Abstract
We report unique desiccation-associated ABA signaling transduction through which the Rop (Rho GTPase of plants) and its target LLP12-2 are regulated during the stage of pollen maturation and tube growth. Overexpression of LLP12-2 drastically inhibited pollen germination and tube growth. Studies on the germination inhibitors, Ca2+ influx blocking agents LaCl3 and EGTA and an actin-depolymerizing drug, latrunculin B (LatB), revealed that the LLP12-2-induced inhibition of germination and tube growth is significantly suppressed by LaCl3 and EGTA in the LLP12-2-overexpressing pollen but not by LatB. These results suggested that LLP12-2 is associated with Ca2+ influx in the cytoplasm and may be not with actin assembly. With the addition of LaCl3 and EGTA, LLP12-2-overexpressing pollen increased germination and tube growth compared with the one without addition, whereas pollen expressing GFP decreased germination and tube growth. Thus, an optimum level of [Ca2+]cyt influx is crucial for normal germination and tube growth. Studies on the inhibitors, staurosporine and okadaic acid in the LLP12-2-overexpressing pollen, showed no appreciable increase in germination when compared with the one without addition, suggesting that staurosporine-sensitive protein kinases and dephosphorylation of phosphoproteins may be not involved in the LLP12-2 mediated germination. However, the LLP12-2-induced inhibition of tube length was slightly but significantly suppressed by staurosporine, suggesting that staurosporine-sensitive protein kinases involve in the LLP12-2-induced inhibition of tube growth.
Key words: calcium influx, phosphoprotein, pollen tube growth, RIC protein, rop GTPase
Rop (Rho GTPase of plants) was newly reported as a master regulator for plant signaling.1,2 It participates in concerted actions of many signaling pathways that influence growth and development, and the adaptation of plants to various environmental situations.3–5 In contrast to be negative regulators in ABA signaling,6 Rops might work as positive regulators in auxin signaling pathways.7,8 Recently, we have reported unique desiccation-associated ABA signaling in which the LLP-Rop1 gene is not only negatively regulated by desiccation but also positively regulated by developmental cues independent of ABA during pollen maturation.9 Although LLP-Rop1 and its target, LLP12-2, accumulate in abundance in the matured and dried pollen upon dehydration, the activity of LLP-Rop1 and LLP12-2 is likely restricted at the stage of pollen maturation.9 As pollen germinates, ABA content decreases its level in the growing tube and thus, the activity of Rop is less restricted than that in the dried pollen and subsequently Rops become powerful regulators playing crucial roles during pollen tube growth.
Pollen germination and tube growth are a continuous and highly polarized process characteristic of tip growth. As soon as pollen hydrates and germinates, a tip-focused cytoplasmic Ca2+ gradient is established and sustained while a pollen tube grows forward.10,11 The Ca2+-permeable channels that modulate [Ca2+]cyt influx in germinating pollen grains have been identified in Arabidopsis,12,13 lily14 and pear.15 When a pollen tube grows, Rop-interactive Cdc42/Rac-interactive binding (CRIB) motif-containing proteins (RICs) play an important role as Rop GTPase targets and control a variety of Rop-dependent signaling pathways.16 RICs contain a CRIB motif required for their specific interaction with GTP-bound Rop. They are grouped into five classes that share little sequence similarity outside of the Rop-interactive domain.16 Different RICs expressed in various reproductive and vegetative parts of the Arabidopsis plant may act as Rop targets to control different Rop-dependent pathways in pollen tubes and in other organ development. For instances, RIC4 has been demonstrated to promote F-actin assembly, whereas RIC3 activates Ca2+ signaling by affecting [Ca2+]cyt influx that subsequently results in F-actin disassembly in pollen tube growth.17 The two RICs, both activated by AtRop1, counteract each other to control the actin dynamics and polar pollen tube growth.17
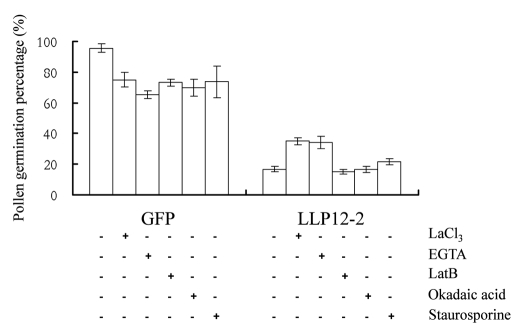
We have demonstrated that LLP12-2, a RIC protein, interacts with active LLP-Rop1 in vivo.9 To examine the function of LLP12-2 in the growing tubes, the purified LLP12-2 PCR product digested with XbaI and SacI was cloned into the corresponding sites of Zm13::GFP construct to generate the Zm13::GFP-LLP12-2 construct. The transient expression of GFP-LLP12-2 in pollen using particle bombardment was investigated. Pollen germination and tube length were measured after particle bombardment and subsequent in vitro germination. With the treatment of Ca2+ influx blocking agents LaCl3 and EGTA, pollen expressing GFP alone significantly decreased germination and tube elongation, suggesting that a decrease in [Ca2+]cyt influx may cause the inhibition of pollen germination and tube growth (Fig. 1 and Table 1). The chemical LaCl3 blocks plasma membrane (PM)-localized inward Ca2+ channels whereas EGTA is a Ca2+ chelating agent.17
Figure 1.
LLP12-2 inhibits pollen germination by regulating the calcium influx channels. Germination percentages were determined 9 h after particle bombardment and subsequent in vitro germination. Equal amounts of GFP and LLP12-2 DNA (7.5 µg) were transiently expressed in lily pollen after which pollen was treated without (control) or with either LaCl3 (1 µM), EGTA (0.5 mM), LatB (0.05 nM), okadaic acid (5 nM) or staurosporine (1 µM) during germination.
Table 1.
Effects of LaCl3, EGTA, LatB, okadaic acid or staurosporine on the length of pollen tube expressing LLP12-2
| Pollen tube length (µm) | ||||||
| Control | LaCl3 | EGTA | LatB | Okadaic acid | Staurosporine | |
| GFP | >1,500 | 1,286 ± 26 | 1,262 ± 23 | 1,248 ± 32 | 1,265 ± 36 | 1,254 ± 31 |
| LLP12-2 | 1,028 ± 22 | 1,305 ± 34 | 1,309 ± 31 | 1,021 ± 24 | 1,089 ± 35 | 1,186 ± 23 |
Pollen tube length was measured 9 h after particle bombardment. Data are mean ± SD (µm) of three individual experiments (n = 10, per experiment).
The inhibition of germination and tube growth was further enhanced in the pollen overexpressing GFP-LLP12-2 when compared with the pollen expressing GFP only (Fig. 1 and Table 1). The LLP12-2-induced inhibition of germination and tube growth in the LLP12-2-overexpressing pollen strongly indicated that LLP12-2 plays a crucial role during pollen germination and growth. The function of LLP12-2 in pollen was further investigated not only by the addition of LaCl3 and EGTA but also by the treatment of an actin-depolymerizing drug, LatB. When LaCl3 or EGTA was applied, pollen germination increased two- to three-fold, and tube length increased by approximately 27% compared with those without drug addition (Fig. 1 and Table 1). On the contrary, no increase in germination percentage and tube length was observed with the application of LatB (Fig. 1 and Table 1). These results suggested that LLP12-2 is indeed involved in [Ca2+]cyt influx but may be not with actin assembly. It is rational to hypothesize that pollen overexpressing LLP12-2 may regulate the activity of PM-localized inward Ca2+ channel proteins, resulting in an increase in Ca2+ influx in the cytoplasm and lead to inhibit germination and tube growth. The hypothesis is supported by the fact that, with the addition of LaCl3, an increase in the level of [Ca2+]cyt influx is adversely affected in the LLP12-2-overexpressing pollen. As a result, both germination and tube growth increase (Fig. 1 and Table 1). A similar result was also obtained with the application of EGTA, a Ca2+ chelating agent where a decrease in the level of [Ca2+]cyt influx alleviates the LLP12-2-induced inhibition of germination and tube growth (Fig. 1 and Table 1). Based on these analyses, it is likely that an optimal level of Ca2+ influx in the cytoplasm of pollen is crucial for normal germination and tube growth as illustrated in Figure 2. Any perturbation of [Ca2+]cyt influx in the pollen would decrease germination and tube growth. The function of LLP12-2 mimics that of RIC3, Group III of Arabidopsis RICs family. It has been reported that RIC3 activates Ca2+ signaling, which leads to F-actin disassembly, whereas RIC4 promotes F-actin assembly.17 Alike RIC3, overexpression of LLP12-2 causes an excess amount of tip-localized calcium in the cytoplasm of the tube and subsequently results in the inhibition of germination and tube growth. It should be noted that the LLP12-2-induced inhibition of germination and tube growth only partially rescued with the treatment of LaCl3 or EGTA, implying that factors other than calcium involves in the modulation of pollen germination and tube growth.18–20
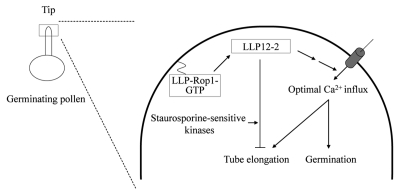
Figure 2.
Schematic diagram of the LLP-Rop1 signaling during pollen germination and tube growth. During germination and tube growth, LLP-Rop1 is activated at the tip and activates LLP12-2, which affects calcium influx in the cytoplasm that in turn promotes germination and tube elongation. In addition, staurosporine-sensitive protein kinases are involved in the LLP12-2-induced inhibition of pollen tube elongation.
Protein kinases such as calcium-dependent protein kinase (CDPK) have been reported to involve in the regulation of pollen germination and tube growth.21,22 Studies have shown that CDPK comprises a kinase domain and a calmodulin-like domain in a single protein. Thus, it acts not only as a Ca2+ sensor but also as an effector affecting growth polarity, elevated cytosolic Ca2+, and plant cytoskeleton during pollen germination and tube growth.21,23 Aside from CDPKs, calcineurin B-like proteins (CBLs), a new family Ca2+ sensor, interact specifically with CBL-interacting protein kinases.24 These putative Ca2+ sensors are responsible for the regulation of calcium-dependent tip growth and growth oscillation in pollen tubes.
To examine the signaling of protein kinases associated with LLP12-2 during germination and tube elongation, bombarded pollen was incubated in the absence or presence of okadaic acid or staurosporine. Okadaic acid is a membrane-permeable inhibitor of serine/threonine protein phosphatases types 1 and 2A,25 whereas staurosporine is a potent broad-spectrum inhibitor of serine/threonine kinases.26 The LLP12-2-overexpressing pollen did not exhibit appreciable increase in germination with the treatment of either staurosporine or okadaic acid when compared with that without treatment (Fig. 1). This implies that staurosporine-sensitive protein kinases and dephosphorylation of phosphoproteins may be not involved in the LLP12-2-regulated germination. Nevertheless, it is intriguing that the LLP12-2-induced inhibition of tube growth was slightly but significantly suppressed by staurosporine, suggesting that staurosporine-sensitive protein kinases involve in the LLP12-2-induced inhibition of tube elongation (Table 1). Thus, staurosporine-sensitive protein kinases play a role governing pollen tube growth as illustrated in Figure 2. It is consistent with the observation that a double mutation of two CDPKs severely reduces tube length but does not reduce germination.27
In conclusion, we report unique desiccation-associated ABA signaling transduction through which the Rop and its target LLP12-2 are regulated during pollen maturation and tube growth. Overexpression of LLP12-2 drastically inhibits pollen germination and tube growth. An optimum level of [Ca2+]cyt influx is crucial for normal germination and tube growth. In addition, staurosporine-sensitive protein kinases also involve in the LLP12-2-induced inhibition of tube growth, but may be not involved in germination.
Acknowledgements
This work was supported by National Science Council [grant NSC 98-2321-B005-003-MY3 to C.S.].
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13466
References
- 1.Berken A. ROPs in the spotlight of plant signal transduction. Cell Mol Life Sci. 2006;63:2446–2459. doi: 10.1007/s00018-006-6197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YJ, Yang Z. Tip growth: signaling in the apical dome. Curr Opin Plant Biol. 2008;11:662–671. doi: 10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, et al. A novel ROP/RAC effector links cell polarity, root-meristem maintenance and vesicle trafficking. Curr Biol. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Zárský V, Potocký M. Recycling domains in plant cell morphogenesis: small GTPase effectors, plasma membrane signalling and the exocyst. Biochem Soc Trans. 2010;38:723–728. doi: 10.1042/BST0380723. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Shiotani K, Togashi T, Miki D, Aoyama M, Wong HL, et al. Analysis of the Rac/Rop small GTPase family in rice: expression, subcellular localization and role in disease resistance. Plant Cell Physiol. 2010;51:585–595. doi: 10.1093/pcp/pcq024. [DOI] [PubMed] [Google Scholar]
- 6.Xin Z, Zhao Y, Zheng ZL. Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiol. 2005;139:1350–1365. doi: 10.1104/pp.105.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao LZ, Cheung AY, Nibau C, Wu HM. RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell. 2005;17:2369–2383. doi: 10.1105/tpc.105.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, et al. A Rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 2010;8:1000282. doi: 10.1371/journal.pbio.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu SW, Cheng CL, Tzen TJ, Wang CS. Rop GTPase and its target CDC42/Rac-interactive-binding motif-containing protein genes respond to desiccation during pollen maturation. Plant Cell Physiol. 2010;51:1197–1209. doi: 10.1093/pcp/pcq076. [DOI] [PubMed] [Google Scholar]
- 10.Holdaway-Clarke TL, Hepler PK. Control of pollen tube growth: role of ion gradient and fluxes. New Phytol. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- 11.Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YF, Fan LM, Zhang WZ, Zhang W, Wu WH. Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol. 2004;136:3892–3904. doi: 10.1104/pp.104.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, et al. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA. 2007;104:14531–14536. doi: 10.1073/pnas.0701781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang ZL, Ma LG, Zhang HL, He RR, Wang XC, Cui SJ, et al. Ca2+ influx into lily pollen grains through a hyperpolarization-activated Ca2+-permeable channel which can be regulated by extracellular CaM. Plant Cell Physiol. 2005;46:598–608. doi: 10.1093/pcp/pci063. [DOI] [PubMed] [Google Scholar]
- 15.Qu HY, Shang ZL, Zhang SL, Liu LM, Wu JY. Identification of hyperpolarization-activated calcium channels in apical pollen tubes of Pyrus pyrifolia. New Phytol. 2007;174:524–536. doi: 10.1111/j.1469-8137.2007.02069.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu G, Gu Y, Li S, Yang Z. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell. 2001;13:2841–2856. doi: 10.1105/tpc.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169:127–138. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boavida LC, McCormick S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007;52:570–582. doi: 10.1111/j.1365-313X.2007.03248.x. [DOI] [PubMed] [Google Scholar]
- 19.Sivitz AB, Reinders A, Ward JM. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008;147:92–100. doi: 10.1104/pp.108.118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol. 2008;148:1201–1211. doi: 10.1104/pp.108.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon GM, Dowd PE, Gilroy S, McCubbin AG. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell. 2006;18:867–878. doi: 10.1105/tpc.105.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, McCormick S. Regulation of pollen tube polarity: Feedback loops rule. Plant Signal Behav. 2008;3:345–347. doi: 10.4161/psb.3.5.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putnam-Evans C, Harmon AC, Palevitz BA, Fechheimer M, Cormier MJ. Calcium-dependent protein kinase is localized with F-actin in plant cells. Cell Motil Cytoskel. 1989;12:12–22. [Google Scholar]
- 24.Zhou L, Fu Y, Yang Z. A genome-wide functional characterization of Arabidopsis regulatory calcium sensors in pollen tubes. J Integr Plant Biol. 2009;51:751–761. doi: 10.1111/j.1744-7909.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- 25.Kong L, Wang M, Wang Q, Wang X, Lin J. Protein phosphatases 1 and 2A and the regulation of calcium uptake and pollen tube development in Picea wilsonii. Tree Physiol. 2006;26:1001–1012. doi: 10.1093/treephys/26.8.1001. [DOI] [PubMed] [Google Scholar]
- 26.Barwe SP, Sathiyabama M, Jayabaskaran C. Induction of chitinase activity by exogenous cytokinins in excised dark-grown cucumber cotyledons: involvement of Ca2+ and staurosporine-sensitive protein kinase(s) in cytokinin signaling. J Plant Physiol. 2001;158:1–7. [Google Scholar]
- 27.Myers C, Romanowsky SM, Barron YD, Garg S, Azuse CL, Curran A, et al. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 2009;59:528–539. doi: 10.1111/j.1365-313X.2009.03894.x. [DOI] [PubMed] [Google Scholar]