Abstract
The shade avoidance syndrome serves to improve the competitive power of plants growing in crowded plant communities. An important element of avoiding shade is to rapidly elongate shoots and outgrow competing neighbors. We investigated the role of cell wall modifying proteins expansins and xyloglucan endotransglucosylase/hydrolases (XTHs) in mediating this vital elongation growth in Arabidopsis thaliana. These proteins act on the cell wall and modify it to make it more extensible thereby facilitating cellular expansion. We found that XTHs are essential for shade-induced growth in Arabidopsis. Expansin activity on the other hand was not regulated in plants exposed to shade. Shade also resulted in rapid apoplastic acidification, which is necessary for the optimal activity of cell wall modifying proteins such as XTHs and expansins.
Key words: shade avoidance, cell wall modification, expansins, XTHs, Arabidopsis thaliana, apoplastic acidification, low red to far-red, low blue
Plants growing in densely vegetated habitats have to compete with their neighbors for light and nutrients.1 The imposition of shade by taller plants in a canopy results in characteristic changes to the light spectrum. The absorption of certain wavelengths of light by chlorophyll pigments causes a reduction in the red to far-red ratio (low R: FR) and a depletion of blue light (Fig. 1). Some plants can detect these light quality changes and translate it to a suite of morphological changes including shoot elongation and leaf hyponasty.1,2 This phenomenon is part of the shade avoidance syndrome and it can improve the light foraging capacity of these plants in a crowded canopy.
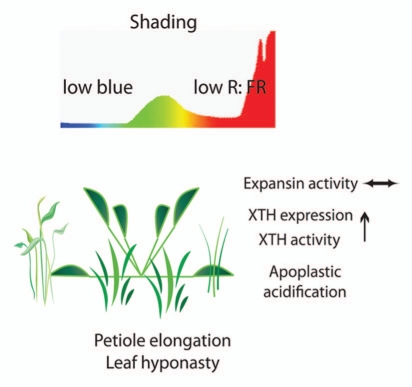
Figure 1.
Shade avoidance in Arabidopsis thaliana. Characteristic changes to the visible light spectrum (low R: FR and low blue) occur due to the presence of neighboring plants. These shade signals trigger increased transcript abundance and activity of specific XTHs. Shade also triggers an acidification of the apoplast. This provides the optimal milieu for the action of XTHs and other cell wall modifying proteins such as expansins. The coordinated action of these proteins modifies the cell wall thereby facilitating cellular expansion and resulting in enhanced petiole elongation and hyponasty. These morphological responses help a plant overtop competing vegetation.
Shade-induced shoot elongation has to be timely and rapid so a plant can quickly outgrow its neighbors. Rapid elongation of the shoot requires turgor-assisted cellular expansion. This expansion, although fuelled by an increase in the cell's turgor, also requires the normally rigid cell wall to weaken and thus allow continued expansion. This occurs via modification of the cell wall through the action of certain proteins and consequent cell wall extension.3 Cell wall extensibility is therefore an important factor regulating growth. Expansins4 and XTHs5 are two well characterized cell wall modifying proteins that are implicated in cellular expansion. We characterized the roles of XTHs and expansins in shade-induced petiole elongation in Arabidopsis.
We subjected Arabidopsis to two distinct shade treatments. Low R: FR ratios imply neighbor proximity while a ‘green shade’ treatment (combined reductions in low R: FR, blue light and total light intensity) mimicked a closed canopy.1,6 Arabidopsis responded to shade signals with enhanced petiole elongation and an upward elevation of its leaves (hyponasty) (Fig. 1). Furthermore, it could distinguish between the two shade signals that we used since the magnitude of the shade avoidance response was greater in green shade than in low R: FR.
Expansin Activity Is Not Differentially Regulated during Shade Avoidance in Arabidopsis
Expansin activity and gene expression in most instances show a strong correlation with cellular growth.4 Despite approximately two and three times higher petiole growth rates in low R: FR and green shade petioles (relative to controls) respectively, it was surprising to find no corresponding differences in expansin activity. There were also no significant differences between petioles from shade treated and control plants in extractable expansin activity or expansin sensitivity.6 Thus although we cannot rule out a shade-induced differential regulation of expansin genes, shade does not seem to regulate the activity of expansins in Arabidopsis petioles.
Shade-Induced Petiole Elongation in Arabidopsis Requires XTHs
In contrast to expansin activity, the petioles of shade treated plants had higher XTH activity relative to controls. Furthermore, specific XTH genes (the Arabidopsis genome has 33 XTH open reading frames) were upregulated in response to low R: FR and green shade. Five XTH genes (XTH 9, −15, −16, −17 and −19) were upregulated in response to low R: FR. In green shade, in addition to the latter four XTH genes, XTH 22 was also upregulated.6 In both treatments, there was a significant upregulation of transcript levels already at the first time point measured (5 h) indicating a rapid transcriptome response. In addition, two xth mutants—xth15 and xth17- had absent, low-R: FR responses and a reduced green shade response (relative to wild type plants) showing that XTH activity is required for shade-induced petiole elongation in Arabidopsis.6
Cell Wall Modification is a Multi-Factorial Coordinated Process
With the exception of some reports,7 expansin gene expression and activity show positive correlations with growth. In the context of shade avoidance as well, we have shown that in the plant species Stellaria longipes, the regulation of low R:FR-induced extension growth was via control of expansin gene expression and activity and not XTH activity.8,9 These reciprocal results between two plant species indicate that it would be inaccurate to label any single cell wall modifying protein family as the sole regulator in the cellular expansion process. The cell wall is an intricate, supramolecular structure composed of a myriad of complex polymers and chemical interactions.10 The structure and composition of the cell wall can be both species- and organ-specific. It can also change with different developmental stages and in response to various biotic and abiotic stresses. Since cell wall proteins have very specific activities and sites of action, the existing cell wall composition is very likely to determine what protein activities are required to modify it. Furthermore, coordinated multiple protein activities are probably required to modify cell wall structure.11 In conclusion, the type of cell wall would dictate the complement of wall modifying activities needed to change it.12
The pH of the apoplast where most cell wall modifying proteins act is another crucial factor regulating cell expansion. We found that in Arabidopsis petioles, green shade induced a rapid (within minutes) efflux of protons into the apoplastic space.6 Since most wall modifying proteins are optimally active at an acidic pH, light quality regulation of the apoplastic pH, likely via activation of plasma membrane proton pumps, would be crucial to the cellular expansion process.
Conclusions
The cell wall is a key control point during shade-induced shoot elongation in plants. Modification of the cell wall mediated by XTHs is required to facilitate cellular expansion and thus shoot elongation. Characteristic changes in the spectral quality of light brought about by shading regulate XTH activity both by affecting gene expression as well as by acidifying the apoplastic pH (Fig. 1).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13643
References
- 1.Franklin KA. Shade avoidance. New Phytol. 2008;79:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 2.Keuskamp DH, Sasidharan R, Pierik R. Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal Behav. 2010;5:655–662. doi: 10.4161/psb.5.6.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosgrove DJ. Expansive growth of plant cell walls. Plant Physiol Biochem. 2000;38:109–124. doi: 10.1016/s0981-9428(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 5.Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002;43:1421. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- 6.Sasidharan R, Chinnappa CC, Staal M, Elzenga JT, Yokoyama R, Nishitani K, et al. Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by XTHs. Plant Physiol. 2010;154:978–990. doi: 10.1104/pp.110.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caderas D, Muster M, Vogler H, Mandel T, Rose JK, McQueen-Mason S, et al. Limited correlation between expansin gene expression and elongation growth rate. Plant Physiol. 2000;123:1399–1414. doi: 10.1104/pp.123.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinnappa CC, Donald GM, Sasidharan R, Emery RJN. The biology of Stellaria longipes (Caryophyllaceae) Can J Bot. 2005;83:1367–1383. [Google Scholar]
- 9.Sasidharan R, Chinnappa CC, Voesenek LACJ, Pierik R. The regulation of cell wall extensibility during shade avoidance: a study using two contrasting ecotypes of Stellaria longipes. Plant Physiol. 2008;148:1557–1569. doi: 10.1104/pp.108.125518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 11.Rose JKC, Bennett AB. Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999;4:176–183. doi: 10.1016/s1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- 12.Nishitani K. A genome-based approach to study the mechanisms by which cell-wall type is defined and constructed by the collaborative actions of cell-wall-related enzymes. J Plant Res. 2002;115:303–307. doi: 10.1007/s10265-002-0032-z. [DOI] [PubMed] [Google Scholar]