Abstract
Egg-laying hens are important candidate bioreactors for pharmaceutical protein production because of the amenability of their eggs for protein expression. In this study, we constructed an oviduct-specific vector containing tissue plasminogen activator (tPA) protein and green fluorescent protein (pL-2.8OVtPAGFP) and assessed its expression in vitro and in vivo. Oviduct epithelial and 3T3 cells were cultured and transfected with pL-2.8OVtPAGFP and pEGP-N1 (control vector), respectively. The pL-2.8OVtPAGFP vector was administered to laying hens via a wing vein and their eggs and tissues were examined for tPA expression. The oviduct-specific vector pL-2.8OVtPAGFP was expressed only in oviduct epithelial cells whereas pEGP-N1 was detected in oviduct epithelial and 3T3 cells. Western blotting detected a 89 kDa band corresponding to tPA in egg white and oviduct epithelial cells, thus confirming expression of the protein. The amount of tPAGFP in eggs ranged 9 to 41 ng/mL on the third day after vector injection. The tPA expressed in egg white and oviduct epithelial cells showed fibrinolytic activity, indicating that the protein was expressed in active form. GFP was observed only in oviducts, with no detection in heart, muscle, liver and intestine. This is the first study to report the expression of tPA in egg white and oviduct epithelial cells using an oviduct-specific vector.
Keywords: green fluorescent protein, human tissue plasminogen activator, laying hens, oviduct-specific expression
Introduction
Transgenic chickens have several advantages over mammalian expression systems for the production of therapeutically important proteins that can be expressed in egg yolk or egg white (Lillico et al., 2005). Genetically selected hens lay ∼330 eggs/year, with 6.5 g of protein and 3.5–4.0 g of egg white per egg, The ovalbumin gene is exclusively expressed in oviduct tissue, with approximately 105 copies of ovalbumin mRNA per cell. The protein encoded by this gene accounts for approximately 54% of egg white or ∼2.2 g of protein/egg (Kohler et al., 1968; Palmiter, 1975; Gilbert, 1984; Burley and Vadehra, 1989; Dougherty and Sanders, 2005). Naturally sterile eggs contain a high concentration of egg white protein that provides recombinant proteins with a long shelf life and no loss of activity (Tranter and Board, 1982; Harvey et al., 2002). Tissue plasminogen activator (tPA) is one of the major protein involved in fibrinolysis since it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. tPA is used to dissolve thrombi associated with heart attacks, strokes, pulmonary obstruction, ischemic strokes and brain injury, in addition to the treatment of cancer. Individuals with frostbite treated with (tPA) show fewer amputations than untreated patients (Ichinose et al., 1986; Tsurup and Medved 2001; Bruen et al., 2007).
In this study, we examined the expression of tPA in vitro and in vivo using an oviduct-specific vector containing human recombinant tPA coupled to green fluorescent protein (GFP). For analysis in vitro, oviduct epithelial cells and 3T3 cells were transfected with the vectors pL-2.8OVtPAGFP and pEGP-N1 (control), respectively. Expression in vivo was assessed by injecting the vector pL-2.8OVtPAGFP intravenously via a wing vein in laying hens and then examining the egg white and selected tissues for tPA expression.
Materials and Methods
Experimental Animals
Sixteen-month-old Isa Brown laying hens that had reached 70% of their egg producing capacity were purchased from Qinglongshan Farm in Nanjing Jiangning District. The hens were fed a standard diet for birds (NRC 1994).
Vector construction
Blood was collected from a wing vein and DNA was extracted using an Invitrogen kit (Carlsbad, California, USA). A 2.8 kb fragment of the chicken ovalbumin gene was amplified using the sense primer 5′CATCTTGTCAT ATGTCCTCAGACTTGGC3′ and the antisense primer 5′GAGCCTCGAGTGAACTCTGAGTTGTCTAG3′ that contained Nde1 and Xho1 restriction enzyme sites, respectively. The 5′ and 3′ regulatory regions of the ovalbumin gene were amplified from chicken genome DNA by high fidelity PCR. The 2.8 kb PCR product was sub-cloned into the vector pGEM-T (Promega) and the correctness of the insertion was confirmed by sequencing. The 5′ and 3′ regulatory regions were sub-cloned into the vector pL-CMVtPAGFP (previously produced in our laboratory) and the resulting vector was named pL-2.8OVtPAGFP. The pL-2.8OVtPAGFP plasmid was digested using the restriction enzymes Afl11 and ClaI and then run on agarose gels to confirm the digestion.
Plasmid DNA preparation and purification
Single colonies of E. coli transformants of the pL-2.8OVtPAGFP vector were grown overnight in 500 mL of Luria – Bertani (LB) broth containing 100 μg of ampicillin/mL. The plasmid DNA was prepared using a standard alkaline lysis method and purified by PEG (BBI, Toronto, Canada) precipitation (Sambrook et al., 1989). The plasmid DNA was resuspended in 5% glucose solution.
Cell culture
A laying hen was decapitated and the magnum portion of the oviduct was removed aseptically. The oviduct tissue was minced finely, washed several times in phosphate-buffered saline (PBS) and suspended in Dulbeccos minimum essential medium (DMEM) containing 20 mL of 10 mM HEPES and collagenase (0.5 mg/mL). The tissue suspension was incubated for 1 h at 37 °C with shaking, after which undigested tissue was removed by filtering the mixture through layers of gauze. Epithelial cells were collected by centrifugation at 500 g for 2 min and then washed three times with DMEM containing 10% fetal bovine serum, 50 μg of penicillin/mL and 50 μg of streptomycin/mL. The primary oviduct cells were then immediately transfected and grown at 41 °C in a 5% CO2 atmosphere in DMEM supplemented with 10 mM HEPES at pH 7.4, and antiobiotics, 8% chicken serum, 2% fetal calf serum, 7–10 mol/L 17β-estradiol (Sigma Chemical Co., St. Louis, MO, USA), 6–10 mol/L corticosterone (Sigma), and 50 μg of insulin/L (Sigma).
DNA transfection in vitro
For DNA transfection in vitro, 4 μg of oviduct-specific and control vector was used per well of cells. Lipofectamine 2000 reagent (Invitrogen) was used as the transfection reagent (ratio of 1 μg of DNA:1.5 μg of lipofectamine), according to the manufacturers instructions. The cells were examined 48 h after transfection. The presence of GFP was assessed by examining the cells with a fluorescence microscope.
Transfection in vivo
For transfection in vivo, seven Isa Brown hens were used, with six being treated and one serving as a control. Seven days after being purchased, 2.0 mL of DNA:lipofectamine mixture containing 1 μg DNA and 2.0 μL lipofectamine was injected into six chickens via wing vein on two consecutive days. The DNA concentration was 1.0 mg/mL. The day after the last injection, the eggs were collected, labeled (treated/control) and dated.
Western blotting
Protein from transfected oviduct epithelial cells was extracted as described by Selbert and Rannie (2002). On the third day after transfection, the treated and control eggs were carefully opened and 15 mL of yolk-free egg white was recovered from each egg. The egg white was stirred for at least 1 h at 4 °C with four volumes of ice-cold 50 mM sodium acetate buffer (pH 5.0) to remove the bulk of the ovomucin fraction (Lillico et al., 2007). Subsequently, 15 μL aliquots of protein from transfected oviduct epithelial cells and from egg whites were mixed with an equal volume of 2 x SDS-PAGE loading buffer, boiled for 5 min and then applied 12% polyacrylamide gels for SDS-PAGE. The separated proteins were subsequently transferred to polyvinylidene dithioride membranes (Hoffmann-La Roche, Basel, Switzerland). Western blotting was done as described by Selbert and Rannie (2002) using a GFP-specific primary antibody and a specific secondary antibody (both diluted 1:1000).
Quantification of tPAGFP
For the quantification of tPAGFP, the egg white from eggs three days post-transfection was manually separated from the yolk (Lillico et al., 2007). Ovalbumin and ovotransferrin were removed by affinity column chromatography on Sepharose Blue HP (GE Healthcare). Egg white (15 μL) was diluted 200-fold in PBS-T (0.1% Tween 20 in PBS) and the amount of tPAGFP was determined with a GFP ELISA kit (Cell Biolabs Inc., San Diego, California, USA) with the absorbances being read at 450 nm in a multiwell spectrophotometer.
Detection of GFP in tissue sections
Four days after egg collection, hens E1, E2, E4 and E6 of the treated group were re-injected with the pL2.8OVtPAGFP plasmid. One day after injection, the hens were killed and tissue samples (50 mg of oviduct, heart, skeletal muscle, liver and intestine) were collected, snap-frozen and stored at −80 °C. Two days later, 8 μm thick tissue sections were obtained by using a cryomicrotome and mounted on gelatin-coated slides. The slides were examined with an Olympus SZX12 astereozoom microscope equipped with a GFP lter set (Olympus SZX-FGFPA) to confirm tPA expression in the tissues.
Fibrinolytic activity of tPA
The fibrinolytic activity of tPA was determined by the fibrin clot lysis assay using fibrin-containing agarose plates. The plates were prepared by pouring 5 mL of low-melting-temperature agarose solution (3% in TBS) containing 2.5 U of thrombin mixed with 5 mL of fibrinogen (5 mg/mL) into petri dishes at 37 °C. The plates were incubated at 37 °C for 30 min until the fibrin clot became visible. For the assay, 20 μL of egg white from eggs three days post-transfection, protein from transfected oviduct cells and bovine serum albumin (BSA) was added to wells (3.5 mm dia x 4.5 mm deep) cut in the solidified fibrin-agarose gel and incubated at 30 °C overnight. Fibrinolysis was quantified by comparing the size of the zone of hydrolysis around each well. The plates were photographed after 24 h at 20–22 °C.
Results
Oviduct-specific vector
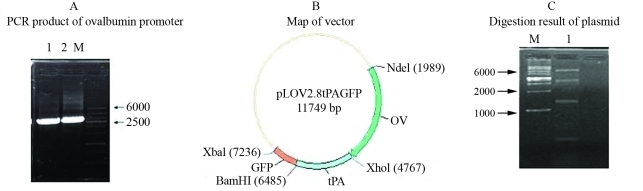
Figure 1 shows the construction map of pL-2.8OVtPAGFP. Panel A shows the 2800 bp PCR product obtained by amplification of the ovalbumin gene (results of two agarose gels), panel B shows the vector map, and panel C shows that plasmid digestion resulted in fragments of 1502 bp, 209 bp, 6382 bp and 3656 bp.
Figure 1-.
A, PCR product of the ovalbumin promoter separated in agarose gel. Lane M, 100–6000 bp wide range markers, lanes 1 and 2, ovalbumin promoter. B, Construction map of the pL-2.8OVtPAGFP vector. C, Digestion fragments (1502 bp, 209 bp, 6382 bp and 3656 bp) of the plasmid.
Cell cultures
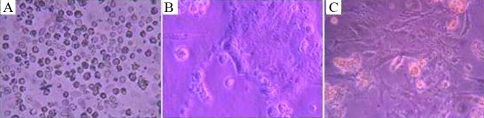
Oviduct epithelial cells obtained by collagenase digestion and cultured for up to seven days were polygonal or fusiform in shape and formed clumps or clusters; the cells showed spontaneous beating similar to cardiac cells. Upon adherence to the plastic substrate the cells maintained close contact with each other and formed tubular structures. Figure 2 shows the appearance of oviduct epithelial cells after 0, 2 and 7 days in culture.
Figure 2-.
Images of oviduct epithelial cells after 0, 2 and 7 days in culture, shown in panels A, B and C, respectively.
Temporal expression of EGFP gene in cultured oviduct cells
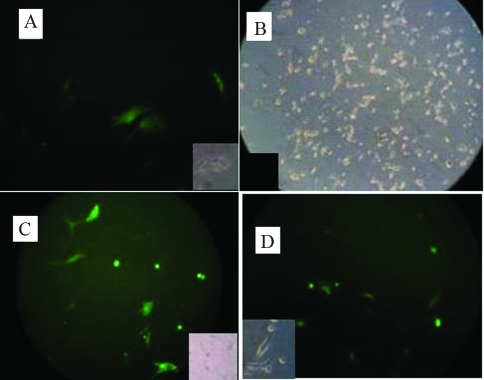
The pLl-2.8OVtPAGFP vector was expressed only in oviduct epithelial cells whereas the pEGFP-N1 vector was detected in oviduct epithelial cells and 3T3 cells. Figure 3 reveals the green and bright field images of oviduct epithelial cells and 3T3 cells transfected with pL-2.8OVtPAGFP and control vector, respectively.
Figure 3-.
A, Fluorescence image of oviduct cells transfected with the pL-2.8OVtPAGFP vector. B, Bright field image of 3T3 cells transfected with the pL-2.8OVtPAGFP vector. C and D, Fluorescence images of oviduct epithelial cells and 3T3 cells, respectively, transfected with the pEGFP-N1 vector. The small images are bright-field/black photographs for A, B, C and D.
Molecular mass of tPA
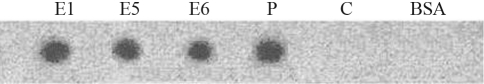
Western blotting for tPA identified a 89 kDa band except in samples from E3 and the control eggs (Figure 4). In this figure, P indicates oviduct epithelial cells protein transfected with pL-2.8OVtPAGFP, and E1 to E6 and C denote egg white from eggs three days after transfection and from the control group, respectively.
Figure 4-.
Western blot of proteins from oviduct epithelial cells (P) and egg white from transfected (E1 – E6) and control (C) eggs collected on the third day post-transfection.
Quantification of tPAGFP
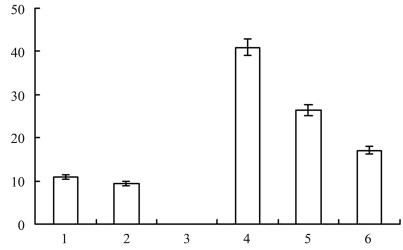
ELISA quantification of tPAGFP in egg white on the third day after transfection showed that the eggs contained 9–41 ng of tPAGFP/mL (Figure 5); no tPAGFP was seen in the egg white of egg E3 or in the control group.
Figure 5-.
Quantification of tPAGFP in egg white of eggs collected from hens E1 to E6 on the third day post-transfection.
Fibrinolytic activity of tPA
Fibrin selectivity is the major reason for the preferred use of tPA over other thrombolytic agents. The clear zones of hydrolysis seen around the tPA showed that the protein was expressed in an active form in transfected eggs (E1, E5 and E6) and transfected oviduct epithelial cells (P) (Figure 6); no fibrinolytic activity was observed in the control egg and BSA samples.
Figure 6-.
Fibrinolytic activity of tPA in egg white of eggs collected from hens E1, E5 and E6 on the third day post-transfection, in protein extracted from transfected oviduct cells (P), in egg white (C) of control eggs collected on same day as the transfected group and in BSA.
Temporal expression of GFP gene in hen tissues
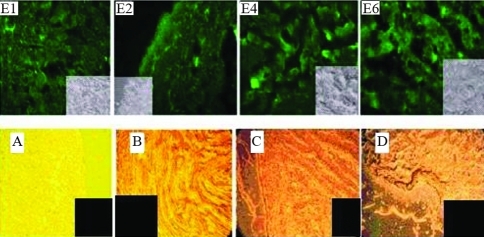
GFP was expressed only in oviduct tissue, with none being detected in heart, skeletal muscle, liver and intestine. Figure 7 shows fluorescence and bright field images of oviduct, heart, skeletal muscle, liver and intestine of vector injected hens.
Figure 7-.
Fluorescence images of GFP in oviduct tissue of hens E1, E2, E4 and E6. Small figures are bright field images of E1, E2, E4 and E6. A, B, C and D are bright field images of heart, muscle, liver and intestine, respectively.
Discussion
Oviduct epithelial cells cultured for up to seven days showed characteristics typical of these cells, i.e., polygonal or fusiform shape, spontaneous beating, formation of clusters attached to the bottom of the plastic culture dishes and organization into tubular structures. These characteristics agree with those reported by Ouhibi et al. (1989) and Moll et al. (1986), who noted that epithelial cells remain closely associated with each other to maintain their structural integrity. This close association is attributed to the presence of intermediate cytoskeletal laments that hold the epithelium together and are a characteristic epithelial marker.
DNA transfection in chicken oviduct cells is problematic since it depends on obtaining a primary cell culture as few standard cell lines are available, and also because expression of the chicken ovalbumin gene is strictly limited to oviduct epithelial cells in the laying season (Ochiai et al., 1998). As shown here, expression of the pL-2.8OtPAGFP vector in primary chicken oviduct epithelial cells was highest 48 h post-transfection, whereas there was no expression in 3T3 cells. In contrast, the control plasmid was expressed in oviduct epithelial cells and 3T3 cells. These findings indicate that in oviduct epithelial cells the oviduct-specific expression vector only drives the expression of exogenous genes. This expression system is regulated by various factor including steroid hormones, type of substratum, and cell-cell interactions (Muramatsu et al., 1997). Gao et al. (2005) constructed an oviduct-specific expression vector (pOV) containing 3.0 kb of the 5′-flanking sequence of the chicken ovalbumin gene. These authors used various transfection procedures, including electroporation, liposomes and polyethyleneimine, to determine the best method for transfecting primary oviduct epithelial cells. Slightly higher transfection rates were obtained with polyethyleneimine compared to the other two methods. They also showed that exogenous gene expression was specific for oviduct cells when an oviduct-specific vector was used.
Western blotting with a GFP-specific antibody confirmed the expression of tPA in egg white and transfected oviduct epithelial cells. The molecular mass of the fusion protein was ∼89 kDa, which agreed with the theoretical value calculated from the tPA amino acid sequence. No immunoreactive band was seen in hen E3, perhaps because of the influence of factors such as the vector integration site, genetic background of the hens, and epigenetic factors. Our result agrees with Gao et al. (2006), who reported preliminary characterization of the expression of recombinant human tissue kallikrein in egg white of laying hens based on an oviduct-specific promoter. Expression of the oviduct-specific promoter was confirmed by western blotting and showed a specic band of ∼52 kDa in the GST-hK1 fusion protein and two bands of ∼37 kDa and 43 kDa in the egg white of vector-injected hens. These finding also agreed with Zhu et al. (2005) who used an oviduct-specific promoter driven by GFP to check the expression of monoclonal antibodies in hen egg white; as in other studies, expression of the monoclonal antibody was confirmed by western blotting.
Variation in the level of recombinant protein expression among hens containing the same vector integration site has been observed (Rapp et al., 2003). As shown above, the amount of tPAGFP ranged from 9 to 41 ng/mL on the third day post-transfection, with vector expression generally being maximal 24 h after transfection. The levels of recombinant protein observed here were much lower than those reported by Kwon et al. (2010) (88.7–233.8 ng of human recombinant protein/mL in quail) and Lillico et al. (2007) (38 μg of human recombinant protein/mL in chickens). These discrepancies probably reflect variations in the method of transfection used, e.g., injection of virus into fertilized eggs versus direct injection of the vector via a wing vein.
GFP expression was observed in oviduct tissue but not in heart, muscle, liver or intestine. This finding agrees with Scott and Carlos (2005) who reported that GFP expression was specific to neurons and consistent with multiple generation. GFP expression has been observed in other tissues when large oviduct-specific promoters are used. For example, Zhu et al. (2005) reported GFP expression in oviduct and intestinal tissue of hens when large oviduct-specific promoters (7.5 kb and 15 kb) were used. As shown here, tPA was detected in eggs of transfected hens and in protein extracts from oviduct epithelial cells. The tPA expressed in these systems was biologically active since it showed fibrinolytic activity. These results agree with other reports in which the expression of active enzyme was also observed after transfection with oviduct-specific vectors (Liang et al., 2000; Gao et al., 2006; Ying et al., 2006).
To our knowledge, this is the first report to describe the expression of tPA in oviduct cells, oviduct tissue and eggs of hens transfected with an oviduct-specific expression vector. The level of tPA expression observed here can probably be increased by further research. The use of oviduct-specific vectors should provide a useful approach for producing therapeutically important proteins in birds in vitro and in vivo.
Acknowledgments
This study was supported by the National High Technology Research and Development program of China (883 Key program no. 2007AA100504), Annhui Natural Science Foundation (Grant no. 050410201), Scientific Research Foundation for Doctors, Jinling Institute of Technology (Grant no. 403010004) and Natural Science Foundation of the Educational Commission of Jiangsu province, China (Grant no. 09kjd230034).
Footnotes
Associate Editor: Carlos F.M. Menck
References
- Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle JR. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546–51. doi: 10.1001/archsurg.142.6.546. [DOI] [PubMed] [Google Scholar]
- Burley RW, Vadehra DV. The Avian Egg: Chemistry and Biology. John Wily and Sons; New York: 1989. pp. 68–71. [Google Scholar]
- Dougherty DC, Sanders MM. Estrogen action: Revitalization of the chick oviduct model. Trends Endocrinol Metab. 2005;16:414–419. doi: 10.1016/j.tem.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Gao B, Huai-Chang S, Cheng-Yi S, Zhi-Yue W, Qin C, Hong-Qin S. Transfection and expression of exogenous gene in laying hens oviduct in vitro and in vivo. J Zhejiang Univ Sci B. 2005;6:137–141. doi: 10.1631/jzus.2005.B0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Sun HC, Fang HX, Qian K, Zhao MS, Qiu HL, Song CY, Wang ZY. Expression and preliminary characterization of recombinant human tissue kallikrein in egg white of laying hens. Poultry Sci. 2006;85:1239–1244. doi: 10.1093/ps/85.7.1239. [DOI] [PubMed] [Google Scholar]
- Gilbert AB. Egg albumin and its formation. In: Bell DJ, Free BM, editors. Physiology and Biochemistry of the Domestic Fowl. Academic Press; London: 1998. pp. 1291–1329. [Google Scholar]
- Ichinose A, Takio K, Fujikawa K. Localization of the binding sites of tissue-type plasminogen activator to fibrin. J Clin Invest. 1986;78:163–169. doi: 10.1172/JCI112546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AJ, Speksnijder G, Baugh LR, Morris JA, Ivarie R. Expression of exogenous protein in the egg white of transgenic chicken. Nat Biotechnol. 2002;20:396–399. doi: 10.1038/nbt0402-396. [DOI] [PubMed] [Google Scholar]
- Kohler PO, Grimley PM, O′Malley BW. Protein synthesis: Differential stimulation of cell specific proteins in epithelial cells of chick oviduct. Science. 1968;160:86–87. doi: 10.1126/science.160.3823.86. [DOI] [PubMed] [Google Scholar]
- Kwon SC, Choi JW, Jang HJ, Shin SS, Lee SK, Park TS, Choi IY, Lee GS, Song G, Han JY. Production of biofunctional recombinant human interleukin 1 receptor antagonist (rhIL1RN) from transgenic quail egg white. Biol Reprod. 2010;82:1057–1064. doi: 10.1095/biolreprod.109.081687. [DOI] [PubMed] [Google Scholar]
- Liang JF, Yong TL, Maureen EC, Victor CY. Synthesis and characterization of positively charged tPA as prodrug using a heparin/protamine-based drug-delivery system. AAPS Pharmsci. 2000;2:59–67. doi: 10.1208/ps020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillico SG, McGrew MJ, Sherman A, Sang HM. Transgenic chicken as bioreactors for transgenic drug. Drug Discov Today. 2005;10:191–196. doi: 10.1016/S1359-6446(04)03317-3. [DOI] [PubMed] [Google Scholar]
- Lillico SG, Sherman A, McGrew MJ, Robertson CD, Smith J, Haslam C, Bamard P, Radcliffe PA, Mitrophanous KA, Elliot EA, et al. Oviduct-specific expression of two therapeutic proteins in transgenic hens. Proc Natl Acad Sci USA. 2007;104:1771–1776. doi: 10.1073/pnas.0610401104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Cowin P, Kapprell HP, Franke WW. Desmosomal proteins: New markers for identification and classification of tumors. Lab Invest. 1986;54:4–25. [PubMed] [Google Scholar]
- Muramatsu T, Yoshimoto M, Yasushige O, Jun-ichi O. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochem Biophys Res Commun. 1997;230:376–380. doi: 10.1006/bbrc.1996.5882. [DOI] [PubMed] [Google Scholar]
- NRC. Nutrient Requirements of Poultry 9th revised edition. National Academy Press; Washington: 1994. pp. 19–34. [Google Scholar]
- Ochiai H, Hyi-Man P, Akihiro N, Ryuzo S, Jun-Ichi O, Tatsuo M. Synthesis of human erythropoietin in vivo in the oviduct of laying hens by localized in vivo gene transfer using electroporation. Poultry Sci. 1998;77:299–302. doi: 10.1093/ps/77.2.299. [DOI] [PubMed] [Google Scholar]
- Ouhibi N, Yves M, Gerard B, Bernard N. Culture of epithelial cells derived from the oviduct of different species. Hum Reprod. 1989;4:229–235. doi: 10.1093/oxfordjournals.humrep.a136877. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Quantitation of parameters that determine rate of ovalbumin synthesis. Cell. 1975;4:189–197. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Rapp JC, Harvey AJ, Speksnijder GL, Hu W, Ivarie R. Biologically active human interferon produced in the egg white of transgenic hens. Transgenic Res. 2003;12:569–575. doi: 10.1023/a:1025854217349. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 1989. [Google Scholar]
- Scott BB, Carlos L. Generation of tissue-specific transgenic birds with lentiviral vectors. Proc Natl Acad Sci USA. 2005;102:16443–16447. doi: 10.1073/pnas.0508437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbert S, Rannie D. Analysis of transgenic mice. Methods Mol Biol. 2002;180:305–341. doi: 10.1385/1-59259-178-7:305. [DOI] [PubMed] [Google Scholar]
- Tranter HS, Board RG. Antimicrobial defense of avian eggs: Biological perspective and chemical basis. J Appl Biochem. 1982;4:295–338. [Google Scholar]
- Tsurupa G, Medved L. Identification and characterization of novel tPA- and plasminogen-binding sites within fibrin(ogen) alpha C-domains. Biochemistry. 2001;40:801–808. doi: 10.1021/bi001789t. [DOI] [PubMed] [Google Scholar]
- Ying SH, Si-Guo L, Jian-Quan C, Ai-Min Z, Guo-Xiang C. Expression of a variant of human tissue-type plasminogen activator in transgenic mouse milk. J Exp Anim Sci. 2006;43:211–218. [Google Scholar]
- Zhu L, van der Lavoir MC, Albanese J, Beenhouwer DO, Cardarelli PM, Cuison S, Deng DF, Deshpande S, Diamond JH, Green L, et al. Production of human monoclonal antibody in eggs of chimeric chickens. Nat Biotechnol. 2005;23:1159–1169. doi: 10.1038/nbt1132. [DOI] [PubMed] [Google Scholar]