Abstract
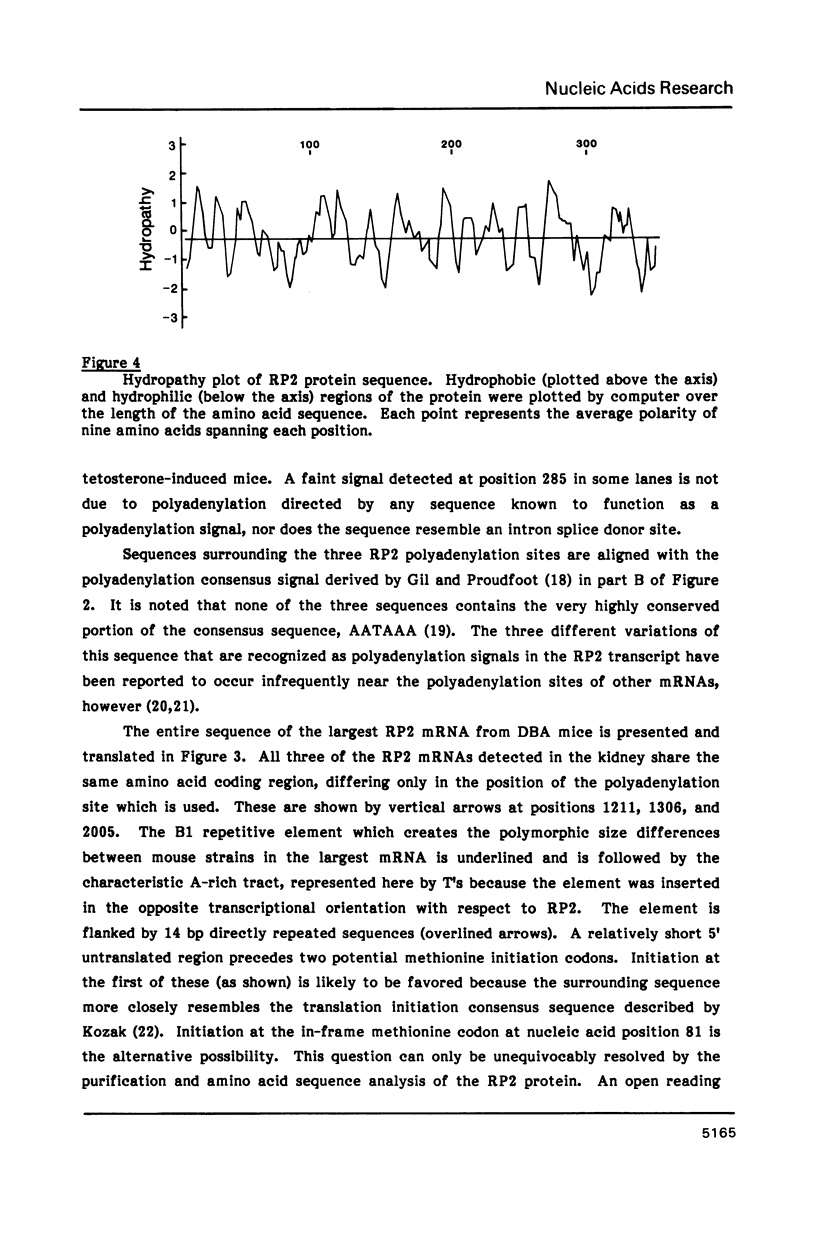
The major forms of testosterone-regulated RP2 messenger RNA (also known as MAK mRNA and pMK908) in the mouse kidney were characterized by examining cDNA and genomic clones. Three sizes of RP2 mRNA are detected by Northern blot analysis and these were shown to result from polyadenylation at three distinct sites within the primary transcript of this single-copy gene. The complete RP2 mRNA sequence was obtained from overlapping cDNA clones, revealing an open reading frame of 357 amino acids that corresponds to a protein of 40,365 daltons. The detection of RP2 mRNA in all tissues examined to date suggests that the RP2 protein may function in a housekeeping role in all cells. This is supported by the finding of a high percentage of G + C residues at the 5' end of the gene, including a sequence homologous to the binding site of the transcription factor Sp1, which has been suggested to affect the regulation of other housekeeping genes that have been characterized. An examination of the amino acid sequence indicates that the RP2 protein is proline-rich and is composed of alternating alpha-helix and beta-sheet regions. RP2 is probably not integrated into a membrane structure in the cell as it does not appear to contain hydrophobic regions capable of spanning a membrane.
Full text
PDF





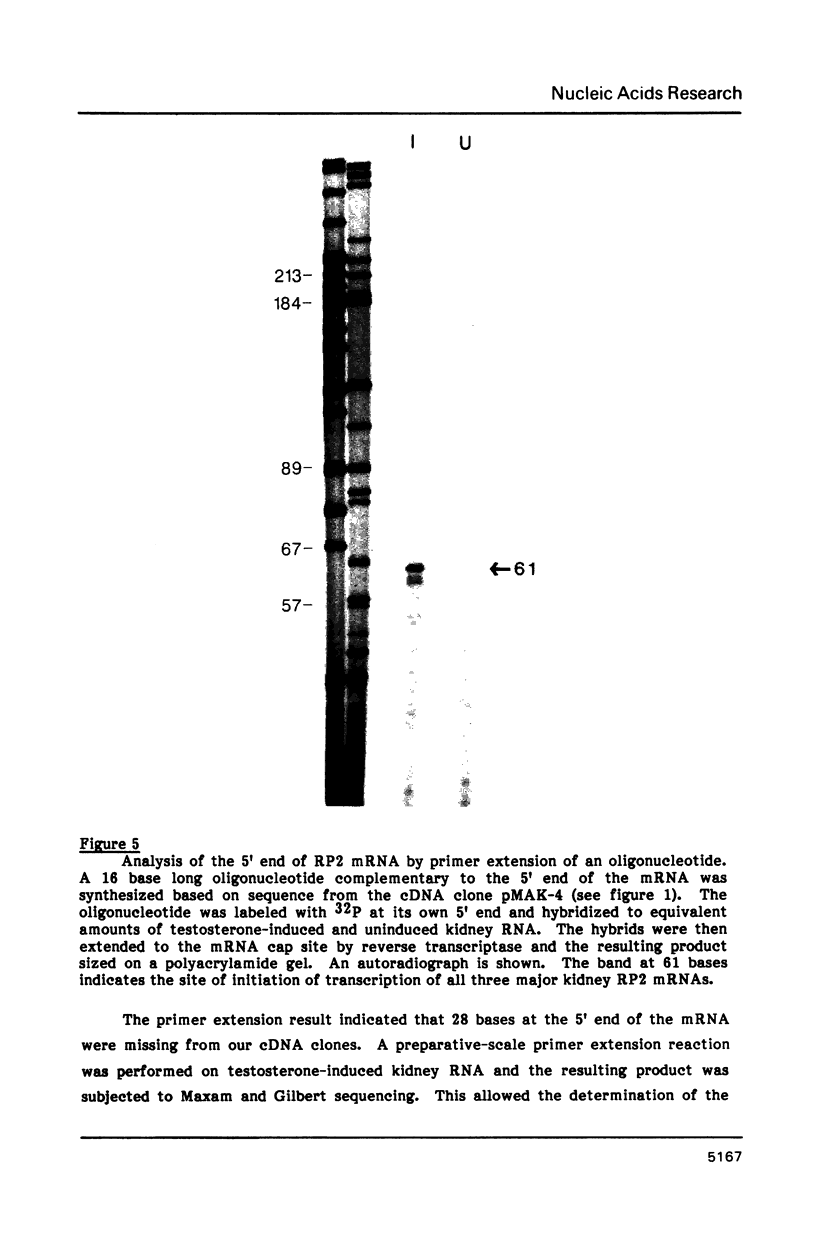
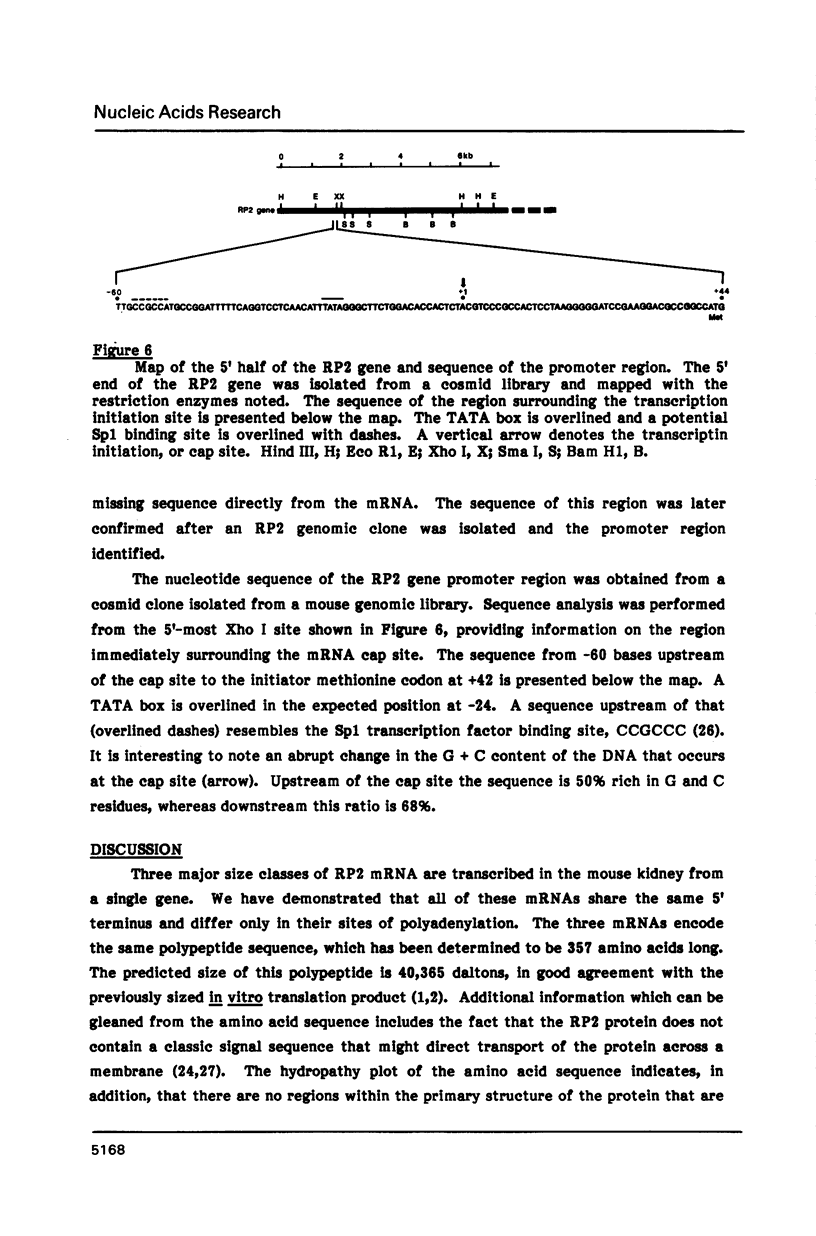


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger F. G., Gross K. W., Watson G. Isolation and characterization of a DNA sequence complementary to an androgen-inducible messenger RNA from mouse kidney. J Biol Chem. 1981 Jul 10;256(13):7006–7013. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. R., Daar I. O., Krug J. R., Maquat L. E. Characterization of the functional gene and several processed pseudogenes in the human triosephosphate isomerase gene family. Mol Cell Biol. 1985 Jul;5(7):1694–1706. doi: 10.1128/mcb.5.7.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock L. P. Androgen and progestin stimulation of ornithine decarboxylase activity in the mouse kidney. Endocrinology. 1983 Jun;112(6):1903–1909. doi: 10.1210/endo-112-6-1903. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dush M. K., Sikela J. M., Khan S. A., Tischfield J. A., Stambrook P. J. Nucleotide sequence and organization of the mouse adenine phosphoribosyltransferase gene: presence of a coding region common to animal and bacterial phosphoribosyltransferases that has a variable intron/exon arrangement. Proc Natl Acad Sci U S A. 1985 May;82(9):2731–2735. doi: 10.1073/pnas.82.9.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Elliott R. W., Berger F. G. DNA sequence polymorphism in an androgen-regulated gene is associated with alteration in the encoded RNAs. Proc Natl Acad Sci U S A. 1983 Jan;80(2):501–504. doi: 10.1073/pnas.80.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
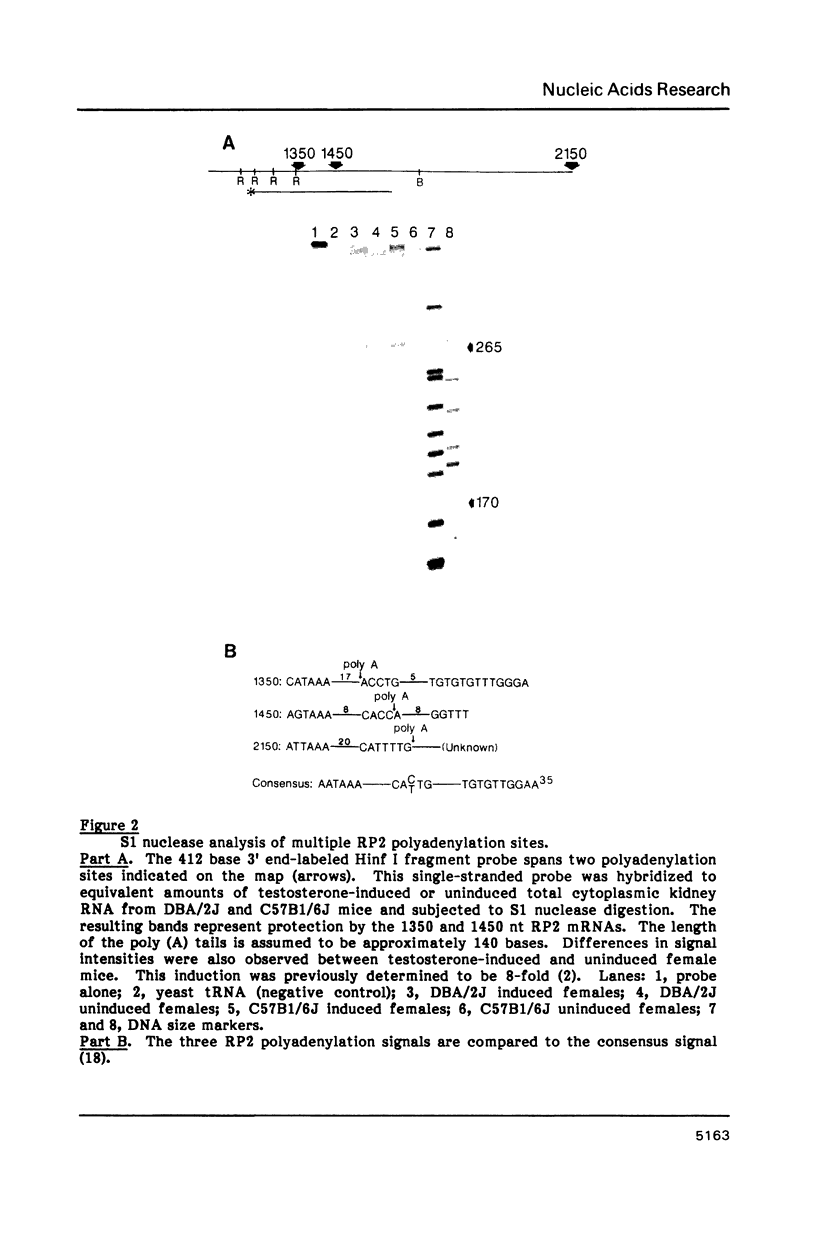
- Gil A., Proudfoot N. J. A sequence downstream of AAUAAA is required for rabbit beta-globin mRNA 3'-end formation. 1984 Nov 29-Dec 5Nature. 312(5993):473–474. doi: 10.1038/312473a0. [DOI] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Sherr C. J., Galibert F. Nucleotide sequence of the feline retroviral oncogene v-fms shows unexpected homology with oncogenes encoding tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Jan;81(1):85–89. doi: 10.1073/pnas.81.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D., Snider L. D., Lingrel J. B. Polymorphism in an androgen-regulated mouse gene is the result of the insertion of a B1 repetitive element into the transcription unit. Mol Cell Biol. 1986 Jan;6(1):209–217. doi: 10.1128/mcb.6.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochakian C. D., Mayumi T. Androgen regulation of transferase II activity in the mouse kidney. Mol Cell Endocrinol. 1977 Feb;6(4-5):309–318. doi: 10.1016/0303-7207(77)90105-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Snider L. D., King D., Lingrel J. B. Androgen regulation of MAK mRNAs in mouse kidney. J Biol Chem. 1985 Aug 15;260(17):9884–9893. [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Sun Y. H., Goodenow R. S., Hood L. Molecular basis of the dm1 mutation in the major histocompatibility complex of the mouse: a D/L hybrid gene. J Exp Med. 1985 Nov 1;162(5):1588–1602. doi: 10.1084/jem.162.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Paigen K., Ganschow R. E. Genetic control of glucuronidase induction in mice. J Mol Biol. 1973 Dec 5;81(2):225–243. doi: 10.1016/0022-2836(73)90191-5. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Hastie N. D., Held W. A. An abundant androgen-regulated mRNA in the mouse kidney. Cell. 1979 Jun;17(2):441–448. doi: 10.1016/0092-8674(79)90170-3. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Proudfoot N. J. Transcriptional activity of the human pseudogene psi alpha globin compared with alpha globin, its functional gene counterpart. Nucleic Acids Res. 1983 Nov 25;11(22):7717–7733. doi: 10.1093/nar/11.22.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. K., Masters J. N., Attardi G. Human dihydrofolate reductase gene organization. Extensive conservation of the G + C-rich 5' non-coding sequence and strong intron size divergence from homologous mammalian genes. J Mol Biol. 1984 Jun 25;176(2):169–187. doi: 10.1016/0022-2836(84)90419-4. [DOI] [PubMed] [Google Scholar]