Abstract
Mitogen-activated protein kinase (MAPK) pathways play crucial roles in developmental and adaptive responses. Depending on the stimulus, MAPK activation regulates a wide variety of plant cell responses, such as proliferation, differentiation and cell death, which normally require precise spatial and temporal control. In this context, protein phosphatases play important roles by regulating the duration and magnitude of MAPK activities. During infection by non-host and incompatible host microorganisms, MAPK activity can promote a local cell death mechanism called hypersensitive response (HR), which is part of the plant defence response. HR-like responses require sustained MAPK activity and correlate with oxidative burst. We recently showed that MAPK phosphatase MKP2 positively controls biotic and abiotic stress responses in Arabidopsis. MKP2 interacts with MPK6 in HR-like responses triggered by fungal elicitors, suggesting that MKP2 protein is part of the mechanism involved in MAPK regulation during HR. Here we discuss the interplay of MAPK and MKP2 phosphatase signaling during cell death responses elicited by host-pathogen interactions.
Key words: Arabidopsis, hypersensitive response (HR), MAPK, MPK6, MKP2, ROS
Different studies have identified conserved components of MAPK pathways in plants and have provided evidence that MAPK signaling regulates a wide variety of plant biological responses.1 For example, MAPK signaling is required for the regulation of stomatal functions,2–4 hormone signaling5,6 and innate immunity responses.7–9 An increasing number of reports indicate that plant MAPKs, in particular tobacco SIPK/Ntf4 and WIPK and their Arabidopsis orthologs, MPK6 and MPK3, are converging points for signals elicited by different pathogens and play regulatory roles in disease responses.10
One of the most efficient and immediate immune responses dependent on MAPK signaling is a mechanism of cell death called hypersensitive response (HR). HR is a rapid, localized cell death process at the site of pathogen infection, which is associated with specific molecular effects such as the generation of reactive oxygen species (ROS) and protein phosphorylation.11 The best evidence implicating MAPK activity in HR comes from gain-of-function studies overexpressing SIPK/Ntf4 and WIPK in tobacco leaves. In these experiments, activation of SIPK/Ntf4 kinases efficiently induces HR-like cell death,12,13 but the absence of endogenous WIPK function causes delayed induction of this HR phenotype, suggesting that WIPK activity facilitates or potentiates the SIPK signal.14 Similarly, overexpression analyses of Arabidopsis MPK3 and MPK6 proteins, either alone or co-expressed with activated upstream regulators (MKK proteins), also triggers a cell death phenotype,15 suggesting a coordinated role of MKK/MAPK signaling modules in HR.15 Thus, the involvement of MAPK activities such as SIPK/MPK6 in HR cell death responses is supported by different studies; however their regulation by phosphatases remains less understood.
The main regulators of MAPKs are specific phosphatases belonging to various families, including PP2C Ser/Thr phosphatases, Tyr phosphatases (PTPs) or dual specificity phosphatases (DSPs) such as the MAPK phosphatase (MKP) subgroup.16,17 In general, dephosphorylation of MAPKs inactivates their function in many metabolic, developmental or adaptive responses. In the context of HR, we have recently shown that Arabidopsis MKP phosphatase MKP2 interacts with MPK6 in the response triggered by fungal elicitors. In particular, co-expression of MPK6 and MKP2 proteins in infected tobacco leaves significantly attenuates the cell death phenotype produced by expressing MPK6 alone, suggesting that MKP2 negatively regulates MAPK activities in this process.18
Role of MKP2 Activity in HR Responses
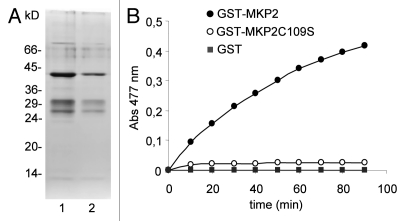
MKP2 is a MKP-family member, structurally related to Arabidopsis DsPTP1 (64% of identity), a MAPK phosphatase that has been shown to dephosphorylate MPK4 in vitro.19 Both proteins probably represent a duplication that occurred in Brassicacea,17 and are also related to other low-molecular weight MAPK phosphatases such as mammalian VHR and PAC-1 proteins.20,21 These enzymes share a simple structural organization consisting of a conserved catalytic region without additional functional domains. A common feature of this catalytic region is the presence of a conserved Cys residue that is essential for activity.19,22 We and others23 have confirmed the requirement of this residue (Cys109) in the case of MKP2 (Fig. 1).
Figure 1.
Cys109 is essential for MKP2 activity in vitro. (A) SDS-polyacrylamide gel electrophoresis of GST-MKP2 (lane 1) and GST-MKP2C109S (lane 2) fusion proteins. Positions of molecular weight markers are indicated on the left. (B) Phosphatase activity of wild-type and mutant GST-MKP2 proteins. Reactions were performed using the artificial substrate OMPF and 12 mg of each fusion protein. Phosphatase activity was assayed at 30°C in 0.8 ml of reaction buffer (50 mM 3,3-dimethylglutaric acid, pH 7, 1 mM EDTA , 0.15 M NaCl, 500 µM OMPF). The amount of 3-O-methylfluorescein was measured by absorbance at 477 nm.
Studies in animal systems suggest that MAPK phosphates are often involved in negative feedback regulation of MAPK signals.22,24 Accordingly, expression of many MKPs correlates with MAPK activation and rapidly increases in response to MAPK-dependent stimuli. However, there is less evidence for the existence of similar feedback loops in plants. We find significant induction of MKP2 transcripts in seedlings that are exposed to a variety of stimuli known to involve MAPK activation. As shown in Figure 2, there is clear upregulation of MKP2 expression in response to stress conditions caused by ABA or salt treatment, both of which are associated with activation of MAPK pathways. In addition, ubiquitous expression of an MKP-GFP chimera in transgenic plants revealed a tissue-specific pattern of MKP2-GFP accumulation that might correspond to cell-types with elevated MAPK activation18 (e.g., the stomata and meristematic cells), suggesting that MKP2 is stabilized by MAPK activity. Therefore, it appears that MKP2 expression can be induced by MAPK signaling via transcriptional and/or post-transcriptional mechanisms, and we suggest that this induction represents a feedback inhibitory mechanism to restrict the magnitude or duration of MAPK activation, for example during HR-like responses. Indeed, our genetic analyses of MKP2 function in Arabidopsis support a role of MKP2 in limiting the spread of the HR response in vivo.18
Figure 2.
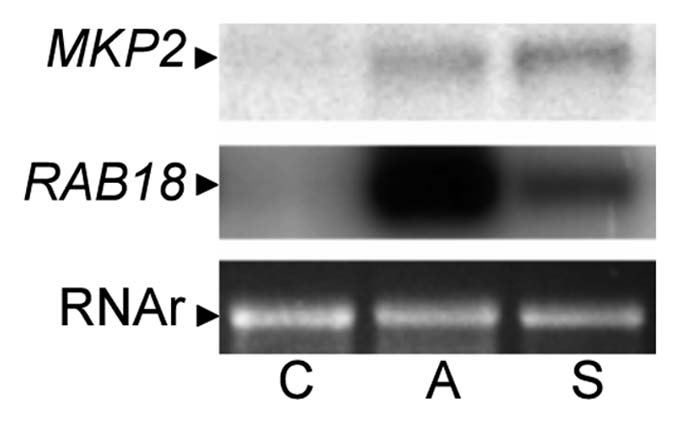
ABA/salt treatments induce MKP2 expression. Northern blot analysis of 7-day-old seedlings treated during 3 h with 100 µM ABA (A) and 250 mM NaCl (S). RAB18 expression is also shown as a positive control induced by ABA and salt treatments.
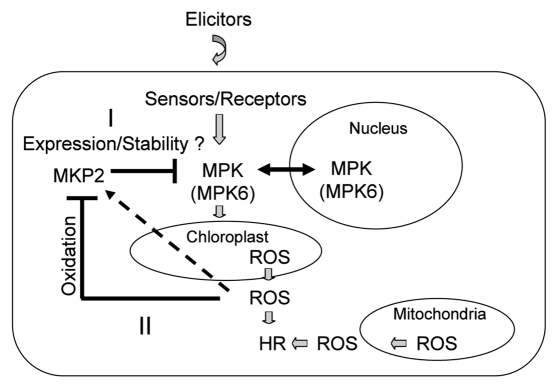
Nevertheless, MKP2 activity may also be inhibited during the HR response as a result of ROS-mediated oxidation. Studies in animal systems have shown that oxidation of the conserved Cys residue characteristic of MKP-family enzymes inhibits their catalytic activity, for example upon treatment with ROS agents.22 It is therefore conceivable that ROS accumulation during HR responses also leads to oxidation and inhibition of MKP2 (Fig. 3). Perhaps this negative effect on MKP2 activity plays a role in ensuring the correct balance of MAPK signaling that leads to localized cell-death induction. Interestingly, other phosphatases that have been implicated in regulating MAPK activities in plants, including the PP2C-family proteins, AP2C1,25 and ABI,26 are also highly sensitive to the redox status of the cell.27,28 These phosphatases, which do not contain the same Cys in their active site motif, may also undergo oxidation or ROS-dependent inhibition, and it will be interesting to ascertain whether they play a regulatory role in the responses to pathogen infection.
Figure 3.
Hypothetical model of MKP2 function in plant HR cell death. Different mechanisms may be converging such as transcriptional (I) and post-transcriptional regulation (II), which may take place to balance and control the induction of cell-death response.
Conserved Regulation of HR and Animal Apoptosis?
Interestingly, VHR and PAC-1 have been implicated in the negative inhibition of MAPK activities during programmed cell death responses.29,30 This raises the possibility that related MAPK phosphatases play similar functions during animal and plant cell death responses. Although HR cell death shows molecular differences to animal apoptosis such as the absence in plants of some key components;31 several morphological and biochemical traits including cellular shrinking, cytoskeletal rearrangements or chromatin processing are conserved hallmarks.31 Consequently, signaling by MAPK cascades and their negative regulation by specific phosphates may also represent a conserved mechanism of cell death control in animals and plants, although further studies are necessary before we can understand this apparent conservation, and the links between MAPK signaling and other hormonal pathways in cell death processes.
Conclusions
Recent progress by different groups is revealing a complex regulatory network controlling plant responses to pathogen infection. This network depends on different regulatory pathways and effectors, the functions of which are beginning to be elucidated. Signaling by MAPK modules appear to play a central role in HR-like responses, although the precise contribution of individual MAPK isoforms and their downstream effectors in this context remain largely unknown. Similarly, increasing evidence indicates that regulation of MAPK activity by specific phosphatases such as MKP2 is essential for achieving a correct balance of HR-mediated cell death. This probably requires the precise regulation of MAPK phosphatase activity by both transcriptional and post-transcriptional mechanisms, which may take place at different steps in the HR response (Fig. 3). The challenge for future studies will be to define the specific components and rate-limiting steps of MAPK cascades in HR processes, as well as the mechanisms that integrate MAPK-dependent activities with other signaling pathways during host-pathogen interactions.
Acknowledgements
We thank Sami Irar for help with MKP2 activity. B.V. was financed by Fundação para a Ciência e Tecnologia Ph.D., grant SFRH/BD/62070/2009. This work was supported by Consolider-Ingenio 2010CSD2007-00036 from MCYT (Spain) and grants BIO2009-13044 from MCYT (Spain) and CIRIT2009SGR626 from Comissionat per Universitats i Recerca de la Generalitat de Catalunya to M.P.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13645
References
- 1.Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 2.Gudesblat GE, Iusem ND, Morris PC. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol. 2007;173:713–721. doi: 10.1111/j.1469-8137.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson KM, Rychel AL, Torii KU. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. Plant Cell. 2010;22:296–306. doi: 10.1105/tpc.109.072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JS, Wang S, Sritubtim S, Chen JG, Ellis BE. Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant J. 2009;57:975–985. doi: 10.1111/j.1365-313X.2008.03741.x. [DOI] [PubMed] [Google Scholar]
- 6.Han L, Li GJ, Yang KY, Mao G, Wang R, Liu Y, et al. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 2010 doi: 10.1111/j.1365-313X.2010.04318.x. In press. [DOI] [PubMed] [Google Scholar]
- 7.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 8.Pedley KF, Martin GB. Role of mitogen-activated protein kinases in plant immunity. Curr Opin Plant Biol. 2005;8:541–547. doi: 10.1016/j.pbi.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Asai S, Yoshioka H. The role of radical burst via MAPK signaling in plant immunity. Plant Signal Behav. 2008;3:920–922. doi: 10.4161/psb.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitzschke A, Schikora A, Hirt H. MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol. 2009;12:421–426. doi: 10.1016/j.pbi.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Lam E. Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol. 2004;5:305–315. doi: 10.1038/nrm1358. [DOI] [PubMed] [Google Scholar]
- 12.Ren D, Yang KY, Li G, Liu Y, Zhang S. Activation of Ntf4, a tobacco MAPK, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol. 2006;141:1482–1493. doi: 10.1104/pp.106.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Liu Y, Klessig DF. Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J. 2000;23:339–347. doi: 10.1046/j.1365-313x.2000.00780.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Jin H, Yang KY, Kim CY, Baker B, Zhang S. Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J. 2003;34:149–160. doi: 10.1046/j.1365-313x.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 15.Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, et al. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 17.Bartels S, Besteiro MA, Lang D, Ulm R. Emerging functions for plant MAP kinase phosphatases. Trends Plant Sci. 2010;15:322–329. doi: 10.1016/j.tplants.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Lumbreras V, Vilela B, Irar S, Solé M, Capellades M, Valls M, et al. MAPK phosphatase MKP2 mediates disease responses in Arabidopsis and functionally interacts with MPK3 and MPK6. Plant J. 2010;63:1017–1030. doi: 10.1111/j.1365-313X.2010.04297.x. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R, Huang Y, Kieber J, Luan S. Identification of a dual-specificity protein phosphatase that inactivates a MAP kinase from Arabidopsis. Plant J. 1998;16:581–589. doi: 10.1046/j.1365-313x.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- 20.Yuvaniyama J, Denu JM, Dixon JE, Saper MA. Crystal structure of the dual specificity protein phosphatase VHR. Science. 1996;272:1328–1331. doi: 10.1126/science.272.5266.1328. [DOI] [PubMed] [Google Scholar]
- 21.Farooq A, Plotnikova O, Chaturvedi G, Yan S, Zeng L, Zhang Q, et al. Solution structure of the MAPK phosphatase PAC-1 catalytic domain. Insights into substrate-induced enzymatic activation of MKP. Structure. 2003;11:155–164. doi: 10.1016/s0969-2126(02)00943-7. [DOI] [PubMed] [Google Scholar]
- 22.Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Ellis BE. Arabidopsis MAPK phosphatase 2 (MKP2) positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 MAPKs. J Biol Chem. 2007;282:25020–25029. doi: 10.1074/jbc.M701888200. [DOI] [PubMed] [Google Scholar]
- 24.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death-apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 25.Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H, et al. The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid and ethylene levels in Arabidopsis. Plant Cell. 2007;19:2213–2224. doi: 10.1105/tpc.106.049585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung J, Orfanidi S, Chefdor F, Mészaros T, Bolte S, Mizoguchi T, et al. Antagonistic interaction between MAP kinase and protein phosphatase 2C in stress recovery. Plant Sci. 2006;171:596–606. [Google Scholar]
- 27.Meinhard M, Rodriguez PL, Grill E. The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta. 2002;214:775–782. doi: 10.1007/s00425-001-0675-3. [DOI] [PubMed] [Google Scholar]
- 28.Meinhard M, Grill E. Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 2001;508:443–446. doi: 10.1016/s0014-5793(01)03106-4. [DOI] [PubMed] [Google Scholar]
- 29.Arnoldussen YJ, Lorenzo PI, Pretorius ME, Waehre H, Risberg B, Maelandsmo GM, et al. The mitogen-activated protein kinase phosphatase vaccinia H1-related protein inhibits apoptosis in prostate cancer cells and is overexpressed in prostate cancer. Cancer Res. 2008;68:9255–9264. doi: 10.1158/0008-5472.CAN-08-1224. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Jin YJ, Calaf GM, Huang WL. Yin Y, PAC1 is a direct transcription target of E2F-1 in apoptotic signaling. Oncogene. 2007;26:6526–6535. doi: 10.1038/sj.onc.1210484. [DOI] [PubMed] [Google Scholar]