Abstract
Background:
The study is to show the advantages of preservation of a calvarial bone flap in the abdominal pocket after decompressive craniotomy. Decompressive craniectomy is an option in the surgical management of refractory hypertension when maximal medical treatment (sedation, drainage of cerebrospinal fluid, moderate cooling, etc) has failed to control refractory high intracranial pressure.
Methods:
We have prospectively analyzed 82 consecutively operated cases decompressive craniotomies done at the University Neurosurgical Clinic in Prishtina/KOSOVA over a period of eight years (June 1999 to Aug 2008). Of the 75 who had their grafts replaced (7 patient died before replacement of bone graft), 62 patients had hemicraniectomy (fronto-parieto-temporal) 7 of them were bilateral.
Results
In 66 out of 75 patients was achieved a satisfactory and cosmetically reconstruction, in 9 cases was required augmentation with methyl methacrylate to achieve cosmetic needs. Two patients had infection and the bone was removed; 6 months later these patients had cranioplasty with methyl methacrylate. The duration of storage of calvarial bone in abdominal pouch before reimplantation was 14 – 232 days (range 56 days).
Conclusion:
We think that storage of the patients own bone flap in the abdominal pocket is a safe, easy, cheap, sterile, histocompatible, and better cosmetic results.
Keywords: Autogenous bone, bone flap, cranioplasty, subgaleal pocket
INTRODUCTION
Decompressive craniectomy can be defined as the removal of a large area of the skull with opening of the dura to increase the volume of the cranial cavity, facilitating a reduction in intracranial pressure. Decompressive craniectomy is an option in the surgical management of refractory hypertension when maximal medical treatment (sedation, drainage of cerebrospinal fluid, moderate cooling, etc) has failed to control refractory high intracranial pressure, especially in brain trauma, stroke, and post-operative edema after brain surgery. The technique of storing the craniectomy graft in a subcutaneous location in the patient offers a theoretical advantage in that the patient's own body might provide a storage environment, thereby reducing graft devitalization.[2,6,8,9] Autologous calvarial bone graft has been widely accepted as the best and safest method for reconstruction and also provides the best cosmesis.
Various preservation techniques include deep freezing, preservation in bactericidal solutions, sterilization, and preservation in a subgaleal pouch.[1,10,11] The cosmetic outcome of some of these is poor because of the adverse effects of the technique on graft preservation.[1,5,10,11]
In 1920, Kreider[9] reported the first case of preservation of calvarial bone in the left hypochondrium. This was in a 4-year-old boy who had sustained a compound skull fracture. Destination of a bone flap after craniotomy has many possible fates.
The aim of the present clinical study was to assess the efficacy of preservation of calvarial bone in an abdominal pouch.
MATERIALS AND METHODS
We have prospectively analyzed 82 consecutively operated cases decompressive craniotomies done at the University Neurosurgical Clinic in Prishtina / KOSOVA over a period of 8 years (June 1999 to Aug 2008). Of the 75 who had their grafts replaced (7 patient died before replacement of bone graft), 62 patients had hemicraniectomy (fronto-parieto-temporal), 7 of them were bilateral. The rest of the 13 patients required smaller craniectomy for decompression. Dura was always opened in a stellate fashion and after brain herniation it was closed with fascia pericranialis. Six patients were thin, 57 were normal, and 12 were obese. The mean age of patients was 39 years (range, 1 to 68 years). Male were 49 and female were 33. Out of 82, children were 16. Etiology of brain swelling requiring decompressive craniectomy in 82 patients is listed in Table 1.
Table 1.
Etiology of brain swelling requiring decompressive craniectomy
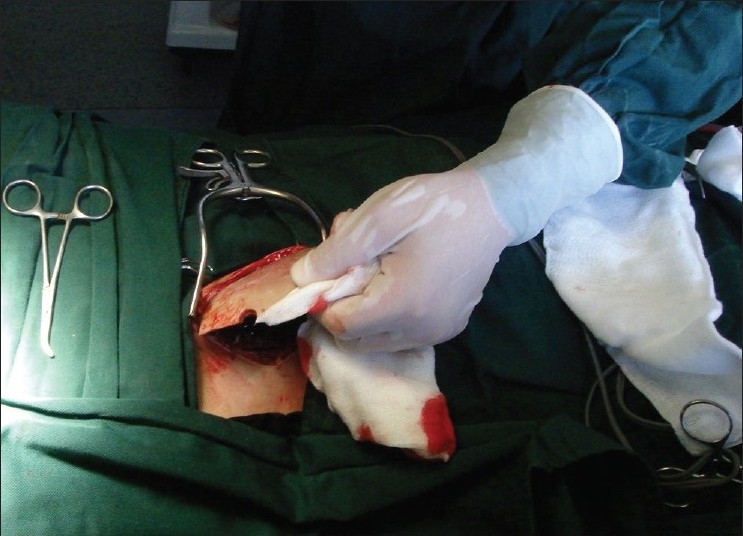
In the region of mesogastrium of left abdomen, we performed a linear horizontal incision with length between 8 and 12 cm (depend on the size of bone graft). With surgical scissors, we prepare the so-called abdominal pocket. After meticulous surgical hemostasis, we placed the bone graft with the convex part on upper site, because the bone edges can injure the skin. Under skin and skin are sutured with interrupted sutures. We do not prefer the right sight because in the future the scar might suggest the procedures like cholecystectomia or appendectomia.
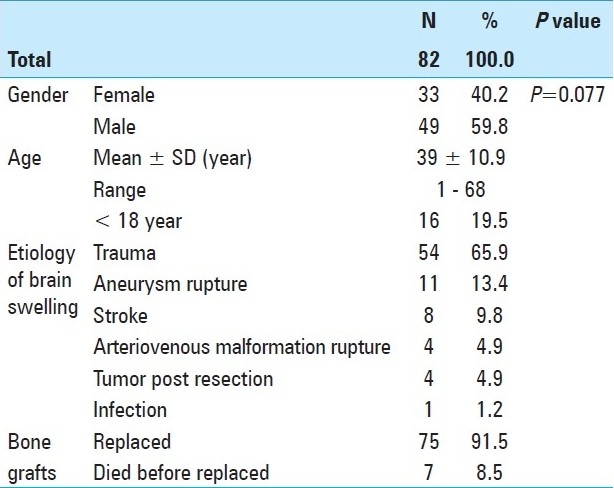
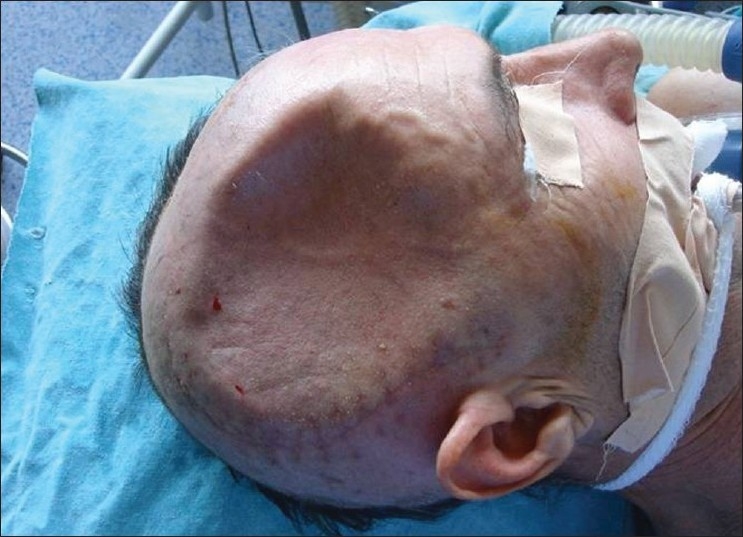
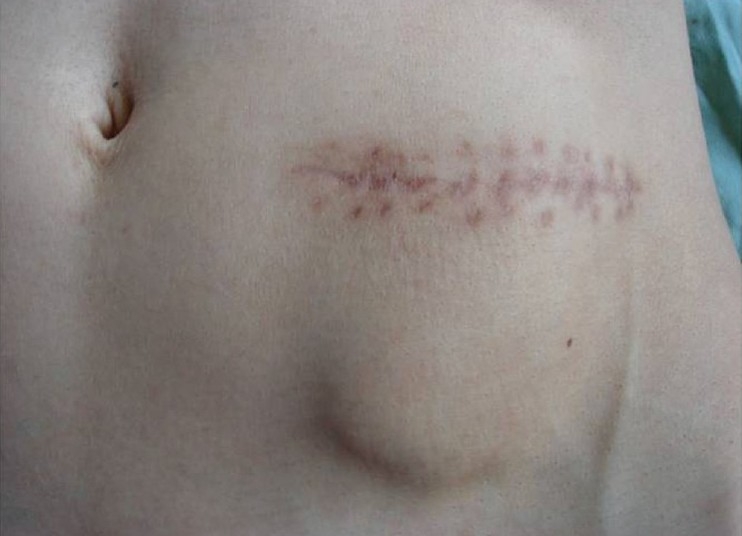
Presentation of a case: Figure 1. Cranial defect before graft replacement. Figure 2. Bone graft in abdominal pocket. Figure 3. Taking the bone out of abdomen. Figure 4. Quality of bone after six months. Figure 5. Three weeks after replacement of bone flap – perfect surgical/cosmetically results L/L view. Figure 6. Three weeks after replacement of bone flap perfectsurgical/cosmetically results A/P view.
Figure 1.
Cranial defect before graft replacement
Figure 2.
Bone graft in abdominal pocket
Figure 3.
Taking the bone out of abdomen
Figure 4.
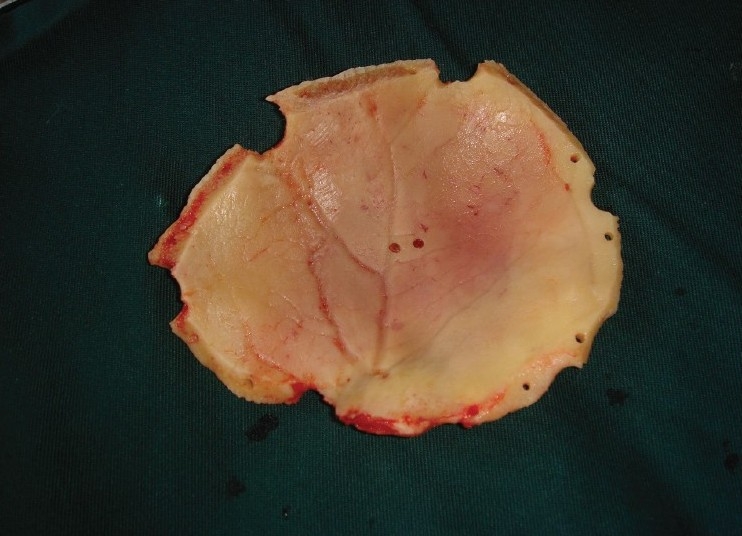
Quality of bone after six months
Figure 5.
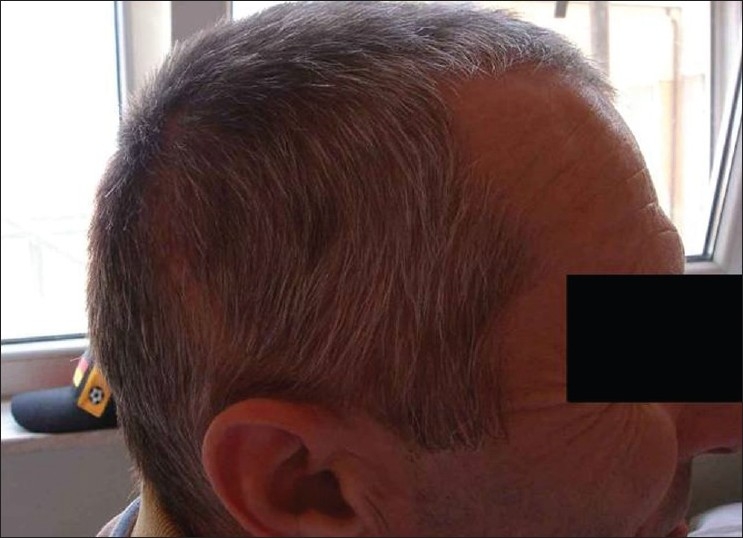
Three weeks after replacement of bone flap – perfect surgical/cosmetically results L/L view?
Figure 6.

Three weeks after replacement of bone flap – perfect surgical/cosmetically results A/P view
The post operative evaluation was based on adequacy of the recovered craniotomy graft to achieve satisfactory reconstruction.
RESULTS
In 66 out of 75 patients was achieved a satisfactory and cosmetically reconstruction, and in 9 cases was required augmentation with methyl methacrylate to achieve cosmetic needs. During surgery the temporo-basal region was removed in small pieces with rongeurs the part that required augmentation with methyl methacrylate. Two patients had infection and the bone was removed; 6 months later these patients had cranioplasty with methyl methacrylate. The duration of storage of calvarial bone in an abdominal pouch before reimplantation was 14 – 232 days (range 56 days).
At the time of cranioplasty done and after a few months, we did not encounter microscopic resorption of the bone flap in any of our patients. The average of GCS on admission was 6 (range 3 - 15). After calvarial bone replacement hematomas occurred in 4 patients: 3 were subgaleactic and 1 in abdominal pouch, there was no need for percutaneous aspiration. After the onset of symptoms and mass effect on computed tomography, timing of decompressive craniectomy was 1 – 6 h (range 2 hours). Involvement of dominant hemisphere was in 51 cases. Average extra time required for preparing abdominal pouch to insert calvarial bone was 14 min (range 9 – 21 minutes) average size of bone was 9 × 11cm (range 8 – 12 × 10 – 13).
Two patients were complaining because of unpleasant abdominal pressure sensation. In seven cases of bilateral craniectomy, calvarial bones were preserved on both sides each.
CONCLUSION
When there is a high intracranial pressure and is not linked to evacuable mass lesions, such as in patients with massive bilateral brain swelling or unilateral massive brain swelling, medical treatment in such cases is frequently ineffective in controlling high intracranial pressure. Decompression craniotomy should be seen as a last resort therapeutic option. Routine decompression craniotomy should not be recommended for all patients with brain swelling. Early decompressive craniotomy is a therapeutic option in the management of these patients.
The size of craniectomy is of critical importance. Small craniectomies risk brain herniation with venous infarction and increased edema at the bone margins.
Replacement of the bone removed at craniotomy, a fresh skull autograft, is superior to all alternative forms of cranioplasty.[2,3,4,7,12,14,15] Recently, custom alloplastic implants prefabricated from computed tomography data have made alloplastic reconstruction of these large defects more precise and less time consuming. However, the risks of complications related to a large foreign body remain. For these reasons, returning the bone removed during decompressive craniectomy to the defects at a later date is an attractive reconstructive alternative.[1,5,13,14] While cranioplasty with a frozen or freeze-dried craniectomy graft results in an initial perfect reconstruction, it is subject to a high rate of graft resorption after replacement. In Hauptli and Segantini's[8] series of 143 craniectomy grafts undergoing cryogenic preservation, 86 (60%) had osteolysis significant enough to produce instability or unsatisfactory cosmetic results and 23 patients (16%) required surgical revision. In contrast, Hauptli and Segantini reported that only 3 of 42 (7%) of subcutaneous preserved grafts required revision cranioplasty. Three of our thin patients experience some discomfort from the stored graft in abdominal pouch. Bone flap was stored between the fat and muscle tissue. Autogenic bone flap is alive, not expensive, and perfectly fit with the size of the defect. Brain protection and cosmetic aspects are the major indications of cranioplasty.
We think that storage of the patients own bone flap in the abdominal pocket is a safe, easy, cheap, sterile, histocompatible, and better cosmetic results. When replacement the bone removed at craniotomy, a fresh skull autograft is superior to all alternative forms of cranioplasty such as methylmetacrylate or some metals like tantalum. When compared to the use of synthetic cranioplasty materials, a personal bone flap has very low percentage of inflammatory complications. This procedure is standardized in our University Neurosurgical Clinic of Kosova (post war country) from 1999 and we kindly prefer this procedure to other centers.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/72/81735
Contributor Information
Arsim Morina, Email: koneso@gmail.com.
Fatos Kelmendi, Email: dr.kelmendifatos@gmail.com.
Qamile Morina, Email: morinaq@gmail.com.
Shefki Dragusha, Email: shefkidr@hotmail.com.
Feti Ahmeti, Email: feti__@hotmail.com.
Dukagjin Morina, Email: skallperi@yahoo.com.
Kushtrim Gashi, Email: drkushtrimi@hotmail.com.
REFERENCES
- 1.Abbott KH. Use of frozen cranial bone flaps for autogenous and homologous grafts in cranioplasty and spinal interbody fusion. J Neurosurg. 1953;10:380–8. doi: 10.3171/jns.1953.10.4.0380. [DOI] [PubMed] [Google Scholar]
- 2.Açikgöz B, Ozcan OE, Erbengi A, Bertan V, Ruacan S, Açikgöz HG. Histopathologic and microdensitometric analysis of craniotomy bone flaps preserved between abdominal fat and muscle. Surg Neurol. 1986;26:557–61. doi: 10.1016/0090-3019(86)90339-3. [DOI] [PubMed] [Google Scholar]
- 3.Cutting CB, McCarthy JG, Knize DM. Repair and grafting of bone. In: McCarthy JG, editor. Plastic Surgery. I. Philadelphia: Saunders; 1990. pp. 583–629. [Google Scholar]
- 4.Delashaw JB, Persing JA. Cranial defects and their repair. In: Youmans JR, editor. Youmans Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurosurgical Problems. 3rd ed. Vol. 4. Philadelphia: Saunders; 1990. pp. 2290–304. [Google Scholar]
- 5.Elliot H, Scott HJ. The bone-bank in neurosurgery. Br J Surg. 1951;39:31–4. doi: 10.1002/bjs.18003915305. [DOI] [PubMed] [Google Scholar]
- 6.Goel A, Deogaonkar M. Subgaleal preservation of calvarial flaps. Surg Neurol. 1995;44:181–2. doi: 10.1016/0090-3019(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 7.Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110:488. doi: 10.1097/00000658-193910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauptli J, Segantini P. New tissue preservation method for bone flaps following decompressive craniotomy. Helv Chir Acta. 1980;47:121. [PubMed] [Google Scholar]
- 9.Kreider GN. Repair of cranial defect by a new method. JAMA. 1920;74:1024. [Google Scholar]
- 10.Kreuz FP, Hyatt GW, Turner TC, Bassett AL. The preservation and clinical use of freeze-dried bone. J Bone Joint Surg AM. 1951;33:863–72. [PubMed] [Google Scholar]
- 11.Krishnan P, Bhattacharyya AK, Sil K, De R. Bone flap preservation after decompressive craniectomy- Experience with 55 cases. Neurol India. 2006;54:291–2. doi: 10.4103/0028-3886.27156. [DOI] [PubMed] [Google Scholar]
- 12.Longacre JJ. Deformities of the forehead, scalp, and cranium. In: Converse JM, editor. Reconstructive Plastic Surgery. Vol. 2. Philadelphia: Saunders; 1964. pp. 564–97. [Google Scholar]
- 13.Odom GL, Woodhall B, Wrenn FR. The use of refrigerated autogenous bone flaps for cranioplasty. J Neurosurg. 1952;9:606. doi: 10.3171/jns.1952.9.6.0606. [DOI] [PubMed] [Google Scholar]
- 14.Prolo DJ, Burres KP, McLaughlin WT, Christensen AH. Autogenous skull cranioplasty: Fresh and preserved (frozen), with consideration of the cellular response. Neurosurgery. 1979;4:18. doi: 10.1227/00006123-197901000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Salyer KE, Taylor DP. Bone grafts in craniofacial surgery. Clin Plast Surg. 1987;14:27. [PubMed] [Google Scholar]


