Abstract
Gamete fusion activates the egg in animals and plants, and the gamete fusion site on the zygote might provide a possible cue for zygotic development and/or embryonic patterning. In angiosperms, a zygote generally divides into a two-celled proembryo consisting of an apical and a basal cell with different cell fates. This is a putative step in the formation of the apical-basal axis of the proembryo. We observed the positional relationship between the gamete fusion site and the division plane formed by zygotic cleavage using an in vitro fertilization system with rice gametes. There was no relationship between the gamete fusion site and the division plane leading to the two-celled proembryo. Thus, the gamete fusion site on the rice zygote does not appear to function as a determinant for positioning the zygote division plane, and the zygote apparently possesses autonomous potential to establish cell polarity along the apical-basal axis for its first cleavage.
Key words: asymmetric division, egg cell, fertilization, gamete fusion, rice, sperm cell, two-celled proembryo, zygote
Gamete Fusion in the Embryo Sac
In the embryo sac of angiosperms, the egg cell is generally covered with a cell wall at the micropylar portion and the cell wall is incomplete over the chalazal one-third to two-thirds of the cell, exposing a large area of the egg cell plasma membrane adjacent to the synergids and central cell.1–3 Regarding the gamete fusion site on the egg embedded in the embryo sac, it has been shown that the egg cell appears to fuse with a sperm cell in the zone where the synergid cells degenerate.4,5 However, it is unclear whether there is a restricted or a specific gamete fusion point on the chalazal-side plasma membrane of an egg cell located in the embryo sac. Because the egg and sperm cells exist as a hemi-protoplast and as protoplasts in the embryo sac, respectively, to allow membrane fusion between gametes, it is possible that gamete fusion can occur around the chalazal surface of the egg cell, except for where it is attached to synergids (Fig. 1A).
Figure 1.
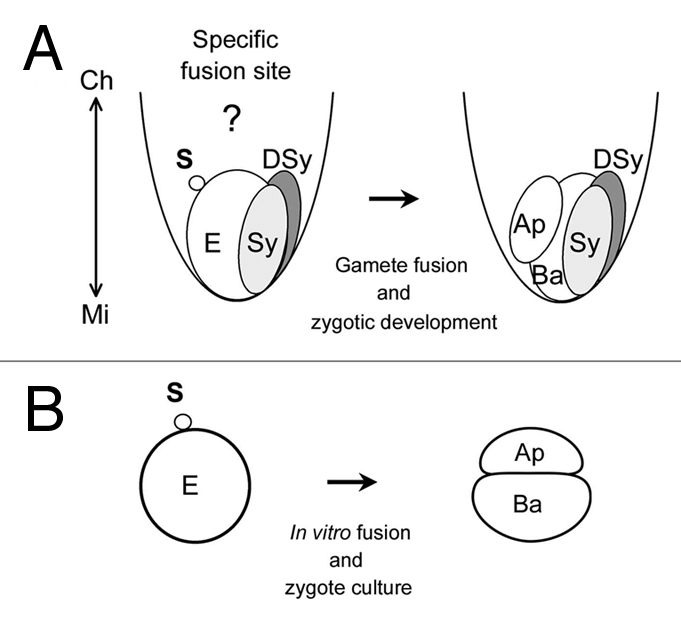
Sperm fusion site on an egg cell and two-celled proembryo embedded in rice embryo sac (A) or produced by in vitro fertilization (IVF) system with rice gametes (B). (A) One of two sperm cells released into an embryo sac fuses with an egg cell. Because the egg and sperm cells exist as hemi-protoplasts and protoplasts in the embryo sac, respectively, to allow gamete fusion, it is possible that gamete fusion can occur around the chalazal surface of the egg cell. After gamete fusion, the zygote divides parallel or slightly oblique to the region facing the synergid and the resulting two-celled embryo comprises a small apical cell with dense cytoplasm and a larger vacuolated basal cell.25 The double arrowed line shows the chalazal-micropylar axis. (B) the zygote produced by IVF divides into an asymmetric two-celled embryo consisting of a small apical cell with dense cytoplasm and a large basal cell with well-developed vacuoles, as in planta. Ap, apical cell; Ba, basal cell; DSy, degenerated synergid; E, egg cell; S, sperm cell; Sy, intact synergid.
Post Fusion Events: Egg Activation and Zygote Division
In animals, sperm entry activates the oocyte via cascades of ionic and biochemical events, and in some cases the sperm entry position provides a cue for subsequent embryonic patterning.6–9 As for plant gamete fusion, it has been shown that the Ca2+ influx triggered at the gamete fusion point propagates in the zygote as a wavefront10 and that the elevated Ca2+ levels within the zygote induces a post-fusion event: the rapid formation of the zygotic cell wall.11 Moreover, using free-living gametes of brown algae, the sperm entry site has been shown to provide positional information for establishing an intermediate default axis of the zygote, which is then overridden by light stimuli that cause the formation of the thallus-rhizoid axis.12,13 These suggest that gamete fusion activates the egg cell and that the gamete fusion site marks the apical-basal axis orientation of zygote and proembryo.
In angiosperms, it has been shown that the apical cell of the two-celled proembryo produced by zygotic cell division develops into the embryo proper, while the basal cell develops into the suspensor and hypophysis.14–19 In addition to cytological observations of cleavage in plant zygotes, it has been reported that the two daughter cells from a zygote possess different transcriptional profiles.20,21 Moreover, the YODA-dependent MAPKK signaling pathway and the temporal accumulation of a phytohormone auxin via PIN7—an auxin efflux carrier protein—are thought to be crucial for specifying the fate of the basal and apical cells of the Arabidopsis two-celled proembryo, respectively.22,23 These cytological and genetic analyses suggest that the first cell division of the zygote is closely related to cell fate specification of the two daughter cells and is a key step in the formation of the apicalbasal axis of proembryo.
Positional Relationship between the Gamete Fusion Site and the First Division Plane in the Rice Zygote
Elucidating the relationship between the gamete fusion point on the zygote and the position of the cleavage plane in the two-celled proembryo would provide basic insight into whether the gamete fusion point functions as a putative positional cue for positioning of the zygote cleavage plane. The rice in vitro fertilization (IVF) system is a suitable system for such analysis. This is because the IVF-produced rice zygote divides into an asymmetric two-celled proembryo consisting of a small apical cell with dense cytoplasm and a large basal cell with well-developed vacuoles, in a highly similar manner to the zygote located within the embryo sac (Fig. 1A and B).24,25 We visualized the gamete fusion site on the rice zygote by performing IVF with an egg cell and a sperm cell, which was prestained with Alexa Fluor 488-conjugated concanavalin A (Fig. 2A and B).26 Thereafter, the fusion site-labeled zygote was cultured to the two-celled proembryo stage and the positional relationship between the gamete fusion site and the zygotic first division plane was monitored (Fig. 2C–F). Figure 2G indicates a representative two-celled proembryo, on which the positions of putative fusion sites from observations of 33 independent experiments have been plotted based on the relative distance from the first cell division plane. The sites were positioned randomly, indicating that there is no spatial relationship between the gamete fusion site on the zygote and the position of the division plane in the two-celled proembryo.
Figure 2.
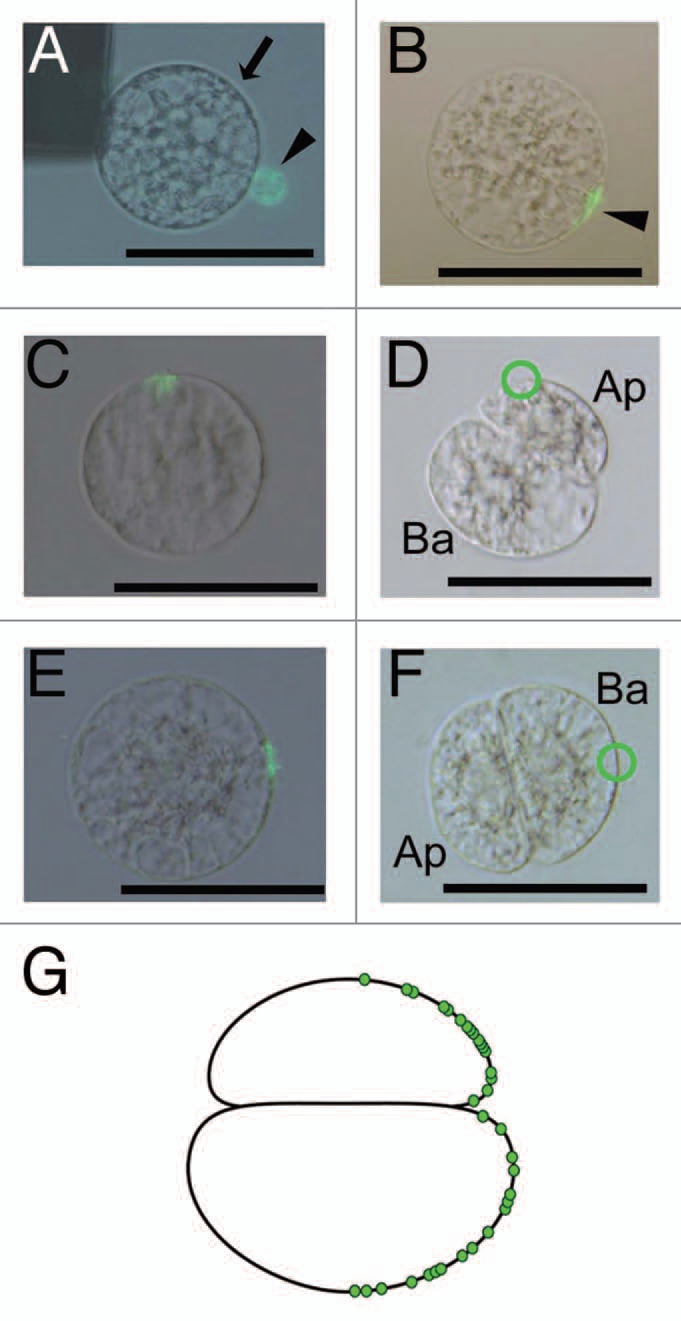
Fluorescent labeling of the gamete fusion site on the rice zygote (A and B) and subsequent tracing of the position of the gamete fusion site on the two-celled proembryo (C–G). (A) Alignment of an egg cell with a sperm cell stained with Alexa Fluor 488-conjugated concanavalin A (ConA 488) on one of the electrodes under an alternating current (AC) field in a fusion droplet. Bright-field and fluorescent images are merged. The arrow and arrowhead indicate an egg cell and a sperm cell, respectively. (B) Fusion of gametes in (A) following a negative direct current (DC) pulse. Bright-field and fluorescent images are merged. Arrowhead indicates fluorescent-labeled fusion point. (C and D) A zygote with the ConA 488-labeled fusion site in (C) was cultured. The zygote divided to produce a two-celled proembryo as shown in (D). A putative trace of the gamete fusion site is enclosed by a green circle. Bright-field and fluorescent images are merged in (C). (D) is a bright-field image. (E and F) a zygote with the ConA 488-labeled fusion site in (E) was cultured. The zygote divided to produce a two-celled proembryo as shown in (F). The green circle indicates a possible trace of the gamete fusion site on the two-celled embryo. (G) A schematic diagram of a two-celled embryo on which possible gamete fusion sites obtained from 33 independent experiments have been plotted. Ap, apical cell; Ba, basal cell. Bars = 50 µm.
Gamete Fusion Site on the Egg Cell
Our IVF study suggested that, even if gamete fusion might occur at any point on the surface of an egg cell, the resulting zygote can still divide into a two-celled proembryo in a highly similar manner to a zygote within the embryo sac. This observation suggests that the gamete fusion site does not affect positioning of the zygotic division plane, and indicates a possibility that there is no specific or restricted point on the egg cell surface for gamete fusion. In addition to the in vitro situation, the possibility is consistent with reports on the gene GENERATIVE CELL SPECIFIC 1 (GCS1), encoding a key male transmembrane protein for the gamete fusion/recognition process.27 In the embryo sac, two sperm cells with a gcs mutation remained attached to an egg cell without undergoing cell fusion. Notably, the attachment of the sperm cell was not restricted to the egg cell surface at points of degenerating synergid contact but was observed to be placed randomly on the egg cells. Taking these in vitro and in planta results into account, we suggest that sperm cell fusion in angiosperms is only needed for activation of the egg cell and for delivering the male genome to the female gamete. Although the putative molecule(s) for gamete recognition and/or fusion on plant egg cells have not yet been identified, these might not be distributed at a specific region but localized over the entire plasma membrane of the egg cell.
Autonomous Establishment of Cell Polarity in Rice Zygotes
In the rice egg cell, the nucleus and cytoplasm are located at the micropylar end (basal side), whereas vacuoles were localized at the micropylar end (apical side), suggesting that distinct polarity and possible apical-basal axis exist in the cell (Fig. 3).24,25,28,29 After gamete fusion, the nuclei and putative cytoplasm-rich regions at basal side of the rice egg cell appears to move toward the apical pole during zygotic development, and the zygote divides into the two-celled embryo comprising a small apical cell with dense cytoplasm and a larger vacuolated basal cell.25,30
Figure 3.
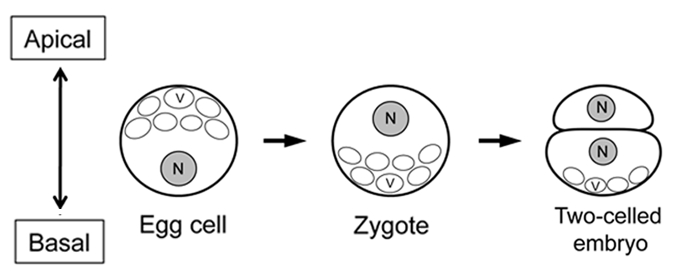
Possible apical-basal axis in rice egg cell, zygote and two-celled proembryo. N and V indicate nucleus and vacuoles, respectively. The double arrowed line shows the apical-basal axis. The illustrations were made on the basis of anatomical studies for rice egg cell, zygote and two-celled proembryo.25,28–30
Because fertilization and subsequent embryogenesis and endosperm formation progress in the embryo sac, deeply embedded in ovular tissue, it has been believed that zygotic development is affected and probably controlled by extrinsic signals or molecules from tissues surrounding the zygote, such as endosperm and maternal tissues. In addition to the surrounding tissue-derived extrinsic signals, the sperm cell fusion site on the egg cell is also considered as a potential extrinsic signal for zygote development. However, our results suggest that rice zygotes produced by IVF develop into asymmetrical two-celled proembryos in surrounding tissue- and gamete fusion site-independent manner. This indicates the possibilities that the two possible extrinsic cues—from surrounding tissues/cells or from the gamete fusion point—do not function as determinants for positioning of the first division plane in the rice zygote, and that the zygote possesses an autonomous potential to establish cell polarity along the apical-basal axis, which is putatively predetermined in the egg cell. The apical-basal axis will be then fixed by division of the zygote into a two-celled proembryo.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13468
References
- 1.Jensen WA. The ultrastructure and composition of the egg and central cell of cotton. Amer J Bot. 1965;52:781–797. [Google Scholar]
- 2.Huang BQ, Russell SD. Female germ unit: Organization, reconstruction and isolation. Int Rev Cytol. 1992;140:233–293. [Google Scholar]
- 3.Russell SD. The egg cell: Development and role in fertilization and early embryogenesis. Plant Cell. 1993;5:1349–1359. doi: 10.1105/tpc.5.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dresselhaus T. Cell-cell communication during double fertilization. Curr Opin Plant Biol. 2006;9:41–47. doi: 10.1016/j.pbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Spielman M, Scott RJ. Polyspermy barriers in plants: from preventing to promoting fertilization. Sex Plant Reprod. 2008;21:53–65. [Google Scholar]
- 6.Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107:37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein B, Hird SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–1474. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- 8.Bowerman B. Maternal control of polarity and patterning during embryogenesis in C. elegans. In: Moody SA, editor. Cell Lineage and Fate Determination. San Diego: Academic Press; 1999. pp. 97–117. [Google Scholar]
- 9.Piotrowska K, Zernicka-Goetz M. Role for sperm in spatial patterning of the early mouse embryo. Nature. 2000;409:517–521. doi: 10.1038/35054069. [DOI] [PubMed] [Google Scholar]
- 10.Antoine AF, Faure JE, Cordeiro S, Dumas C, Rougier M, Feijo JA. A calcium influx is triggered and propagates in the zygote as a wavefront during in vitro fertilization of flowering plants. Proc Natl Acad Sci USA. 2000;97:10643–10648. doi: 10.1073/pnas.180243697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kranz E, von Wiegen P, Lörz H. Early cytological events after induction of cell division in egg cells and zygote development following in vitro fertilization with angiosperm gametes. Plant J. 1995;8:9–23. [Google Scholar]
- 12.Kropf DL. Induction of polarity in fucoid zygotes. Plant Cell. 1991;9:1011–1020. doi: 10.1105/tpc.9.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hable WE, Kropf DL. Sperm entry induces polarity in fucoid zygotes. Development. 2000;127:493–501. doi: 10.1242/dev.127.3.493. [DOI] [PubMed] [Google Scholar]
- 14.Pritchard NH. A cytochemical study of embryo development in Stellaria media. Amer J Bot. 1964;51:472–479. [Google Scholar]
- 15.Schulz R, Jensen WA. Capsella embryogenesis: The egg, zygote and young embryo. Amer J Bot. 1968;55:807–819. [Google Scholar]
- 16.Tykarska T. Rape embryogenesis: I. The proembryo development. Acta Soc Bot Pol. 1976;45:3–16. [Google Scholar]
- 17.Schel JHN, Kieft H, van Lammeren AAM. Interactions between embryo and endosperm during early developmental stage of maize caryopses (Zea maize) Can J Bot. 1984;62:2842–2853. [Google Scholar]
- 18.Mansfield SG, Briarty LG. Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can J Bot. 1991;69:461–467. [Google Scholar]
- 19.Lindsey K, Topping JE. Embryogenesis: a question of pattern. J Exp Bot. 1993;259:359–374. [Google Scholar]
- 20.Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, et al. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T, Scholten S, Lörz H, Kranz E. Identification of genes that are up or downregulated in the apical or basal cell of maize two-celled embryos and monitoring their expressions during zygote development by a cell manipulation- and PCR-based approach. Plant Cell Physiol. 2005;46:332–338. doi: 10.1093/pcp/pci032. [DOI] [PubMed] [Google Scholar]
- 22.Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 2004;116:109–119. doi: 10.1016/s0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- 23.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 24.Uchiumi T, Uemura I, Okamoto T. Establishment of an in vitro fertilization system in rice (Oryza sativa L.) Planta. 2007;226:581–589. doi: 10.1007/s00425-007-0506-2. [DOI] [PubMed] [Google Scholar]
- 25.Sato A, Toyooka K, Okamoto T. Asymmetric cell division of rice zygotes located in embryo sac and produced by in vitro fertilization. Sex Plant Reprod. 2010;23:211–217. doi: 10.1007/s00497-009-0129-9. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima K, Uchiumi T, Okamoto T. Positional relationship between the gamete fusion site and the first division plane in the rice zygote. J Exp Bot. 2010;61:3101–3105. doi: 10.1093/jxb/erq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- 28.Jian D, Yang HY. An ultrastructural study embryo sac in Oryza sativa L. Acta Bot Sin. 1989;31:81–88. [Google Scholar]
- 29.Maeda E, Maeda K. Ultrastructure of egg apparatus of rice (Oryza sativa) after anthesis. Jpn J Crop Sci. 1990;59:179–197. [Google Scholar]
- 30.Jones TJ, Rost TL. Histochemistry and ultrastructure of rice (Oryza sativa) zygotic embryogenesis. Amer J Bot. 1989;76:504–520. [Google Scholar]


