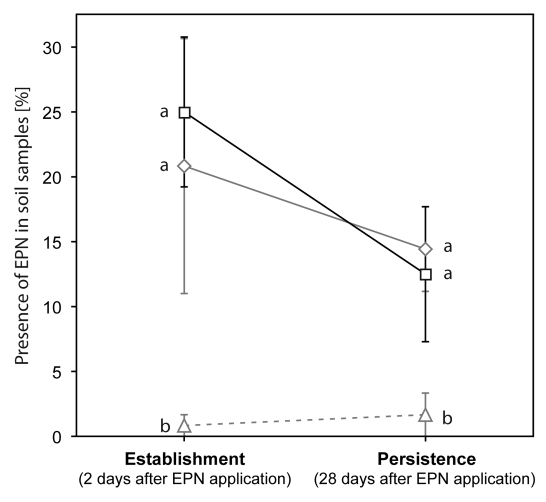
To determine if the selection for enhanced responsiveness to EβC went at a cost for establishment and persistence we compared these key traits for the original and the EβC-selected stains. Using a metal auger (2 cm diam.; 20 cm high), 310 soil samples were dug out either two days (establishment) or 28 days (persistence) after EPN application. The soil was placed in plastic boxes (4.5 cm diam.; 60 cm high) and as previously described26 Tenebrio molitor (Coleoptera: Tenebrionidae) larva was placed as bait in the boxes. Presence/absence of EPN was evaluated by visually checking T. molitor larvae for EPN infection. Soil samples from areas where no EPN were applied served as controls. No significant differences were found between the original and selected strain of H. bacteriophora strain (factor “strain”), neither in establishment after two days nor in persistence after 28 days (factor “time”) (Fig. 1, two-way ANOVA, Ftime1,35 = 2.937, p = 0.097; Fstrain2,35 = 10.359, p < 0.001; Ftime × strain2,35 = 1.202, p = 0.315, statistical differences within factors were calculated using a Bonferoni post-hoc test). Hence, the selection of H. bacteriophora for a better response to EβC had no consequence for how the nematodes settled in the experimental fields. Future efforts to improve the effectiveness H. bacteriophora against WCR might also include selection for increased persistence in soil. This would allow lower application rates and could provide growers with an affordable and efficient control strategy against this voracious pest.
Figure 1.
Establishment and persistence of the original and a selected strain of H. bacteriophora. The selected strain (squares) established and persisted as well as the original strain (diamonds). The triangles represent control samples from plots where no nematodes were released. Establishment (after two days) and persistence (after 28 days) was equal for both strains. Moreover, the number of soil samples containing EPN after 28 days was not significantly lower than after 2 days, independently of treatment. A few nematodes were detected in the control samples but again no differences over time were detected. Error bars indicate the SEM. Different lower-case letters indicate statistical differences within establishment (after 2 days) or persistence (after 28 days) (p <0.05).
So far, manipulation of tritrophic systems in order to improve biological control has been largely theoretical.27–29 We show here that for EPN this approach is realistic and that their responsiveness to root-produced foraging signals can be enhanced without significant costs for other relevant traits. It has also been shown that the emissions of the signals by the plants can be enhanced.30 Combining these strategies opens new perspectives for the development of ecologically sound strategies in pest management.
Acknowledgements
We thank all the members of the FARCE and e-vol labs at the University of Neuchâtel for support, in particular Matthias Held. We also thank Marie-Eve Wyniger and Sergio Rasmann for advice and help in developing the experimental set-ups. The fieldwork was possible thanks to the hospitality of the Plant Health Service in Hodmezovasarhely in Hungary, offered by Ibolya Zseller, Jozsef Gavallier, Kataline Buzas, Erzsebet Dormannsne, Piroska Szabo, Andras Varga and others. We thank Hungarian summer students for their help with field experiments. The foundation strain H. bacteriophora PS8 was kindly provided by R.U. Ehlers (Christian-Albrechts Universität, Kiel, Germany). This study was funded by the CTI Innovation and Technology Fund of Switzerland (CTI project no. 7487.1 LSPP-LS).
Abbreviations
- EPN
entomopathogenic nematode
- WCR
western corn rootworm
- EβC
(E)-β-caryophyllene
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13363
References
- 1.Meinke LJ, Sappington TW, Onstad DW, Guillemaud T, Miller NJ, Judith K, et al. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric For Entomol. 2009;11:29–46. [Google Scholar]
- 2.Krysan JL. Selected topics in the biology of Diabrotica. In: Cox ML, editor. Advances in Chrysomelidae Biology. Leiden, The Netherlands: Backhuys Publisher; 1999. pp. 479–513. [Google Scholar]
- 3.Krysan JL, Miller TA. Methods for study of pest Diabrotica. New York: Springer-Verlag; 1986. [Google Scholar]
- 4.Miller N, Estoup A, Toepfer S, Bourguet D, Lapchin L, Derridj S, et al. Multiple transatlantic introductions of the western corn rootworm. Science. 2005;310:992. doi: 10.1126/science.1115871. [DOI] [PubMed] [Google Scholar]
- 5.Wesseler J, Fall EH. Potential damage costs of Diabrotica virgifera virgifera infestation in Europe—the ‘no control’ scenario. J Appl Entomol. 2010;134:385–394. [Google Scholar]
- 6.Meinke LJ, Siegfried BD, Wright RJ, Chandler LD. Adult susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to selected insecticides. J Econom Entomol. 1998;91:594–600. [Google Scholar]
- 7.Levine E, Spencer JL, Isard SA, Onstad DW, Gray ME. Adaptation of the western corn rootworm, Diabrotica vigifera virgifera LeConte (Coleoptera: Chrysomelidae), to crop rotation: evolution of a new strain in response to a cultural management practice. Am Entomol. 2002;48:94–107. [Google Scholar]
- 8.Krysan JJ, Foster DE, Branson TF, Ostlie KR, Cranshaw WS. Two years before the hatch: rootworms adapt to crop rotation. Bull Entomol Soc Am. 1986;32:250–258. [Google Scholar]
- 9.Kuhlmann U, van der Brugt WACM. Possibilities for biological control of the western corn rootworm, Diabrotica virgifera virgifera LeConte, in Central Europe. Biocontrol. 1998;19:59–68. [Google Scholar]
- 10.Ali JG, Alborn HT, Stelinski LL. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J Chem Ecol. 2010;36:361–368. doi: 10.1007/s10886-010-9773-7. [DOI] [PubMed] [Google Scholar]
- 11.Aratchige NS, Lesna I, Sabelis MW. Below-ground plant parts emit herbivore-induced volatiles: olfactory responses of a predatory mite to tulip bulbs infested by rust mites. Exp Appl Acarol. 2004;33:21. doi: 10.1023/b:appa.0000030011.66371.3f. [DOI] [PubMed] [Google Scholar]
- 12.Boff MIC, van Tol R, Smits PH. Behavioural response of Heterorhabditis megidis towards plant roots and insect larvae. Biocontrol. 2002;47:67–83. [Google Scholar]
- 13.van Tol RWHM, van der Sommen ATC, Boff MIC, van Bezooijen J, Sabelis MW, Smits PH. Plants protect their roots by alerting the enemies of grubs. Ecol Lett. 2001;4:292–294. [Google Scholar]
- 14.Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 15.Hiltpold I, Turlings TCJ. Below-ground chemical signalling in maize: when simplicity rhymes with efficiency. J Chem Ecol. 2008;34:628–635. doi: 10.1007/s10886-008-9467-6. [DOI] [PubMed] [Google Scholar]
- 16.Hiltpold I, Toepfer S, Kuhlmann U, Turlings TCJ. How maize root volatiles influence the efficacy of entomopathogenic nematodes against the western corn rootworm? Chemoecology. 2010;20:155–162. [Google Scholar]
- 17.Kurzt B, Hiltpold I, Turlings TCJ, Kuhlmann U, Toepfer S. Comparative susceptibility of larval instars and pupae of the western corn rootworm to infection by three entomopathogenic nematodes. Biocontrol. 2009;54:255–262. [Google Scholar]
- 18.Hiltpold I, Baroni M, Toepfer S, Kuhlmann U, Turlings TCJ. Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J Exp Biol. 2010;213:2417–2423. doi: 10.1242/jeb.041301. [DOI] [PubMed] [Google Scholar]
- 19.Kraaijeveld R, Godfray HCJ. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:218–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- 20.Gaugler R, Campbell JF, McGuire TR. Fitness of a genetically improved entomopathogenic nematode. J Invert Pathol. 1990;56:106–116. [Google Scholar]
- 21.Stuart RJ, Lewis EE, Gaugler R. Selection alters the pattern of emergence from the host cadaver in the entomopathogenic nematode, Steinernema glaseri. Parasitology. 1996;113:183–189. doi: 10.1017/s0031182000067627. [DOI] [PubMed] [Google Scholar]
- 22.Wilson M, Gaugler R. Factors limiting short-term persistence of entomopathogenic nematodes. J Appl Entomol. 2004;128:250–253. [Google Scholar]
- 23.Barbercheck ME. Effect of soil physical factors on biological-control agents of soil insect pests. Florida Entomol. 1992;75:539–548. [Google Scholar]
- 24.Kung SP, Gaugler R, Kaya HK. Influence of soil pH and oxygen on persistenc of Steinernema spp. J Nematol. 1990;22:440–445. [PMC free article] [PubMed] [Google Scholar]
- 25.Susurluk A, Ehlers RU. Field persistence of the entomopathogenic nematode Heterorhabditis bacteriophora in different crops. Biocontrol. 2008;53:627–641. [Google Scholar]
- 26.Kurtz B, Toepfer S, Ehlers RU, Kuhlmann U. Assessment of establishment and persistence of entomopathogenic nematodes for biological control of western corn rootworm. J Appl Entomol. 2007;131:420–425. [Google Scholar]
- 27.Beckendorf SK, Hoy MA. In: Genetic improvement of arthropod naturel enemies through selection, hybridization or genetic engineering techniques. Hoy MA, Herzog DC, editors. Orlando FL: Biological Control in Agricultural IPM Systems: Academic; 1985. pp. 166–187. [Google Scholar]
- 28.Hoy MA. Genetic Improvement of Insects—Fact or Fantasy. Environ Entomol. 1976;5:833–839. [Google Scholar]
- 29.Hoy MA. Transgenic arthropods for pest management programs: Risks and realities. Exp Appl Acarol. 2000;24:463–495. doi: 10.1023/a:1006401225083. [DOI] [PubMed] [Google Scholar]
- 30.Degenhardt J, Hiltpold I, Köllner TG, Frey M, Gierl A, Gershenzon J, et al. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA. 2009;106:13213–13218. doi: 10.1073/pnas.0906365106. [DOI] [PMC free article] [PubMed] [Google Scholar]