Abstract
Cardiac hypertrophy is a stereotypic response of the heart to increased workload. The nature of the workload increase may vary depending on the stimulus (repetitive, chronic, pressure, or volume overload). If the heart fully adapts to the new loading condition, the hypertrophic response is considered physiological. If the hypertrophic response is associated with the ultimate development of contractile dysfunction and heart failure, the response is considered pathological. Although divergent signalling mechanisms may lead to these distinct patterns of hypertrophy, there is some overlap. Given the close relationship between workload and energy demand, any form of cardiac hypertrophy will impact the energy generation by mitochondria, which are the key organelles for cellular ATP production. Significant changes in the expression of nuclear and mitochondrially encoded transcripts that impact mitochondrial function as well as altered mitochondrial proteome composition and mitochondrial energetics have been described in various forms of cardiac hypertrophy. Here, we review mitochondrial alterations in pathological and physiological hypertrophy. We suggest that mitochondrial adaptations to pathological and physiological hypertrophy are distinct, and we shall review potential mechanisms that might account for these differences.
Keywords: Cardiac hypertrophy, Mitochondria, Heart failure, Signal transduction
1. Introduction
Cardiac hypertrophy predominantly develops in response to increased workload, and less commonly in response to genetic mutations or exposure to growth factors. Increased workload augments ventricular wall stress,1 and the hypertrophic response is an adaptation to these changes in wall stress so as to maintain cardiac output. Cardiac hypertrophy can broadly be divided into pathological or physiological hypertrophy. Thus, if after an initial phase of compensation, the growth response leads to contractile dysfunction, ventricular dilation, and heart failure, hypertrophy is considered pathological. This is the case in conditions of arterial hypertension,2 aortic stenosis, aortic regurgitation,3 or following experimental aortic constriction. These examples of pressure overload are considered the main cause of heart failure in the clinical setting.4–6 In contrast, if increased workload is met by a hypertropic response with a new steady state that does not negatively impact contractile function, then the response is considered physiological. This is best exemplified by the impact of chronic exercise training and has been referred to as the ‘athlete's heart’.7,8
Although these definitions tend to hold true, a precise distinction between pathological and physiological hypertrophy is sometimes difficult and the potential mechanisms that drive the pathophysiology may sometimes overlap. Here, we shall focus on the metabolic and specifically mitochondrial alterations found in physiological or pathological hypertrophy (Table 1). To put the findings into perspective, we need to first address the various models that have been used to induce experimental cardiac hypertrophy.
Table 1.
Key features of pathological vs. physiological hypertrophy and their mitochondrial alterations
| Physiological hypertrophy | Pathological hypertrophy | |
|---|---|---|
| Stimuli | Volume overload (isotonic exercise) | Volume overload (valvular disease, aortovenous fistula) |
| Pressure overload (isometric exercise) | Pressure overload (arterial hypertension, aortic constriction) | |
| Pregnancy | ||
| Foetal gene expression | Normal | Up-regulated |
| Cardiac function | Normal/improved | Increasingly impaired during the time course |
| Decompensation | Not occuring | Occuring |
| Cardiac structure | Increased myocyte volume | Increased myocyte volume |
| Formation of new sarcomers | Formation of new sarcomers | |
| Interstitial fibrosis | ||
| Increased rates of apoptosis | ||
| Fatty acid oxidation | Unchanged or increased | Decreased |
| Glucose oxidation | Unchanged or increased | Unchanged |
| Maybe reduced during heart failure | ||
| Mitochondrial biogenesis | In accordance to cellular hypertrophy | Unchanged during compensated hypertrophy |
| Maybe diminished during heart failure | ||
| ATP production | Sufficient | Impaired |
2. Models of hypertrophy
Broadly speaking, hypertrophy can be induced by causing either volume overload or pressure overload. These mechanisms may contribute to the pathophysiology of physiological or pathological hypertrophy. Various animal models for both purposes have been reviewed in the past.9,10 In reviewing metabolic phenotypes in these animal models, it is important to consider changes that are attributable to left ventricular hypertrophy (LVH), vs. left ventricular dysfunction (LVD). This is a distinction which is not always possible as both changes may occur in parallel. However, we have recently characterized cardiac function following transverse aortic constriction in rats and noted three distinct phases in the evolution of LVH and LVD that progressively evolve in relation to the time from the onset of aortic constriction. These are: (i) compensated hypertrophy, (ii) heart failure with preserved ejection fraction, and (iii) heart failure with reduced ejection fraction.11 However, a comprehensive analysis of LV haemodynamics in relation to cardiac metabolism or mitochondrial function has not been performed in most studies of cardiac hypertrophy. Given that the transition between these phases is not abrupt but gradual, in a given study, it remains challenging to distinguish metabolic or mitochondrial alterations that are secondary to LVH vs. LVD.
A commonly used model for the induction of pathological hypertrophy is that of aortic constriction. Narrowing the aorta between the brachiocephalic trunk and the left carotid artery leads to pressure overload that mimics aortic stenosis. This results in the generation of a considerable pressure gradient (>100 mmHg) with a high rate of survival.12,13 Constriction of the ascending aorta eliminates potential effects of cerebral hypertension but is associated with a higher mortality rate and is used less frequently. A third aortic constriction model is constriction of the abdominal aorta, proximal to the renal arteries. Although this model is often also referred to as a model of pressure overload, giving rise to pathological hypertrophy, there is usually no acute significant pressure gradient across the constriction and the underlying mechanism is likely to be secondary to renovascular hypertension that results from renal hypoperfusion.14 Nephrectomy, combined with renal artery stenosis, also induces sustained hypertension, although this model has been used less often as an experimental model of pressure overload hypertrophy.15 Non-surgical experimental models of pressure overload cardiac hypertrophy include inbred rodent strains that are susceptible to hypertension such as the spontaneously hypertensive rat (SHR) or the Dahl salt-sensitive hypertensive rat.16,17
Pathological hypertrophy can also be induced by volume overload that is surgically established by creating an aortovenous fistula. Using this model, the Dhalla group described three stages of cardiac hypertrophy occurring during the first 16 weeks after an aortocaval shunt was induced.18 After the initial phase of rapid hypertrophy, haemodynamic function is normal or only mildly depressed between 2 and 8 weeks after creating the shunt. Subsequently, decompensation occurs with pulmonary congestion and reduced cardiac function. A different model of volume overload that progresses to pathological hypertrophy is aortic regurgitation. While mild chronic aortic regurgitation was suggested to induce compensated hypertrophy, more severe aortic regurgitation resulted in eccentric hypertrophy and the development of congestive heart failure.19
Isometric exercise (e.g. weightlifting) leads to physiological hypertrophy, which likely develops in response to intermittent pressure overload.20 Increased systemic arterial pressure that develops during this type of exercise, results in concentric hypertrophy of the left ventricle, i.e. increased left ventricular wall thickness without alterations in the left ventricular chamber size. In contrast, endurance training (e.g. running, swimming, and cycling) is characterized by isotonic exercise.20 This is associated with increased venous bloodflow to the heart and adrenergic activation that is akin to volume overload. In contrast to volume overload that is secondary to aortic regurgitation or aortovenous fistulae, the eccentric hypertrophy that develops in response to endurance training normally does not decompensate. However, cases of ‘overtraining' have been described.21
Finally, there are a large number of genetically engineered mouse models that have been designed to perturb key signalling pathways that are believed to be involved in physiological or pathological cardiac hypertrophy.22 Some of these models have also been used to address potential metabolic and mitochondrial changes that are associated with pathological or physiological cardiac hypertrophy.23–25 A common theme across the studies that have addressed metabolic changes in cardiac hypertrophy is the description of various changes in mitochondrial structure or function.26 In most cases of pathological hypertrophy, mitochondrial dysfunction has been described. In contrast, physiological cardiac hypertrophy is associated with enhanced mitochondrial function. Thus, a distinct signature found in mitochondria could potentially predict the nature of the hypertrophic response and help to distinguish physiological from pathological hypertrophy prior to the inevitable decompensation that accompanies pathological cardiac hypertrophy.
3. Changes in cardiac metabolism in physiological vs. pathological cardiac hypertrophy
Mitochondria are the major site of substrate oxidation in cardiomyocytes. Thus hypertrophy-related changes in myocardial substrate oxidation are likely related to mitochondrial function. Studies have described distinct patterns in myocardial substrate oxidation in pathological and physiological cardiac hypertrophy.
3.1. Fatty acid oxidation
Fatty acid oxidation (FAO) is the main fuel that drives mitochondrial ATP generation by the adult heart, accounting for ∼60–90% of cardiac ATP that is produced. Following uptake and conjugation with acetyl CoA (FA-CoA), FA-CoA enters the mitochondrion, via the carnitine acyl transferase shuttle (CPT-1 and CPT-2). CPT-1 is subject to allosteric regulation by malonyl CoA, and effective transfer to the mitochondria requires adequate amounts of carnitine. Upon entering the mitochondrial matrix, FA-CoA undergoes beta oxidation.27 Many groups have described reduced myocardial FAO in pressure overload cardiac hypertrophy and heart failure.12,28,29 However, during the early stages of hypertrophy, which precede heart failure, changes in, or potential mechanisms for reduced, FAO are less clear.
Several studies have described reduced mRNA expression of CPT-1 during pathological hypertrophy 30–32 or reduced flux through CPT-1.33 However, some studies have reported unchanged 34 or increased expression of CPT-1.35,36 Interestingly, Sorokina et al.33 noted reduced CPT-1 activity, but described a shift from the mCPT-1 form to the foetal lCPT-1 variant, which was insufficient to increase FAO. Some groups have also described reduced carnitine levels, which might further depress fatty acid import into mitochondria.37,38 Variable results have been described for FAO, with reports of unchanged 34 or reduced oxidation rate.12,30,37 These discrepancies might be partially related to the differences in animal models used for these studies, differences in experimental conditions, variable degrees of cardiac hypertrophy, and varying degrees of carnitine deficiency.39
Fewer studies have examined myocardial FAO in exercise-induced cardiac hypertrophy. Microarray analysis of cardiac genes in treadmill-trained rats revealed a preferential change in gene expression that would predict an increase in FAO.40 This contrasted with the gene expression profile in a model of pathological hypertrophy. Direct measurements of FAO in exercise-trained rats also revealed an increase in FAO 41 with isolated working hearts from exercise-trained rats revealing elevated rates of both glucose oxidation (GO) and FAO. Taken together, these studies suggest that pathological and physiological cardiac hypertrophy exhibit divergent changes in myocardial FA utilization.
3.2. Lipotoxicity
Accumulation of toxic lipid intermediates has been proposed to contribute to mitochondrial dysfunction, particularly in obesity and diabetes.42 Recent studies have suggested that pressure overload cardiac hypertrophy increases the accumulation of myocardial triglycerides, which could represent a marker for lipotoxicity.43 In addition, neurohumoral changes in heart failure, such as increased adrenergic activity,44,45 will increase the delivery of FA to the heart by increasing adipose tissue lipolysis.46 This increase in FA delivery in combination with defective fatty acid utilization in heart failure will promote lipid accumulation in the cardiomyocytes,43,47 which could conrtibute to mitochondrial dysfunction by various mechanisms.42,48,49
3.3. Cardiolipin
Cardiolopin is one of the most abundant mitochondrial phospholipids, and mitochondrial cardiolipin composition plays an important role in regulating the activity of the mitochondrial electron transport chain.50,51 Its high content of unsaturated fatty acids renders it prone to reactive oxygen species (ROS)-induced damage.51–53 Mitochondrial cardioliopin content is reduced in heart failure53–55 and ischaemia/reperfusion56 and correlates with reduced electron transport chain activities.
3.4. Glucose utilization
Many studies have described an increase in glycolysis in pressure overload cardiac hypertrophy,57 without a coordinate increase in glucose oxidation (GO). Most studies, in which LVH was associated with measurable reductions in contractile function, reveal no changes in GO during pathological hypertrophy 37,58 or decreased GO rates.59 Akki et al.30 reported an increase in pyruvate oxidation in a model of abdominal aortic constriction at the stage of compensated cardiac hypertrophy. Thus, by the time that LV dysfunction develops, there is reduced coupling of glycolysis to GO.37,58,59 Interestingly, tricarboxylic acid (TCA) cycle flux in pressure overload hypertrophy that is associated with LV dysfunction is maintained via increased anaplerotic flux via glycolytic pyruvate and malic enzyme, thereby bypassing energy-yielding reactions and leading to reduced energetic efficiency.33 Once overt heart failure has developed, we and others have observed impaired GO.11,12 Although the relative contribution of glucose (via glycolysis and GO) relative to FAO in pressure overload cardiac hypertrophy is increased, it is important to emphasize that this substrate switch is unlikely to fully compensate for the decline in myocardial ATP generation from fatty acids. In contrast, myocardial GO following treadmill training in rats is increased.41 The coordinate increase in GO and FAO following exercise-induced cardiac hypertrophy points to an overall increase in mitochondrial oxidative capacity in physiological cardiac hypertrophy.
4. Changes in mitochondrial function in pathological vs. physiological cardiac hypertrophy
4.1. Mitochondrial morphology and function in the compensated stage of pathological hypertrophy
It is generally accepted that mitochondrial dysfunction develops in the failing heart, and in many of these studies the model used was one of pathological cardiac hypertrophy.26 However, there are a number of lines of evidence that mitochondrial dysfunction might not necessarily be present during the early stages of compensatory LVH. In a canine model of compensated LVH, induced by ascending aortic banding, there was no evidence of changes in mitochondrial morphology or mitochondrial function.60 Although pigs with compensated LVH (secondary to ascending aortic banding) revealed a reduction in phosphocreatine (PCr)/ATP ratios, this might have been due to reduced mitochondrial creatine kinase protein and not to impaired mitochondrial oxidative capacity per se as these animals maintained the ability to increase myocardial oxygen consumption (MVO2) in the presence of the mitochondrial uncoupler dinitrophenol.61,62
We recently completed a study in rats following transverse aortic constriction (TAC), and monitored cardiac and mitochondrial function between 2 and 20 weeks. LV function was normal 2 weeks post TAC, and despite a reduction in mitochondrial FAO, the respiratory capacity of mitochondria exposed to glutamate was increased. Between 6 weeks and 10 weeks post TAC, these animals developed congestive heart failure as evidenced by increased lung weights, and diastolic dysfunction, but with preserved ejection fraction. At the 6 week time point, mitochondrial respirations remained elevated but subsequently declined and were similar to sham controls at 10 weeks. Mitochondrial dysfunction only developed in parallel, with the onset of systolic dysfunction and reduced ejection fraction, indicating progressive heart failure.12 Thus, these observations suggest that diastolic dysfunction precedes systolic dysfunction in the progression from compensated to decompensated LVH and that this diastolic dysfunction was not associated with mitochondrial dysfunction.
In contrast, in the neonatal banded rabbit model, activities of mitochondrial complex I and III were reduced at the phase of compensated cardiac hypertrophy, but this was accompanied by reduced mitochondrial ROS and oxidative damage that only became evident after the transition to heart failure.63 A recent study in dogs with compensated LVH suggested that relative to controls, hypertrophied hearts did not lose mitochondrial membrane potential in the context of ischaemia and re-perfusion injury. However, the increase in mitochondrial membrane potential correlated with an increased risk of arrhythmias.64 In these studies, diastolic function was not measured, thus, it is not possible to determine whether the associated mitochondrial dysfunction could have contributed to diastolic impairment. Thus, additional studies are required to elucidate the contribution of mitochondrial function and energetics to diastolic impairment in LVH.
4.2. Mitochondrial morphology and function after the onset of LV systolic dysfunction
By the time heart failure ensues in models of pathological LVH, altered mitochondrial morphology and function are evident. Thus, pigs with hypertrophic cardiomyopathy revealed swollen cardiac mitochondria with disrupted cristae and substantial mtDNA depletion. Complex I- and Complex IV-activity were also reduced in this model of hypertrophy.65 Swelling of mitochondria is known to correlate with mitochondrial dysfunction and damage, but relatively few studies have systematically examined mitochondrial morphology in parallel with measures of mitochondrial function in the evolution of LVH and heart failure, and the few studies in the literature have not reported consistent results. For example, ultrastructural changes (increased mitochondrial size distribution) were noted in the hearts of stroke-prone SHR rats; however, these changes preceded the onset of hypertension, suggesting that they could be independent of LVH.66 In contrast, no changes in mitochondrial morphology were noted in a dog model of right ventricular hypertrophy and congestive heart failure.67 Disorganization of mitochondrial cristae combined with a reduction in cristae density has been reported for failing rat hearts after aortic constriction.11 This study also revealed significant remodelling of the mitochondrial proteome, characterized by reduced content of the majority of FAO proteins and >50% of the electron transport chain subunits that were detected.11 Rosca et al.68 described reduced mitochondrial function due to a decrease in respirasomes, in which several complexes of the electron transport chain unite to facilitate mitochondrial respiration. Many other studies in varied models of LVH and heart failure have confirmed the existence of mitochondrial dysfunction.26,69–73
The adenine nucleotide translocase (ANT) mediates nucleotide transfer from mitochondria to cytosol. Thus, impaired ANT activity could contribute to deficient energy availability in pathological cardiac hypertrophy. Reduced levels of cardiac ANT has been reported in some, but not all, models of pressure overload cardiac hypertrophy.36,74 In contrast, overexpression of ANT ameliorated cardiac dysfunction in angiotensin II-mediated cardiac hypertrophy, despite the absence of any baseline changes in ANT expression relative to control rats.75 Thus, a role for ANT in mediating mitochondrial dysfunction in pathological cardiac hypertrophy is not clear, but modulation of ANT could potentially represent a therapeutic strategy that may enhance mitochondrial function in pathological LVH.
Under physiological conditions, mitochondria are responsible for generation of most of the superoxides in the cell. Under pathological conditions such as heart failure, extra-mitochondrial sources of ROS such as NADPH oxidase also contribute to myocardial oxidative stress and mitochondrial dysfunction.76,77 Myocardial oxidative stress has been implicated in the transition from compensated LVH to heart failure, and evidence exists to support a role for mitochondrial and non-mitochondrial sources of ROS.76–78 Oxidative stress can further impair mitochondrial function by leading to oxidative modifications of mitochondrial proteins, mutations of mitochondrial DNA and activation of the permeability transition pore.79
ROS-induced mitochondrial uncoupling has been hypothesized to contribute to decreased mitochondrial energetics in conditions associated with increased FA availability such as obesity and diabetes.79–81 Given the increased circulating concentrations of FA in heart failure, it is theoretically possible that ROS-mediated mitochondrial uncoupling could contribute to mitochondrial energetic dysfunction. Increased levels of mitochondrial uncoupling proteins and decreased mitochondrial efficiency have been described in the infarction model of heart failure.82 However, pressure overload hypertrophy is associated with repression of uncoupling protein 3 expression.83 Thus, the role of mitochondrial uncoupling in pressure overload cardiac hypertophy remains to be definitively established.
Taken together, it is reasonable to conclude that compensated LVH is associated with relatively preserved mitochondrial function and that the development of mitochondrial dysfunction occurs in parallel with the development of heart failure. It remains to be definitively proved if the mitochondrial dysfunction precedes heart failure and plays a role in the transition from compensated LVH to heart failure (i.e. causative) or if the change in mitochondrial function represents one of a number of pathophysiological processes that either contribute to, or are associated with the transition to heart failure.
4.3. Mitochondrial adaptations to exercise-induced cardiac hypertrophy
Exercise -induced cardiac hypertrophy is associated with increased mitochondrial biogenesis in various animal models.26,84 We recently showed that physiological cardiac hypertrophy induced by swim training in mice was associated with increased palmitoyl carnitine-supported mitochondrial respirations and ATP production rates in permeabilized cardiac fibres.25 The signalling mechanisms that may contribute to these mitochondrial adaptations will be discussed in detail in the next section. A recent study by Huang et al.85 suggested that a bout of exhaustive exercise in untrained or trained rats could precipitate mitochondrial DNA deletions and increased levels of cytochrome C and caspace 3. These observations raise the possibility that intermittent bouts of exercise might induce mitochondrial damage that may serve as a signal to activate the mitochondrial biogenesis programme.
5. Mechanisms linking pathological or physiological cardiac hypertrophy with altered mitochondrial function
5.1. Signalling pathways
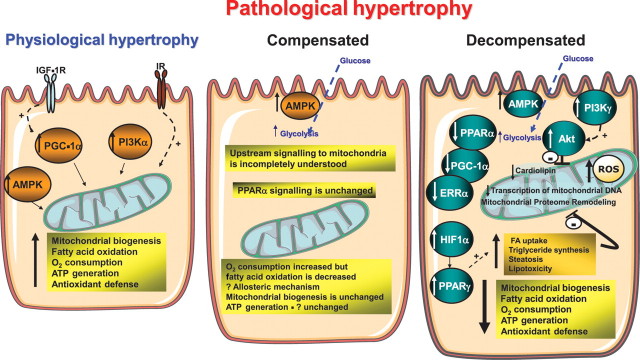
Most of the studies examining mitochondrial dysfunction in the context of pathological cardiac hypertrophy have been performed after the onset of LV dysfunction. Thus, caution is warranted in ascribing changes in signal transduction that may impact mitochondrial function in these studies, as the possibility exists that they could be the result of LV dysfunction and heart failure. Nevertheless, an examination of differences or similarities in changes in myocardial signal transduction pathways in pathological vs. physiological cardiac hypertrophy might provide some clues to potential mechanisms that might lead to the divergent mitochondrial phenotypes (Figure 1).
Figure 1.
Schematic representation of signalling pathways that may influence mitochondrial function in physiological and pathological cardiac hypertrophy. In physiological cardiac hypertrophy, as develops in response to exercise, there is increased activation of Class 1A PI3Kα, PGC-1α, and AMPK, all of which can promote mitochondrial biogenesis and increase mitochondrial oxidative capacity. Growth factors such as insulin (IR) and IGF-1R signalling might be required for the mitochondrial adaptation to exercise-induced cardiac hypertrophy. The signalling mechanisms that are activated in the compensated stage of pathological cardiac hypertrophy are relatively understudied. AMPK activation will increase glucose uptake and glycolysis. Although mitochondrial respiratory capacity remains relatively intact, FAO rates are decreased despite normal expression of PPAR-α target genes, suggesting allosteric regulatory mechanisms. There is scant published evidence to support any increase in mitochondrial biogenesis at this stage. Most studies have focused on the models in which LV dysfunction is present (decompensated). In this stage, there are perturbations in many signalling pathways that conspire to impair mitochondrial function. These include decreased expression or activity of transcriptional regulators that govern mitochondrial biogenesis and oxidative capacity (i.e. PGC-1α, ERRα, and PPAR-α) and decreased transcription of mitochondrial DNA. Increased G-protein-coupled receptor signalling activates Class1B PI3Kγ that leads to constitutive activation of Akt, which may repress mitochondrial function. Activation of HIF-1α leads to a PPAR-α-mediated increase in FA uptake and lipogenesis that may promote lipotoxicity, which could further impair mitochondrial function. Reduced cardiolipin content and remodelling of the mitochondrial proteome also contribute to mitochondrial dysfunction. Mitochondrial dysfunction promotes oxidative stress that leads to a vicious cycle of progressive mitochondrial damage.
As reviewed by many groups, pressure overload hypertrophy activates a number of signalling pathways, which are not specifically activated in relation to exercise-induced or physiological cardiac hypertrophy.20,86 Moreover, transcriptional profiling has also suggested that gene expression patterns differ between physiological hypertrophy, the compensated stage of pressure overload hypertrophy, and heart failure.40,87,88 Pathological cardiac hypertrophy is associated with activation of many signalling pathways, exemplified by the calineurin/nuclear factor of activated T-cell pathway, histone deacetylases, phosphatidyl inositol 3 kinase (PI3K)/Akt/FOXO1 (forkhead)/mammalian target of rapamycin signalling networks, the ERK-MAPK signalling pathways, G-protein-coupled signalling pathways, and others. There has been no systematic evaluation of the interaction of these signalling networks with regulatory networks such as peroxisome proliferator activated receptor gamma coactivator 1α (PGC-1α) that directly regulate mitochondrial biogenesis and mitochondrial function.
It is clear that in many experimental models of pressure overload-induced LV dysfunction there is a repression of PGC-1α expression and/or down-regulation of gene targets of transcription factors such as peroxisome proliferator activated receptor α (PPAR-α) or estrogen-related receptor α (ERRα) that are co-activated by PGC-1α.89 Indeed, mice with genetic deletion of PGC-1α exhibit increased susceptibility to heart failure when they are subjected to pressure overload.23,90 The interactions between pro-hypertrophic signalling pathways and PGC-1α signalling are incompletely understood. For example, activation of p38MAPK has been shown to increase PGC-1α activity via direct phosphorylation of PGC-1α and by releasing its association with a repressor molecule.91 Thus, activation of p38 MAPK cannot account for the reduction in PGC-1α signalling that is observed in many models of pathological cardiac hypertrophy and LV dysfunction. Hypoxia inducible factor-1α (HIF-1α) and PPAR-γ have recently been proposed to mediate in part the substrate switch away from FAO to glycolysis and to contribute to lipotoxicity in pressure overload hypertrophy.43 However, the interaction of the HIF-1α/PPAR-γ signalling axis in LVH -associated mitochondrial dysfunction remains to be explored.
Class I and Class II histone de-acetylases (HDACs) have been implicated in the regulation of cardiac hypertrophy, with Class I HDACs promoting hypertrophic remodelling and Class II HDACs suppressing cardiac hypertrophy.92 Indeed, HDAC inhibitors exhibit profound effects to suppress pathological cardiac hypertrophy.93 Whether or not HDAC signalling contributes to the metabolic and mitochondrial disorders that characterize pathological cardiac hypertrophy are less well understood. A recent study of cardiomyocyte-restricted deletion of HDAC3, a Class I HDAC, revealed dramatic cardiac hypertrophy, and significant up-regulation of PPAR-α targets that resulted in increased FAO, mitochondrial uncoupling, and lipotoxicity, and premature death from heart failure.24 The increase in PPAR-α activity is opposite to what is observed in pressure overload cardiac hypertrophy. Moreover, transgenic overexpression of HDAC3 did not induce cardiac hypertrophy but instead increased cardiomyocyte proliferation.94 This contrasts with HDAC2 transgenic mice that clearly develop cardiac hypertrophy.95 Taken together, these models suggest distinct roles for HDAC isoforms in the regulation of cardiac hypertrophy and mitochondrial function. However, our knowledge of how HDACs might or might not contribute to the mitochondrial dysfunction that accompanies pathological cardiac hypertrophy remains incomplete. The observations of profound mitochondrial dysfunction and lipotoxicity in HDAC3-deficient hearts should raise caution regarding the use of non-specific HDAC inhibitors in the treatment of pathological cardiac hypertrophy. Prior to adoption of this therapeutic strategy, a detailed analysis of the role of respective Class I and Class II HDAC isoforms on mitochondrial function in the heart under non-stressed conditions and following induction of pressure overload hypertrophy is clearly warranted.
Although activation of Akt occurs in pathological and physiological cardiac hypertrophy, activation of the PI3K-Akt signalling pathway has been shown to be necessary and sufficient for the development of physiological cardiac hypertrophy.96 Exercise training is associated with activation of PI3K and Akt signalling in the heart, and is also associated with induction of PGC-1α content.25 We recently demonstrated that the activation of mitochondrial respiratory capacity in the context of physiological cardiac hypertrophy requires PI3K signalling but is not mediated via activation of Akt. Blocking PI3K signalling prevented the exercise-induced increase in mitochondrial function, but did not prevent the induction of PGC-1α.25 These observations suggest that PI3K and PGC-1α signalling act in a coordinated fashion to enhance mitochondrial respiratory capacity in physiological cardiac hypertrophy. In contrast, constitutive Akt activation was associated with repression of mitochondrial function and indeed eventually leads to heart failure that resembles pathological cardiac hypertrophy.25,96,97 These data raise the possibility that sustained Akt activation, which in pressure overload cardiac hypertrophy is mediated by G-protein-coupled activation of Class 1B PI3Kγ, could play a role in the associated reduction in mitochondrial function. In contrast, activation of Class 1A PI3K by exercise or growth factors augments mitochondrial function by activating alternative PI3K-dependent but Akt-independent signalling pathways that are required for increasing mitochondrial capacity.
Our studies in mutant mice with impaired insulin or insulin-like growth factor (IGF)-1 signalling also suggest potential interactions between growth factor signalling acting via Class 1A PI3K and the mitochondrial adaptations to pathological or physiological cardiac hypertrophy. Thus, mice with genetic deletion of insulin receptors in cardiomyocytes develop mitochondrial dysfunction under non-stressed conditions,98 but the mitochondrial function declines more rapidly in these mice when pathological LV remodelling occurs after coronary artery ligation.99 We have also reported that IGF-1 signalling alone or in concert with insulin signalling is required for exercise-induced cardiac hypertrophy.100,101 Of interest, the induction of PGC-1α by exercise training was prevented in mice that lack IGF-1 receptor signalling in cardiomyocytes. The failure to induce PGC-1α was associated with evidence of energetic stress and activation of AMPK kinase.101 Taken together, these observations suggest the existence of signalling networks that link growth factors, their tyrosine kinase receptors, and Class 1A PI3K with the mitochondrial adaptations to physiological cardiac hypertrophy.
AMP kinase (AMPK) signalling may promote mitochondrial biogenesis.102–104 AMPK is activated in the heart in pathological hypertrophy and in response to exercise.105,106 However, it is likely that their contribution to changes in mitochondrial function are distinct in pathological vs. physiological cardiac hypertrophy. In a model of compensated pressure overload cardiac hypertrophy, the activation of AMPK was shown to be due in part to reduced energy generation that occured on the basis of diminished long-chain FAO that could be reversed by supplying short-chain fatty acids.107 In more severe pathological LVH, it is most likely that the reduction in mitochondrial function also contributes to the increase in AMPK activity. The activation of AMPK may play an important role in increasing glycolysis in these hearts but might not be sufficient to offset reduction in other signalling pathways such as PGC-1α, ERRα, or mitochondrial DNA replication pathways whose activities are also impaired in pathological cardiac hypertrophy by mechanisms that are incompletely understood.108 In contrast, we propose that in physiological cardiac hypertrophy, multiple signalling pathways, such as activation of PGC-1α and Class1A PI3K, act in concert with increased AMPK activation to increase mitochondrial respiratory capacity and promote mitochondrial biogenesis (Figure 1).
6. Summary
As summarized in Table 2 and Figure 1, pathological cardiac hypertrophy is associated with reduced myocardial FA utilization that correlates with mitochondrial dysfunction, particularly during the transition to heart failure. Multiple mechanisms might contribute to this such as oxidative stress and repression of transcriptional networks that regulate mitochondrial capacity and biogenesis. However, it is important to emphasize that these defects might not necessarily be present in the compensated stages, and whether or not mitochondrial dysfunction directly contributes to the transition to heart failure or is occurring in parallel with heart failure remains to be definitively established. In contrast, physiological cardiac hypertrophy is associated with enhanced mitochondrial respiratory capacity and increased myocardial substrate utilization that likely reflects the synergistic interactions of multiple signalling pathways such as PI3K, PGC-1α, and AMPK, which work in concert to increase mitochondrial biogenesis and metabolic capacity.
Table 2.
Possible mechanisms of mitochondrial dysfunction in pathological hypertrophy
| Potential mechanisms of mitochondrial dysfunction in pathological hypertrophy |
|---|
| ROS |
| Altered ANT expression |
| Cardiolipin loss or peroxidation |
| Fatty acid and lipid overload |
| Mitochonrial uncoupling |
| Impaired mitochondrial biogenesis |
| Reduced transcriptional signalling of regulators of mitochondria |
Funding
E.D.A. is supported by National Insitutes of Health grants RO1DK092065, UO1HL087947, and is an established investigator of the American Heart Association. T.D. was Heisenberg-Professor of the Deutsche Forschungsgemeinschaft (DFG) until August 2010 and was supported by grants from the DFG (Do602/6-1, 8-1, 9-1).
Acknowledgements
We wish to thank Moritz Osterholt for help preparing the manuscript.
Conflict of interest: none declared.
References
- 1.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. doi:10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papademetriou V. From hypertension to heart failure. J Clin hypertens (Greenwich) 2004;6:14–17. doi: 10.1111/j.1524-6175.2004.03919.x. doi:10.1111/j.1524-6175.2004.03919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu SK, Magid NR, Fox PR, Goldfine SM, Borer JS. Fibrosis, myocyte degeneration and heart failure in chronic experimental aortic regurgitation. Cardiology. 1998;90:101–109. doi: 10.1159/000006827. doi:10.1159/000006827. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR. Mechanisms of development of heart failure in the hypertensive patient. Cardiology. 1999;92(Suppl. 1)):3–6. doi: 10.1159/000047287. discussion 7–9, 20–21 doi:10.1159/000047287. [DOI] [PubMed] [Google Scholar]
- 5.Carabello BA. Aortic stenosis: from pressure overload to heart failure. Heart Fail Clin. 2006;2:435–442. doi: 10.1016/j.hfc.2006.11.001. doi:10.1016/j.hfc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. 2005;26:1790–1796. doi: 10.1093/eurheartj/ehi290. doi:10.1093/eurheartj/ehi290. [DOI] [PubMed] [Google Scholar]
- 7.Fagard R. Athlete's heart. Heart. 2003;89:1455–1461. doi: 10.1136/heart.89.12.1455. doi:10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naylor LH, George K, O'Driscoll G, Green DJ. The athlete's heart: a contemporary appraisal of the ‘Morganroth hypothesis. Sports Med. 2008;38:69–90. doi: 10.2165/00007256-200838010-00006. doi:10.2165/00007256-200838010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39:60–76. doi: 10.1016/s0008-6363(98)00110-2. doi:10.1016/S0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 10.Monnet E, Chachques JC. Animal models of heart failure: what is new? Ann Thorac Surg. 2005;79:1445–1453. doi: 10.1016/j.athoracsur.2004.04.002. doi:10.1016/j.athoracsur.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res. 2010;85:376–384. doi: 10.1093/cvr/cvp344. doi:10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 12.Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, et al. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res. 2010;86:461–470. doi: 10.1093/cvr/cvp414. doi:10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- 13.Zaha V, Grohmann J, Gobel H, Geibel A, Beyersdorf F, Doenst T. Experimental model for heart failure in rats—induction and diagnosis. Thoracic Cardiovasc Surg. 2003;51:211–215. doi: 10.1055/s-2003-42264. [DOI] [PubMed] [Google Scholar]
- 14.Swoap SJ, Boddell P, Baldwin KM. Interaction of hypertension and caloric restriction on cardiac mass and isomyosin expression. Am J Physiol. 1995;268:R33–R39. doi: 10.1152/ajpregu.1995.268.1.R33. [DOI] [PubMed] [Google Scholar]
- 15.Wiesel P, Patel AP, Carvajal IM, Wang ZY, Pellacani A, Maemura K, et al. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1-deficient mice. Circ Res. 2001;88:1088–1094. doi: 10.1161/hh1001.091521. doi:10.1161/hh1001.091521. [DOI] [PubMed] [Google Scholar]
- 16.Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, et al. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. doi:10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- 17.Miyachi M, Yazawa H, Furukawa M, Tsuboi K, Ohtake M, Nishizawa T, et al. Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension. 2009;53:701–707. doi: 10.1161/HYPERTENSIONAHA.108.127290. doi:10.1161/HYPERTENSIONAHA.108.127290. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Ren B, Liu S, Sentex E, Tappia PS, Dhalla NS. Characterization of cardiac hypertrophy and heart failure due to volume overload in the rat. J Appl Physiol. 2003;94:752–763. doi: 10.1152/japplphysiol.00248.2002. [DOI] [PubMed] [Google Scholar]
- 19.Magid NM, Opio G, Wallerson DC, Young MS, Borer JS. Heart failure due to chronic experimental aortic regurgitation. Am J Physiol. 1994;267:H556–H562. doi: 10.1152/ajpheart.1994.267.2.H556. [DOI] [PubMed] [Google Scholar]
- 20.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–262. doi: 10.1111/j.1440-1681.2007.04585.x. doi:10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 21.Halson SL, Jeukendrup AE. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004;34:967–981. doi: 10.2165/00007256-200434140-00003. doi:10.2165/00007256-200434140-00003. [DOI] [PubMed] [Google Scholar]
- 22.Molkentin JD, Robbins J. With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J Mol Cell Cardiol. 2009;46:130–136. doi: 10.1016/j.yjmcc.2008.09.002. doi:10.1016/j.yjmcc.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. doi:10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, et al. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest. 2008;118:3588–3597. doi: 10.1172/JCI35847. doi:10.1172/JCI35847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, et al. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007;6:294–306. doi: 10.1016/j.cmet.2007.09.001. doi:10.1016/j.cmet.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rimbaud S, Garnier A, Ventura-Clapier R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep. 2009;61:131–138. doi: 10.1016/s1734-1140(09)70015-5. [DOI] [PubMed] [Google Scholar]
- 27.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. doi:10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 28.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. doi:10.1161/01.CIR.0000023531.22727.C1. [DOI] [PubMed] [Google Scholar]
- 29.van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81:420–428. doi: 10.1093/cvr/cvn282. doi:10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 30.Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem. 2008;311:215–224. doi: 10.1007/s11010-008-9711-y. doi:10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 31.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. doi:10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimbaud S, Sanchez H, Garnier A, Fortin D, Bigard X, Veksler V, et al. Stimulus specific changes of energy metabolism in hypertrophied heart. J Mol Cell Cardiol. 2009;46:952–959. doi: 10.1016/j.yjmcc.2009.01.013. doi:10.1016/j.yjmcc.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, et al. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. doi:10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 34.Degens H, de Brouwer KF, Gilde AJ, Lindhout M, Willemsen PH, Janssen BJ, et al. Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res Cardiol. 2006;101:17–26. doi: 10.1007/s00395-005-0549-0. doi:10.1007/s00395-005-0549-0. [DOI] [PubMed] [Google Scholar]
- 35.Iemitsu M, Miyauchi T, Maeda S, Sakai S, Fujii N, Miyazaki H, et al. Cardiac hypertrophy by hypertension and exercise training exhibits different gene expression of enzymes in energy metabolism. Hypertens Res. 2003;26:829–837. doi: 10.1291/hypres.26.829. doi:10.1291/hypres.26.829. [DOI] [PubMed] [Google Scholar]
- 36.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. doi:10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 37.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 38.Martin MA, Gomez MA, Guillen F, Bornstein B, Campos Y, Rubio JC, et al. Myocardial carnitine and carnitine palmitoyltransferase deficiencies in patients with severe heart failure. Biochim Biophys Acta. 2000;1502:330–336. doi: 10.1016/s0925-4439(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 39.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. doi:10.1023/A:1015380609464. [DOI] [PubMed] [Google Scholar]
- 40.Strom CC, Aplin M, Ploug T, Christoffersen TE, Langifort J, Viese M, et al. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 2005;272:2684–2695. doi: 10.1111/j.1742-4658.2005.04684.x. doi:10.1111/j.1742-4658.2005.04684.x. [DOI] [PubMed] [Google Scholar]
- 41.Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JC, Antler C, et al. Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2004;287:H1055–H1063. doi: 10.1152/ajpheart.00925.2003. doi:10.1152/ajpheart.00925.2003. [DOI] [PubMed] [Google Scholar]
- 42.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, et al. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. doi:10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–385. doi: 10.1016/j.jacc.2009.03.061. doi:10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 45.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. doi:10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 46.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol. 2009;54:1637–1646. doi: 10.1016/j.jacc.2009.07.024. doi:10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. doi:10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 48.Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, et al. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E678–E685. doi: 10.1152/ajpendo.90287.2008. doi:10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
- 49.Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801:266–271. doi: 10.1016/j.bbalip.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Hatch GM. Regulation of cardiolipin biosynthesis in the heart. Mol Cell Biochem. 1996;159:139–148. doi: 10.1007/BF00420916. doi:10.1007/BF00420916. [DOI] [PubMed] [Google Scholar]
- 51.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. doi:10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. doi:10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 53.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species generated by the mitochondrial respiratory chain affect the complex III activity via cardiolipin peroxidation in beef-heart submitochondrial particles. Mitochondrion. 2001;1:151–159. doi: 10.1016/s1567-7249(01)00011-3. doi:10.1016/S1567-7249(01)00011-3. [DOI] [PubMed] [Google Scholar]
- 54.Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, et al. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–1608. doi: 10.1194/jlr.M800561-JLR200. doi:10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. doi:10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. doi:10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Allard MF. Energy substrate metabolism in cardiac hypertrophy. Curr Hypertens Rep. 2004;6:430–435. doi: 10.1007/s11906-004-0036-2. doi:10.1007/s11906-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 58.Leong HS, Brownsey RW, Kulpa JE, Allard MF. Glycolysis and pyruvate oxidation in cardiac hypertrophy—why so unbalanced? Comp Biochem Physiol A Mol Integr Physiol. 2003;135:499–513. doi: 10.1016/s1095-6433(03)00007-2. doi:10.1016/S1095-6433(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 59.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol. 2000;36:1378–1385. doi: 10.1016/s0735-1097(00)00856-1. doi:10.1016/S0735-1097(00)00856-1. [DOI] [PubMed] [Google Scholar]
- 60.Matlib MA, Rembert JC, Millard RW, Ashraf M, Rouslin W, Asano G, et al. Mitochondrial function in canine experimental cardiac hypertrophy. J Mol Cell Cardiol. 1983;15:221–232. doi: 10.1016/0022-2828(83)90277-8. doi:10.1016/0022-2828(83)90277-8. [DOI] [PubMed] [Google Scholar]
- 61.Gong G, Liu J, Liang P, Guo T, Hu Q, Ochiai K, et al. Oxidative capacity in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H541–H548. doi: 10.1152/ajpheart.01142.2002. [DOI] [PubMed] [Google Scholar]
- 62.Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–1576. doi: 10.1161/01.cir.103.11.1570. [DOI] [PubMed] [Google Scholar]
- 63.Griffiths ER, Friehs I, Scherr E, Poutias D, McGowan FX, Del Nido PJ. Electron transport chain dysfunction in neonatal pressure-overload hypertrophy precedes cardiomyocyte apoptosis independent of oxidative stress. J Thorac Cardiovasc Surg. 2010;139:1609–1617. doi: 10.1016/j.jtcvs.2009.08.060. doi:10.1016/j.jtcvs.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin H, Nass RD, Joudrey PJ, Lyon AR, Chemaly ER, Rapti K, et al. Altered spatiotemporal dynamics of the mitochondrial membrane potential in the hypertrophied heart. Biophys J. 2010;98:2063–2071. doi: 10.1016/j.bpj.2010.01.045. doi:10.1016/j.bpj.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin CS, Sun YL, Liu CY. Structural and biochemical evidence of mitochondrial depletion in pigs with hypertrophic cardiomyopathy. Res Vet Sci. 2003;74:219–226. doi: 10.1016/s0034-5288(02)00189-3. doi:10.1016/S0034-5288(02)00189-3. [DOI] [PubMed] [Google Scholar]
- 66.Tokoro T, Ito H, Maenishi O, Suzuki T. Mitochondrial abnormalities in hypertrophied myocardium of stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol Suppl. 1995;22:S268–S269. doi: 10.1111/j.1440-1681.1995.tb02911.x. doi:10.1111/j.1440-1681.1995.tb02911.x. [DOI] [PubMed] [Google Scholar]
- 67.Walker JG, Bishop SP. Mitochondrial function and structure in experimental canine congestive heart failure. Cardiovasc Res. 1971;5:444–450. doi: 10.1093/cvr/5.4.444. doi:10.1093/cvr/5.4.444. [DOI] [PubMed] [Google Scholar]
- 68.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–39. doi: 10.1093/cvr/cvn184. doi:10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. doi:10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jullig M, Hickey AJ, Chai CC, Skea GL, Middleditch MJ, Costa S, et al. Is the failing heart out of fuel or a worn engine running rich? A study of mitochondria in old spontaneously hypertensive rats. Proteomics. 2008;8:2556–2572. doi: 10.1002/pmic.200700977. doi:10.1002/pmic.200700977. [DOI] [PubMed] [Google Scholar]
- 71.Marin-Garcia J, Goldenthal MJ, Moe GW. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res. 2001;52:103–110. doi: 10.1016/s0008-6363(01)00368-6. doi:10.1016/S0008-6363(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 72.Marin-Garcia J, Goldenthal MJ, Moe GW. Mitochondrial pathology in cardiac failure. Cardiovasc Res. 2001;49:17–26. doi: 10.1016/s0008-6363(00)00241-8. doi:10.1016/S0008-6363(00)00241-8. [DOI] [PubMed] [Google Scholar]
- 73.Sharov VG, Goussev A, Lesch M, Goldstein S, Sabbah HN. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1998;30:1757–1762. doi: 10.1006/jmcc.1998.0739. doi:10.1006/jmcc.1998.0739. [DOI] [PubMed] [Google Scholar]
- 74.Hang T, Huang Z, Jiang S, Gong J, Wang C, Xie D, et al. Apoptosis in pressure overload-induced cardiac hypertrophy is mediated, in part, by adenine nucleotide translocator-1. Ann Clin Lab Sci. 2006;36:88–95. [PubMed] [Google Scholar]
- 75.Walther T, Tschope C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, et al. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation. 2007;115:333–344. doi: 10.1161/CIRCULATIONAHA.106.643296. doi:10.1161/CIRCULATIONAHA.106.643296. [DOI] [PubMed] [Google Scholar]
- 76.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. doi:10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. doi:10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–456. doi: 10.1093/cvr/cvn280. doi:10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 79.Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 2010;88:229–240. doi: 10.1093/cvr/cvq239. doi:10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. doi: 10.1152/physiol.00008.2006. doi:10.1152/physiol.00008.2006. [DOI] [PubMed] [Google Scholar]
- 81.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. doi:10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 82.Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, et al. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol. 2008;44:694–700. doi: 10.1016/j.yjmcc.2008.01.008. doi:10.1016/j.yjmcc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 83.Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, et al. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology. 2002;97:203–209. doi: 10.1159/000063122. doi:10.1159/000063122. [DOI] [PubMed] [Google Scholar]
- 84.White FC, McKirnan MD, Breisch EA, Guth BD, Liu YM, Bloor CM. Adaptation of the left ventricle to exercise-induced hypertrophy. J Appl Physiol. 1987;62:1097–1110. doi: 10.1152/jappl.1987.62.3.1097. doi:10.1063/1.339715. [DOI] [PubMed] [Google Scholar]
- 85.Huang CC, Lin TJ, Chen CC, Lin WT. Endurance training accelerates exhaustive exercise-induced mitochondrial DNA deletion and apoptosis of left ventricle myocardium in rats. Eur J Appl Physiol. 2009;107:697–706. doi: 10.1007/s00421-009-1177-4. doi:10.1007/s00421-009-1177-4. [DOI] [PubMed] [Google Scholar]
- 86.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. doi:10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 87.Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005;21:314–323. doi: 10.1152/physiolgenomics.00185.2004. doi:10.1152/physiolgenomics.00185.2004. [DOI] [PubMed] [Google Scholar]
- 88.van den Bosch BJ, Lindsey PJ, van den Burg CM, van der Vlies SA, Lips DJ, van der Vusse GJ, et al. Early and transient gene expression changes in pressure overload-induced cardiac hypertrophy in mice. Genomics. 2006;88:480–488. doi: 10.1016/j.ygeno.2006.04.012. doi:10.1016/j.ygeno.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 89.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid redox signal. 2010;13:1011–1022. doi: 10.1089/ars.2009.2940. doi:10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. doi:10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 92.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. doi:10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 93.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–2588. doi: 10.1161/CIRCULATIONAHA.106.625467. doi:10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trivedi CM, Lu MM, Wang Q, Epstein JA. Transgenic overexpression of Hdac3 in the heart produces increased postnatal cardiac myocyte proliferation but does not induce hypertrophy. J Biol Chem. 2008;283:26484–26489. doi: 10.1074/jbc.M803686200. doi:10.1074/jbc.M803686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, et al. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med. 2007;13:324–331. doi: 10.1038/nm1552. doi:10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- 96.O'Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. doi:10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. doi:10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boudina S, Bugger H, Sena S, O'Neill BT, Zaha VG, Ilkun O, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. doi:10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sena S, Hu P, Zhang D, Wang X, Wayment B, Olsen C, et al. Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction. J Mol Cell Cardiol. 2009;46:910–918. doi: 10.1016/j.yjmcc.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ikeda H, Shiojima I, Ozasa Y, Yoshida M, Holzenberger M, Kahn CR, et al. Interaction of myocardial insulin receptor and IGF receptor signaling in exercise-induced cardiac hypertrophy. J Mol Cell Cardiol. 2009;47:664–675. doi: 10.1016/j.yjmcc.2009.08.028. doi:10.1016/j.yjmcc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. doi:10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. doi:10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, et al. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol. 2010;588:3551–3566. doi: 10.1113/jphysiol.2010.194035. doi:10.1113/jphysiol.2010.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. doi:10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, et al. Physiologic role of AMP-activated protein kinase (AMPK) in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab. 2003;285:E629–E636. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- 106.Kim AS, Miller EJ, Young LH. AMP-activated protein kinase: a core signalling pathway in the heart. Acta Physiol (Oxf) 2009;196:37–53. doi: 10.1111/j.1748-1716.2009.01978.x. doi:10.1111/j.1748-1716.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 107.Allard MF, Parsons HL, Saeedi R, Wambolt RB, Brownsey R. AMPK and metabolic adaptation by the heart to pressure overload. Am J Physiol Heart Circ Physiol. 2007;292:H140–H148. doi: 10.1152/ajpheart.00424.2006. doi:10.1152/ajpheart.00424.2006. [DOI] [PubMed] [Google Scholar]
- 108.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. doi:10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]