Abstract
Thigmonastic or seismonastic movements in Mimosa pudica, such as the response to touch, appear to be regulated by electrical, hydrodynamical and chemical signal transduction. The pulvinus of Mimosa pudica shows elastic properties, and we found that electrically or mechanically induced movements of the petiole were accompanied by a change of the pulvinus shape. As the petiole falls, the volume of the lower part of the pulvinus decreases and the volume of the upper part increases due to the redistribution of water between the upper and lower parts of the pulvinus. This hydroelastic process is reversible. During the relaxation of the petiole, the volume of the lower part of the pulvinus increases and the volume of the upper part decreases. Redistribution of ions between the upper and lower parts of a pulvinus causes fast transport of water through aquaporins and causes a fast change in the volume of the motor cells. Here, the biologically closed electrochemical circuits in electrically and mechanically anisotropic pulvini of Mimosa pudica are analyzed using the charged capacitor method for electrostimulation at different voltages. Changing the polarity of electrodes leads to a strong rectification effect in a pulvinus and to different kinetics of a capacitor discharge if the applied initial voltage is 0.5 V or higher. The electrical properties of Mimosa pudica's pulvini were investigated and the equivalent electrical circuit within the pulvinus was proposed to explain the experimental data. The detailed mechanism of seismonastic movements in Mimosa pudica is discussed.
Key words: electrophysiology, plant electrostimulation, pulvinus, Mimosa pudica, charged capacitor method, electrical circuits, ion channels
Introduction
The exact mechanism of seismonastic movements in Mimosa pudica L. is unknown. There are three main hypotheses: chemical, muscular and osmotic mechanisms (Table 1). All of these mechanisms of plant movements have experimental support, but each hypothesis does not take into account experimental data supporting the other two mechanisms.
Table 1.
Hypothetical mechanisms of seismonastic movements of Mimosa pudica leaf
| Hypotheses | Pro | Contra |
| Diffusion of a chemical compounds1 referred to turgorins or leaf movement factors.2 | Gallic acid 4-O-(β-D-glucopyranosyl-6′-sulfate) can induce leaf closing.2,3 Gallic acid is a product of acid hydrolysis of the tannins. A sulfotransferase involved in sulfation of the turgorins, gallic acid 4-O-(β-D-glucopyranosyl-6′-sulfate), is pulvini-localized in Mimosa pudica.4 If leaves of Mimosa pudica exposed to 14CO2, considerable amounts of labeled photoassimilates are accumulated in the pulvinus of the stimulated leaf.5 There is a correlation between movements of the leaf and sucrose translocation in the phloem of Mimosa pudica.5 | Mechanoreceptors of Mimosa pudica elicit also electrical and hydraulic signals, which induce transport of K+, Cl−, Ca2+ ions and fast water translocation in the pulvinus. The leaf movements are reversible. Some anesthetics reversibly block the seismonastic movements of a petiole.6 Aquaporins are involved in the seismonastic movements of a petiole.7 The mechanical stimulation triggers a large decrease of K+ ions in the extensor site.8 |
| Muscular movements.6,9–11 | The degree of bending of a peyiole is correlated with actin tyrosine-phosphorylation in the pulvinus.11 Fragmentation of actin filaments and microtubules occurs during bending, although the actin cytoskeleton, but not the microtubules, is involved in the regulation of the movement.12 Cytochalasin B and phallodin inhibit the bending of the petiole.13,14 The ATP level in the pulvinus decreases to 30% during falling of a petiole and returns to the initial level after recovery of a petiole and pulvinus.15 Ca, Mg-dependent ATPase in the pulvinus is similar to the ATPases from muscle and non-muscle motile cells.16 A tubulin from Mimosa pudica may be involved in the regulation the movements in Mimosa pudica.17Mimosa pudica has a gelsolin/fragmin family actin-modulating protein that severs actin filament in a Ca2+-dependent manner.18 The central vacuole of the motor cell contains a contractile protein which undergoes a conformational change during seismonastic movement.19 The seismonastic movement of a petiole may be more efficient than typical animal muscle movements.9 | Some anesthetics reversibly block the seismonastic movements of a petiole.6 Aquaporins are involved in the seismonastic movements of a petiole.7 The mechanical stimulation triggers a large decrease of K+ ions in the extensor site.8 |
| Osmotic motor | Migration of calcium regulates the seismonastic response in Mimosa pudica. Volume and conformational changes of the contractive tannin vacuoles in the pulvinus correlate with the seismonastic leaf movement.19 Stimulation of Mimosa pudica causes the secretion of K+ from the cytoplasm into the apoplast.20 The mechanical stimulation triggers a large decrease of K+ ions in the outer extensor.8,21 Shrinking of extensor cells and swelling of flexor cells bend a pulvinus.22 | The degree of bending of a petiole is correlated with actin tyrosine-phosphorylation in the pulvinus.11 Fragmentation of actin filaments and microtubules occurs during bending, although the actin cytoskeleton, but not the microtubules, is involved in the regulation of the movement.12 Cytochalasin B and phallodin inhibit the bending of the petiole.13,14 Gallic acid 4-O-(β-D-glucopyranosyl-6′-sulfate) can induce leaf closing.2,3 A sulfotransferase involved in sulfation of the turgorins, gallic acid 4-O-(β-D-glucopyranosyl-6′-sulfate) is pulvini-localized in Mimosa pudica.4 Cells in flexor and extensor sites have opposite response to the same action potential. |
Chemical hypothesis.
Pfeffer23 and Ricca1 suggested that an unknown chemical compound is responsible for the seismonastic movements in Mimosa pudica. According to Ricca1 this hydrophilic compound, so called a Ricca factor, moves through the xylem vessels to induce mechanical responses to stimuli. Schildknecht and Bender3 isolated and characterized the Ricca factor from Mimosa pudica L, which is turgorin 4-0-(β-D-glucopyranosyl-6′-sulfate) gallic acid. The binding site of this turgorin is located on the plasma membrane in the pulvinus.4 The mechanism of a turgorin action can be similar to acetylcholine effects in animal nerves.24
Muscular hypothesis.
Gardiner10 found that mechanical properties of the pulvinus are similar to animal muscles. The seismonastic movement of a petiole may be more efficient than typical animal muscle movements.9 The phosphorylation level of actin in the pulvinus affects the dynamic reorganization of actin filaments and causes seismonastic movement.11,12,18 If the flexor of the pulvinus is cut away, the extensor will still work, reacting to stimuli.25
Osmotic motor hypothesis.
Mechanical stimuli induce action potential,6 which can activate K+ and Cl− voltage gated ion channels.26 Ion transport in the pulvinus induces osmotic movement of water and sudden turgor loss in the lower pulvinar cells.27 The mechanism of leaf movement exhibited by Mimosa pudica is different from the movement of guard cells in the stomata.28
The exact mechanism of seismonastic movements in Mimosa pudica L. should include elements of all three hypotheses.
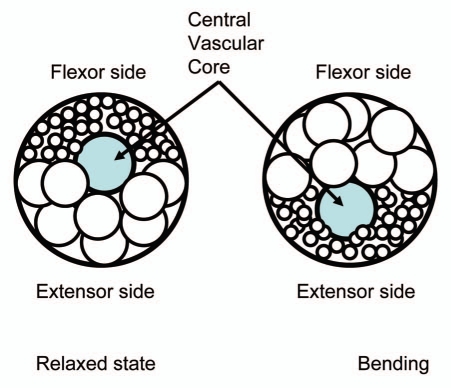
The pulvinus is comprised of three main parts: a central vascular core and two layers of flexor and extensor cells. The action potential activates voltage gated channels and induces the redistribution of K+, Cl−, H+ and Ca2+ ions between extensor and flexor layers which in turn leads to the osmotic movement of water, causing the bending of the pulvinus and movement of the petiole in Mimosa pudica.8,20–22,29–31 The potassium concentration in the apoplast of extensor cells of the Mimosa pudica's pulvinus increases from 30–70 mM to 100 mM. Thus the motor cells shrink and resultantly, the cells from the flexor site take up K+ ions from the apoplast and begin to swell.32 The differential volume changes of flexor and extensor sites result from the transport of ions accompanied by osmotic transport of water. There is a high gradient of osmotic pressure between extensor and flexor cells of about 1 MPa in Samanea pulvini.10,22
The identification and characterization of bioelectrochemical mechanisms for electrical signal transduction in plants would mark a significant step forward in understanding this under-explored area of plant physiology.33,34 Although plant mechanical and chemical sensing and corresponding responses are well known, membrane electrical potential changes in plant cells and the possible involvement of electrophysiology in transduction mediation of these sense-response patterns represents a new dimension of plant tissue and whole organism integrative communication. A short time ago, we discovered the bioelectrochemical mechanisms of electrical signal transduction in biologically closed electrical circuits in the pinnae and petioles of Mimosa pudica and the plant's responses to electrostimulation.35,36 The studies of the mechanisms of concerted movements in plants from electrical signal transduction to cascades of cellular events will have a potentially broad impact on both fundamental sciences and engineering.37,38
When describing the propagation of electrical signals in plants, it is often convenient to represent the real electrical and electrochemical properties of biointerfaces with idealized equivalent electrical circuit models consisting of discrete electrical components.39–42 Biologically closed electrical circuits operate over large distances in biological tissues.43,44 The activation of such circuits can lead to various physiological and biophysical responses.35–37,45 Recently, we investigated the biologically closed electrical circuits in the upper leaf of the Venus flytrap, and found the equivalent electrical circuit.41
Mechanical movements in Mimosa pudica can be induced by electrostimulation if very high applied voltages, 200–400 volts, were briefly applied between the soil and the primary pulvinus to measure the contractile characteristics of a petiole.9 Jonas46 used a 0.5 µF capacitor charged by 50, 100 and 150 volts for electrostimulation and found oscillations of leaves and fast petiole movement in Mimosa pudica after the application of an electrical shock. The petioles bend downward and the pinnae close after the application of 9 volts to Mimosa pudica.14 These high voltages are non-physiological and have a side effect—plant electrolysis. We analyzed, both experimentally and theoretically, the mechanism of mechanical movements in Mimosa pudica induced by low voltage electrostimulation of the petiole and pinna.35,36 The charged capacitor method is a very efficient tool for the study of the bioelectrochemistry of cells, clusters of cells or for electrostimulation of whole plants and evaluation of biologically closed electrical circuits.
Some plants move their leaves upon sudden shaking or touch as seismonastic and thigmonastic movements use osmotic motors, powered by H+-ATPases.11,16,47 The osmotic motor often resides in specialized leaf organs, midribs or pulvini, at the base of the leaves and leaflets. The osmotic motor transfers water through water channels or aquaporins.7,24
In terms of electrophysiology, these responses in Mimosa pudica can be considered in three stages: (1) stimulus perception, (2) signal transmission and (3) induction of response.6,10,23,48–51 Action potentials involve effluxes of K+ and Cl− and a temporary change of turgor, produced by osmotic motors. Like the action potential, a critical threshold depolarization triggers Ca2+ influx, opening of Ca2+-sensitive Cl− channels and K+ channels; effluxes last over a short period of time and result in turgor regulation.26,30,52,53
Plants have many different electrical circuits, which can be activated by different stimuli, such as action potentials, mechanical, thermal or wounding stresses. These circuits are biologically closed and operate during specific reaction of plants to stimuli. In the study reported, we analyzed the biologically closed electrical circuits in a pulvinus of Mimosa pudica through electrostimulation using the new charged capacitor method43,44,54 and observed the effects of electrical or mechanical stimulation on the hydro- elastic properties of a pulvinus. We then evaluated an equivalent electrical scheme of the electrical signal transduction inside the primary pulvinus. The new charge capacitor method permits the study of different steps in signal transmission and responses in the plant kingdom.
Results
Mechanics of petiole movement.
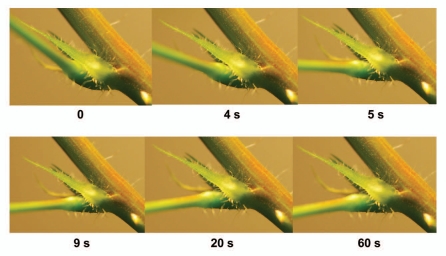
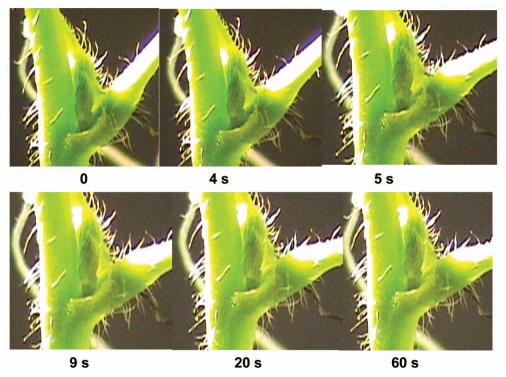
After mechanical stimulation of a petiole or a pulvinus, a petiole falls in a few seconds (Fig. 1) and relaxes to the initial state in 10–12 minutes (Fig. 2). The maximal angle between a petiole and a stem varies from 25 to 100 degrees in different plants. Electrostimulation of a pulvinus by a 47 µF capacitor charged to 1.5 V (+ on upper part, − on lower part of a pulvinus) leads to a petiole bending (Fig. 3) similar to the effect of mechanical stimulation. The lower part of the pulvinus has a higher volume and curvature when a petiole is in a relaxed state (Fig. 2). After mechanical or electrical stimulation of a pulvinus, the volume and curvature in the upper part of the pulvinus increases and a petiole hangs down (Figs. 1 and 3). A pulvinus changes its shape during the hydromechanical movement of a petiole.
Figure 1.
Sequence of photos of a pulvinus of Mimosa pudica after mechanical stimulation.
Figure 2.
Sequence of photos of a relaxation of a pulvinus of Mimosa pudica after mechanical stimulation to the initial state during 20 minutes.
Figure 3.
Sequence of photos of a pulvinus of Mimosa pudica after electrical stimulation by 47 µF charged capacitor with initial voltage of 1.5 V.
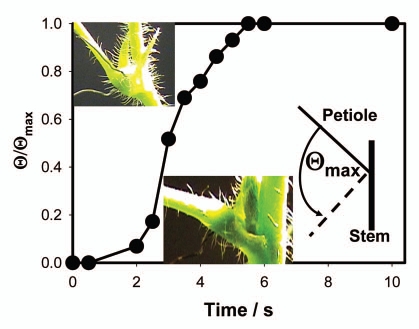
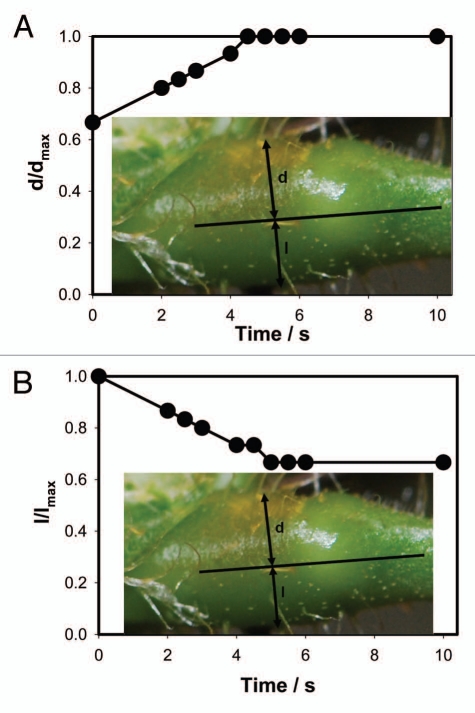
Figure 4 shows the kinetics of a petiole bending, triggered by electrical stimulation. This bending is synchronized with the increased volume of the upper part of a pulvinus (Fig. 5A) and the decreased volume of the lower part of a pulvinus (Fig. 5B).
Figure 4.
Kinetics of a petiole bending triggered by electrical stimulation of Mimosa pudica by 47 µF charged capacitor with initial voltage of 1.5 V.
Figure 5.
Kinetics of a volume changes in the upper and lower part of a pulvinus during a petiole bending triggered by electrical stimulation of Mimosa pudica by 47 µF charged capacitor with initial voltage of 1.5 V. These results were reproduced 16 times.
Electrical processes in pulvinus.
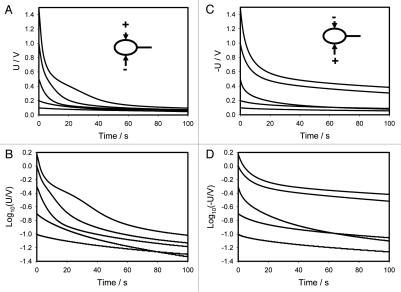
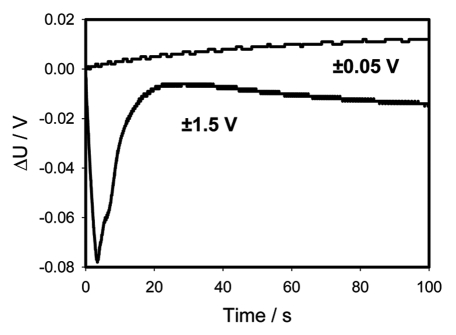
Figures 6A (+ on top of the pulvinus and—in the lower part of the pulvinus) and 6C (− on top of the pulvinus and + in the lower part of the pulvinus) show the time dependencies of a 47 µF charged capacitor discharging in the pulvinus of Mimosa pudica. Figures 6B and D show the same dependencies in logarithmic coordinates. Electrical discharge of a capacitor in the pulvinus is faster when a positive Ag/AgCl electrode is connected to the upper part of the pulvinus and a negative one to the lower part of a pulvinus. This difference increases with an increase of initial voltage on the capacitor (Fig. 6A and B) and is clearly demonstrated on Figure 7—a sum of Figures 6A and B. At an initial voltage of 0.1 V or less, there is no dependence on speed of a capacitor discharge on the polarity of electrodes in upper and lower parts of a pulvinus. At potentials of 0.2 V or higher, there is strong dependence on polarity and amplitude of applied voltage. Since the charge of a capacitor Q is equal to voltage U times capacitance C, this effect can be caused either by voltage or charge of the capacitor.
Figure 6.
Time dependence of electrical discharge between electrodes located across the Mimosa pudica pulvinus and connected to 47 µF charged capacitor (A and C). These results were reproduced 14 times. Voltage between electrodes was measured every 15 ms. Time dependence of electrical discharge in the Mimosa pudica's pulvinus between electrodes connected to 47 µF charged capacitor in logarithmic coordinates (B and D).
Figure 7.
Time dependence of voltage differences (Fig. 6A + C) during electrical discharge in the Mimosa pudica's pulvinus between electrodes of different polarities located across the Mimosa pudica pulvinus and connected to 47 µF charged capacitor.
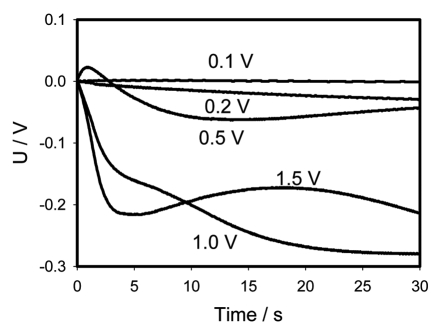
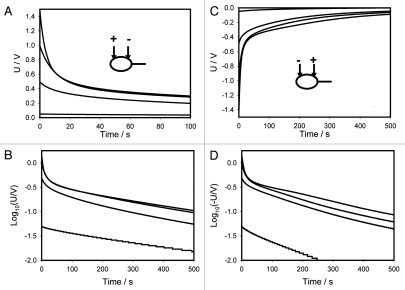
Figures 8A and C show the time dependencies of charged capacitors discharging in the Mimosa pudica pulvinus between electrodes located along the pulvinus. Figures 8B and C show these same values in logarithmic coordinates. If the applied potential is 0.5 V or higher, there is a strong deviation of these dependencies from straight lines according to equation 1 and the equivalent electrical scheme shown on Figure 9A cannot describe the experimental dependencies. Figure 10 shows the dependence of kinetics of a 47 µF capacitor discharging between Ag/AgCl electrodes located along the pulvinus on polarity of electrodes at low and high voltages. If the positive electrode is located in the pulvinus near a stem, and the negative electrode is located near a petiole, the 47 µF capacitor discharge is slightly faster when the applied voltage is 1.5 V.
Figure 8.
Time dependence of electrical discharge in Mimosa pudica pulvinus between electrodes located along the pulvinus and connected to 47 µF charged capacitor (A and C). These results were reproduced 25 times. Voltage between electrodes was measured every 15 ms. Time dependence of electrical discharge in the Mimosa pudica's pulvinus between electrodes located along the pulvinus and connected to a charged capacitor in logarithmic coordinates (B and D).
Figure 9.
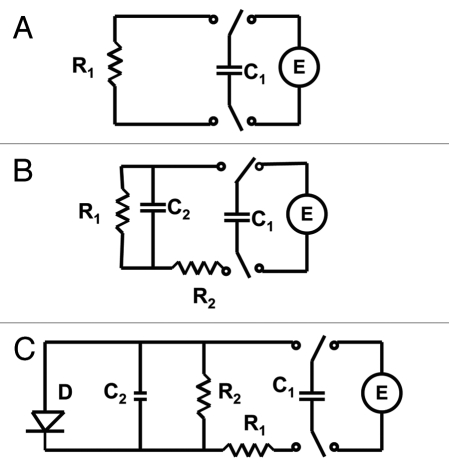
Electrical equivalent schemes of a capacitor discharge in a plant tissue. Abbreviations: C1, charged capacitor from voltage source U; C2, capacitance of plant tissue; R, resistance in the Mimosa pudica tissue.
Figure 10.
Time dependence of voltage differences (Fig. 8A + C) during electrical discharge in the Mimosa pudica's pulvinus between electrodes of different polarities located along the Mimosa pudica pulvinus and connected to 47 µF charged capacitor.
Discussion
Mechanical and electrical stimulation of the leaf movement.
While the mechanism of thigmonastic movement in Mimosa pudica is not clear at the present time, there are a few hypotheses to describe it (Table 1). The osmotic hypothesis states that the thigmonastic movement of Mimosa pudica is powered by a sudden loss of turgor pressure in the motor cells of the pulvinus.
The mechanism of this movement can be explained as follows. The pulvinus is a flexible hinge located at the base of the stalk of the leaf. It has a very anisotropic structure. The motor cells are organized in such a way that they allow changes with changing turgor only in length, but not in circumference.55 As a result, the antagonistic changes in the length of the flexor and extensor cells produce the petiole movements. This mechanism is similar to the work of a bimetallic strip that converts a temperature change into a mechanical displacement. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its normal temperature. In a similar way, the pulvinus uses a hydroelastic mechanism37 that converts a difference of turgor pressure in extensor and flexor cells to the bending and ensuing rotation of the petiole. Bending of the pulvinus also manifests itself by a change in the curvature of the extensor and flexor sides. Movement of the petiole and the change in curvature closely follow each other as demonstrated in Figures 1–3 and 5. During the falling of a petiole, the volume of the lower part of the pulvinus decreases and the volume of the upper part increases in a few seconds. During relaxation of a petiole to its initial state, the volume of the extensor side of a pulvinus increases and the volume of the flexor side decreases in 20 minutes (Fig. 2). This seems to occur due to the redistribution of water between the upper and lower parts of a pulvinus.
Through the usage of nuclear magnetic resonance, the movement of water from the lower half of the pulvinus to the upper half following a mechanical stimulus was observed.56 This observation gives direct evidence for the theory of fast movement of water from the lower half to the upper half of the pulvinus.56 Movement of water from the upper half of the pulvinus to the lower half of the pulvinus during petiole relaxation is slow (Fig. 2).
The details of the mechanism for the concerted action of the flexor and extensor sides of the pulvinus are not yet known, although osmotic pumps are definitely involved in water exchange in these two layers of the pulvinus.
The hydroelastic mechanism of Mimosa movement parallels with the mechanism that closes the Venus fly trap.37 The hydro-elastic model is based on the assumption that the leaf possesses curvature elasticity and consists of outer and inner hydraulic layers where different hydrostatic pressure can build up.37 The open state contains high elastic energy accumulated due to a hydrostatic pressure difference between the outer and inner layers of the leaf. Stimuli induce water flow from one hydraulic layer to another. This very fast process also involves water exchange between two layers of cells in the lobes of the trap with a consequent change of leaf curvature. The closing of the Venus flytrap was described by the hydroelastic curvature (HEC) mechanism.37,54
Cl− and H+ ions also participate in osmotic changes in a pulvinus using anion voltage gated anion channels and proton pumps. There is an opinion that ATPase activity is strongly involved in the thigmonastic movement of Mimosa pudica because a high density of H+-ATPases in the phloem and pulvini was found.15,57
Electrostimulation of a pulvinus can lead to the same response in Mimosa pudica as mechanostimulation.35 Using the charged capacitor method, we can evaluate some electrical properties of plant tissue. The vascular bundle in the petiole is surrounded by a sclerenchyma sheath which gives symplastic isolation.58 Application of a small voltage to the petiole up to 1.5 V does not induce the pulvinus bending or the petiole movement. The equivalent electrical circuit is passive and consists of resistors and capacitors.36 Application of a high voltage of 9 V induces the petiole movement.14
Electrical anisotropy of a pulvinus.
If a capacitor of capacitance C is discharged through a resistor R, the capacitor voltage U is then
| (1) |
and the charge remaining at the capacitor is
| (2) |
Where Q0 = CU0 is the initial charge on the capacitor. The capacitive time constant RC governs the discharging process. At t = τ = RC the capacitor charge is reduced to CU0e−1, which is about 37% of its initial charge. For R in ohms and C in farads, the time constant RC is in seconds. The voltage across the capacitor decreases exponentially with the same time constant τ from the initial value U0 to zero.
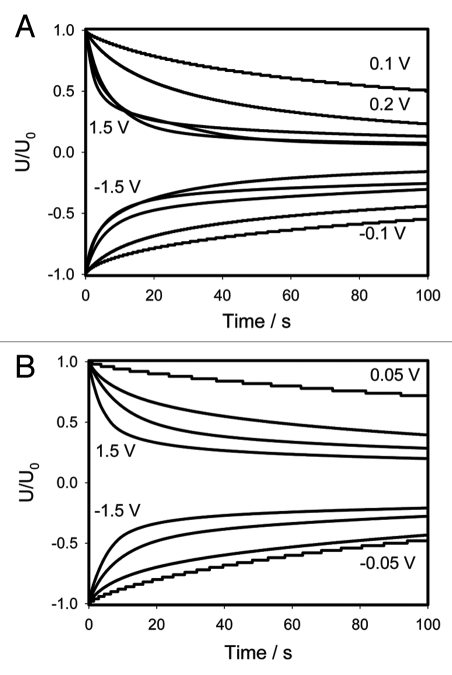
Figures 6 and 8 show the experimental dependencies of voltage of various charged capacitors on time. Logarithmic dependencies in Figures 6B and C, and 8B and D show significant deviations from the linear prediction of equation 1. Such deviations can be described by the equivalent schemes shown on Figure 9B and D. Additional membrane capacitance Cm can cause a deviation from a linear dependence. The equivalent electrical circuit shown in Figure 10A predicts the independence of the results from the polarity of the electrodes during a capacitor discharge in the pulvinus of Mimosa pudica. Changing the polarity of the electrodes leads to different results in the kinetics of the capacitor discharge (Figs. 6A and C, 7 and 8A and C). The time constant τ = RC also depends on the polarity of the electrodes. This effect is similar to the electrical discharge in the Venus flytrap and can be caused by the opening of voltage gated ion channels when the applied voltage exceeds the threshold value. It is convenient to represent electrochemical properties of biologically closed electrical circuits with idealized equivalent electrical circuit models consisting of discrete electrical components. We can simulate the voltage gated K+ and Cl− ion channels using diodes (Fig. 9C). Figures 6A–D and 8A–D show a very large deviation from equation (1) as well as unusual slopes and changes in the curvatures of the time dependencies of the capacitor's discharge. Figures 7 and 9 show the various kinetics of capacitor discharging between the same electrodes in the pulvinus after the polarity of the capacitor was changed. There is a strong rectification effect inside a pulvinus that is sensitive to the polarity of the capacitors (Figs. 7 and 9). This can be caused by a redistribution of K+, Cl− and Ca2+ ions through ion channels in the pulvinus.
Figures 6 and 8 show the kinetics of the discharge when the electrodes are inserted into the pulvinus either across or along this structure. It is useful to normalize these figures by the initial voltage U0 at the capacitor for a better comparison of these dependencies. The results are presented in Figure 11A for data shown in Figure 6 and in Figure 11B for data shown in Figure 8. Ag/AgCl electrodes were inserted across the pulvinus (Fig. 11A) or along the pulvinus (Fig. 11B).
Figure 11.
Normalized presentation of time dependence of electrical discharge in Mimosa pudica's pinna between electrodes connected to 47 µF charged capacitor. U0 is the initial capacitor voltage in volts. (A) experimental data was taken from Figure 6; (B) experimental data was taken from Figure 8.
If the electrical structure of the pulvinus was stationary and consisted of a number of resistors and capacitors, the normalized curves in Figure 11 would overlap. However, this overlapping did not happen: the capacitor with a high initial voltage discharged faster, meaning that the electrical circuits change with applied voltage. Usually this means that some ion channels are open in the plant tissue. Another interesting feature: Figure 11A with electrodes across the pulvinus in contrast to Figure 11B demonstrates the saturation effect. When small voltages are applied, the curves differ from one another, but if U0 exceeds 0.5 V the curves remain very close to each other. This indicates that all ion channels are already open and remain in this state. However, this does not happen in Figure 11B in which the electrodes are placed along the pulvinus indicating that the pulvinus has a very anisotropic electrical structure. This would agree with the fact that, as mentioned above, the mechanical structure of the pulvinus is also very anisotropic.
Another interesting parameter is the input resistance of the tissue that can be defined as
| (3) |
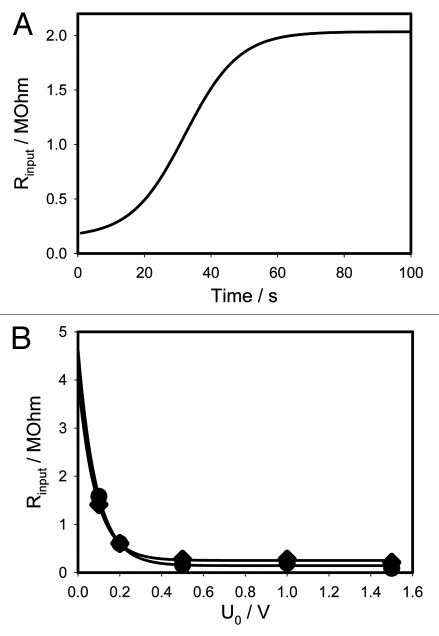
This parameter is often analyzed in electrical impedance spectroscopy studies of biological tissues.36,39,42 Figure 12A presents the time dependence of the input resistance for initial voltage of 1 V with the electrodes placed across the pulvinus. This dependence is perfectly modelled by the circuit in Figure 9C. The input resistance also changes with voltage, thus we analyzed the initial input resistance Rinput(U0) as a function of initial voltage. For data shown in Figure 12B these values are presented in the Table 2.
Figure 12.
Dependence of input resistance for initial voltage of 1 V on 47 µF capacitor on time (A) and dependencies of input resistance on initial voltage (B). Data for b was taken from Table 2.
Table 2.
Input resistance between Ag/AgCl electrodes inserted across the pulvinus
| U0, V | −1.5 | −1.0 | −0.5 | −0.2 | −0.1 | 0.1 | 0.2 | 0.5 | 1.0 | 1.5 |
| Rinput, kOhm | 212.5 | 278.8 | 275.7 | 604.6 | 1410.5 | 1583.0 | 604.0 | 162.1 | 195.6 | 89.9 |
Dependencies of the input resistance on the absolute value of initial voltage are shown in Figure 12B. They are very close to each other and very well fitted with the single exponential functions
| (4) |
for negative initial voltage and
| (5) |
for positive initial voltage. In the Figure 12B to make the comparison more convenient, we plotted the absolute values of voltage. As one can see, both curves sit very close to each other. Therefore, the opening of channels in this plant's tissue does not depend on the polarity of applied voltage.
The activation of biologically closed electrical circuits in Mimosa pudica can lead to different responses in the plant tissue. Mechanical or electrical stimuli can induce different electrical signals such as action potentials, variation potentials, slow wave potentials and streaming potentials which propagate along the conductive bundles. The plasma membranes in phloem cells facilitate the passage of electrical excitations in the form of action potentials. The internal functioning of a plant can be maintained and developed in a continuously varying environment only if all cells, tissues and organs function in concordance. Voltage-gated ionic channels control the plasma membrane potential and the movement of ions across membranes thereby regulating various biological functions. These voltage gated channels play vital roles in signal transduction in higher plants.
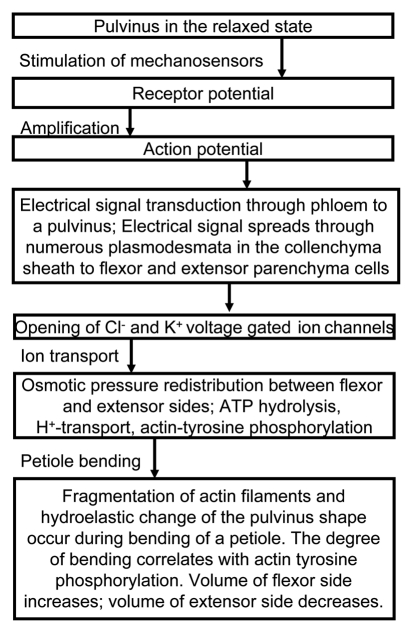
Propagation of an action potential can induce a mechanical response by activating the H+-ATPase, Cl− voltage gated channel, and the voltage gated K+ ion channel in a pulvinus (Fig. 13). Redistribution of ions between the upper and lower parts of a pulvinus induces fast transport of water through aquaporins and causes a fast change in the volume of the motor cells (Fig. 14). Voltage gated K+ and Cl− ion channels can operate as an electrical starter of the osmotic motor in a pulvinus. Ion fluxes activate fast water transport through aquaporins between the lower half of the pulvinus and the upper half of the pulvinus following an electrical stimulus. Our mechanism does not contradict the chemical, muscle and osmotic motor hypotheses and it explains recent observation.
Figure 13.
The mechanism of the seismonastic movements in Mimosa pudica.
Figure 14.
Schematic diagram of a pulvinus cross section in relaxed and bended states.
Materials and Methods
Electrodes.
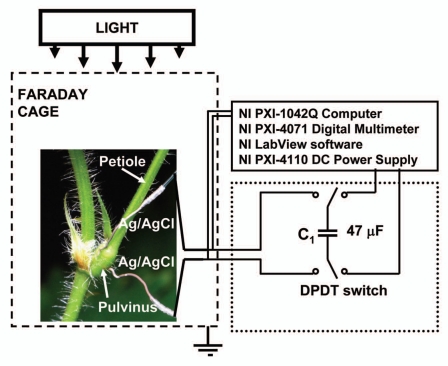
All measurements were conducted in the laboratory at 21°C inside a Faraday cage mounted on a vibration-stabilized table (Fig. 15). Ag/AgCl electrodes were prepared from Teflon coated silver wires (A-M Systems, Inc., USA) according to the method described by Ksenzhek and Volkov.59 The resistance between two Ag/AgCl electrodes that are 2 cm apart in 0.1 M KCl solution was found to be 10 kOhm. The response time of Ag/AgCl electrodes was less than 0.1 µs. As a control experiment, we also used platinum electrodes instead of Ag/AgCl electrodes and received the same results. Platinum electrodes were prepared from Teflon coated platinum wires (A-M Systems, Inc.,). We allowed the plants to rest until the leaves were completely open. The dependence of the electrical discharge on time in Mimosa pudica was measured 3 to 25 hours after insertion of the electrodes.
Figure 15.
Experimental setup.
Plant electrostimulation.
PXI (PCI eXtensions for Instrumentation) is a rugged PC-based platform that offers a high-performance solution for measurement and automation systems. PXI combines the Peripheral Component Interconnect electrical bus with the rugged, modular Eurocard mechanical packaging of CompactPCI and adds specialized synchronization buses and key software features. A NI-PXI-4071 digital multimeter (National Instruments, Austin, TX) connected to 0.2 mm thick nonpolarizable reversible Ag/AgCl electrodes was used to record the digital data. A NI-PXI-4110 DC Power Supply (National Instruments) was the voltage source for capacitor charging. The charged capacitor was used for electrostimulation of Mimosa pudica. The NI-PXI-4110 is a programmable, triple-output precision DC power supply in a single-slot, 3U PXI module. The NI-PXI-4110 has 16-bit resolution for programming the voltage set point and current limit and for using the voltage and current read back/measurement functionality.
The Charged Capacitor Method35,36,41,44,45,54 was used to estimate, with high precision, the amount of electrical energy necessary to induce a response. A double pole, double throw (DPDT) switch was used to connect the known capacitor to the NI-PXI-4110 DC Power Supply during charging, and then to the plant during plant stimulation. Since the charge of a capacitor Q connected to the voltage source U is Q = CU, we can precisely regulate the amount of charge using different capacitors and applying various voltages. By changing the switch position, we can instantaneously connect the charged capacitor to the plant and induce a response.
Images.
Digital video recorders, Sony DCR-HC36 and Sony HDR-XR500 (Sony, USA), were used to monitor the movements of the Mimosa pudica and to collect digital images, which were analyzed frame by frame. A photo camera Nikon DX40DX (Nikon USA Inc.,) with AF-S Micro Nikkor 105 mm 1:2.8 G ED VR lense (Nikon, USA) was used for the photography of Mimosa pudica.
Plants.
The seeds of Mimosa pudica L. were soaked in warm water (30°C) for 48 h. 70 plants were grown in well drained peat moss at 21°C with a 12:12 hr light:dark photoperiod. After growing for two weeks, the seedlings were transplanted into pots in a plant growing chamber. The humidity averaged 45–50%. The plants were watered every day. Two-three month old plants were used for the experiments. Irradiance was 700–800 µmol photons m−2s−1 PAR. All experiments were performed on healthy adult specimens.
Acknowledgements
This work was supported by the National Science Foundation (grant no. HRD0811507).
Abbreviations
- C
capacitance
- DPDT
double pole double throw switch
- HEC
a hydroelastic curvature model
- PXI
PCI eXtensions for instrumentation
- R
resistance
- τ
the circuit time constant
- t
time
- Q
charge of capacitor
- U
voltage
- U0
initial voltage of a capacitor
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12658
References
- 1.Ricca U. Soluzione d'un problema di fisiologia. La propagazione di stimulo nella “Mimosa”. Nuovo Giornale Botanico Italiano Nuovo Serie. 1916;23:51–170. [Google Scholar]
- 2.Schildknecht H, Schumacher K. Ein hoch wirksamer leaf movement factor aus Acacia karoo. Chemische Zeitung. 1981;105:287–290. [Google Scholar]
- 3.Schildknecht H, Bender W. Chemonastisch wirksame leaf factors aus Mimosa pudica. Chemische Zeitung. 1983;107:111–114. [Google Scholar]
- 4.Varin L, Chamberland H, Lafontaine JG, Richard M. The enzyme involved in sulfation of the turgorins, gallic acid 4-O-(β-D-glucopyranosyl-6′-sulfate) is pulvinilocalized in Mimosa pudica. Plant J. 1997;12:831–837. doi: 10.1046/j.1365-313x.1997.12040831.x. [DOI] [PubMed] [Google Scholar]
- 5.Fromm J, Escrich W. Transport processes in stimulated and non-stimulated leaves of Mimosa pudica. I. The movement of 14C-labelled photoassimilates. Trees. 1988;2:7–17. [Google Scholar]
- 6.Bose JC. Movements in Plants. Delhi: B.R. Publishing Corp.; 1918. [Google Scholar]
- 7.Temmei Y, Uchida S, Hoshino D, Kanzawa N, Kuwahara M, Sasaki S, Tsuchia T. Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Letters. 2005;579:4417–4422. doi: 10.1016/j.febslet.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 8.Fromm J, Escrich W. Transport processes in stimulated and non-stimulated leaves of Mimosa pudica III. Displacement of ions during seismonastic leaf movements. Trees. 1988;2:65–72. [Google Scholar]
- 9.Balmer RT, Franks JG. Contractile characteristics of Mimosa pudica L. Plant Physiol. 1975;56:464–467. doi: 10.1104/pp.56.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardiner W. On the power of contractility exhibited by the protoplasm of certain plant cells. Annals Botany. 1888;1:362–367. [Google Scholar]
- 11.Kameyama K, Kishi Y, Yoshomura M, Kanzawa N, Tsuchia T. Thyrosine phosphorylation in plant bending. Nature. 2000;407:37. doi: 10.1038/35024149. [DOI] [PubMed] [Google Scholar]
- 12.Kanzawa N, Hoshino Y, Chiba M, Hoshino D, Kobayashi H, Kamasawa N, et al. Change in the actin cytoskeleton during seismonastic movement of Mimosa pudica. Plant Cell Physiol. 2006;47:531–539. doi: 10.1093/pcp/pcj022. [DOI] [PubMed] [Google Scholar]
- 13.Fleurat-Lessard P, Roblin G, Bonmort J, Besse C. Effects of colchicine, vinblastine, cyclochalasin B and phallodin on the seismonastic movement of Mimosa pudica leaf and on motor cell structure. J Experimental Botany. 1988;39:209–221. [Google Scholar]
- 14.Yao H, Xu Q, Yuan M. Actin dynamics mediates the changes of calcium level during the pulvinus movement of Mimosa pudica. Plant Signaling and Behavior. 2008;3:954–960. doi: 10.4161/psb.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liubimova MN, Deminovskaya NS, Fedorovich IB. The part played by ATP in the motor function of Mimosa pudica leaf. Biokhimia (Moscow) 1964;29:774–779. [Google Scholar]
- 16.Liubimova-Engel'gardt MN, Burnasheva SA, Fain FS, Mitina NA, Poprykina IM. Mimosa pudica adenosine triphosphatase. Biokhimia (Moscow) 1978;43:748–760. [PubMed] [Google Scholar]
- 17.Pal M, Roychaudhury A, Pal A, Biswas S. A novel tubulin from Mimosa pudica. European J Biochem. 1990;192:329–335. doi: 10.1111/j.1432-1033.1990.tb19231.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamashiro S, Kameyama K, Kanzawa N, Tamiya T, Mabuchi I, Tsuchia T. The gelsolin/fragmin family protein indentified in the higher plant Mimosa pudica. J Biochem. 2001;130:243–249. doi: 10.1093/oxfordjournals.jbchem.a002978. [DOI] [PubMed] [Google Scholar]
- 19.Toriyama H, Jaffe M. Migration of calcium and its role in the regulation of seismonasty in the motor cell of Mimosa pudica L. Plant Physiol. 1972;49:72–81. doi: 10.1104/pp.49.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toriyama H. Observational and experimental studies of sensitive plants. VI. The migration of potassium in the primery pulvinus. Cytologia. 1955;20:367–377. [Google Scholar]
- 21.Allen RD. Mechanism of the seismonastic reaction in Mimosa pudica. Plant Physiol. 1969;44:1101–1107. doi: 10.1104/pp.44.8.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorton HL. Water relation in pulvini from Samanea saman. 1. Intact pulvini. Plant Physiol. 1987;83:945–950. doi: 10.1104/pp.83.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeffer W. The Physiology of Plants. Oxford: Clarendon Press; 1905. [Google Scholar]
- 24.Schildknecht H, Meier-Ausgenstein W. Role of turgorins in leaf movement. In: Satter RL, Gorton HL, Vogelman TC, editors. The Pulvinus: Motor Organ For Leaf Movement 101-29. Rockville, Maryland: American Society of Plant Physiologists; 1990. [Google Scholar]
- 25.Dutrochet MH. Memoires pour server a l'histoire anatomique et physiologique des vegetaux et des animaux. Bruxelles, Belgium: 1837. (Fre). [Google Scholar]
- 26.Samejima M, Sibaoka T. Membrane potentials and resistance of excitable cells in the petiole and main pulvinus of Mimosa pudica. Plant Cell Physiology. 1982;23:459–465. [Google Scholar]
- 27.Weintraub M. Leaf movements in Mimosa pudica L. New Phytologist. 1951;50:357–382. [Google Scholar]
- 28.Asprey GF, Palmer JH. A new interpretation of the mechanics of pulvinar movement. Nature. 1955;175:1122. [Google Scholar]
- 29.Gorton HL. Water relation in pulvini from Samanea saman. 2. Effects of excision of motor tissues. Plant Physiol. 1987;83:945–950. doi: 10.1104/pp.83.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roblin G, Fleurate-Lessard P. Redistribution of potassium, chloride and calcium during the gravitropically induced movement of Mimosa pudica pulvinus. Planta. 1987;170:242–248. doi: 10.1007/BF00397894. [DOI] [PubMed] [Google Scholar]
- 31.Satter RL. Leaf movements: an overview of the field. In: Satter RL, Gorton HL, Vogelmann TC, editors. The Pulvinus: Motor Organ for Leaf Movement 1–9. Rockville, Maryland: The American Society of Plant Physiologists; 1990. [Google Scholar]
- 32.Kumon K, Tsurumi S. Ion efflux from pulvinar cells during slow downward movement of the petiole of Mimosa pudica L. induced by photostimulation. J Plant Physiol. 1984;115:439–443. doi: 10.1016/S0176-1617(84)80043-7. [DOI] [PubMed] [Google Scholar]
- 33.Volkov AG. Electrophysiology and Phototropism. In: Balushka F, Manusco S, Volkman D, editors. Communication in Plants. Berlin: Springer: Neuronal Aspects of Plant Life; 2006. pp. 351–367. [Google Scholar]
- 34.Volkov AG, editor. Plant Electrophysiology. Berlin: Springer; 2006. [Google Scholar]
- 35.Volkov AG, Foster JC, Ashby TA, Walker RK, Johnson JJ, Markin VS. Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environm. 2010;33:163–173. doi: 10.1111/j.1365-3040.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 36.Volkov AG, Foster JC, Markin VS. (2010b) Signal transduction in Mimosa pudica: Biologically closed electrical circuits. Plant, Cell Environm. 1984;33:816–827. doi: 10.1111/j.1365-3040.2009.02108.x. [DOI] [PubMed] [Google Scholar]
- 37.Markin VS, Volkov AG, Jovanov E. Active movements in plants: mechanism of trap closure by Dionaea muscipula Ellis. Plant Signaling & Behavior. 2008;3:778–783. doi: 10.4161/psb.3.10.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkov AG, Brown CL. Nanodevices in Nature. In: Kumar CSSR, editor. Nanodevices for Life Sciences. Weinheim: Wiley-VCH; 2006. pp. 440–463. [Google Scholar]
- 39.Nagumo J, Arimoto S, Yoshizawa S. An active pulse transmission line simulating nerve axon. Proc IRE. 1962;50:2061–2070. [Google Scholar]
- 40.Volkov AG. Green plants: Electrochemical interfaces. J Electroanal Chem. 2000;483:150–156. [Google Scholar]
- 41.Volkov AG, Carrell H, Markin VS. Biologically closed electrical circuits in Venus flytrap. Plant Physiol. 2009;149:1661–1667. doi: 10.1104/pp.108.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang MIN, Willison JHM. Electrical impedance analysis in plant tissues: Impedance measurements in leaves. J Exp Bot. 1993;44:1369–1375. [Google Scholar]
- 43.Volkov AG, Adesina T, Markin VS, Jovanov E. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal Behav. 2007;2:139–144. doi: 10.4161/psb.2.3.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146:694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkov AG, Carrell H, Baldwin A, Markin VS. Electrical memory in Venus flytrap. Bioelectrochem. 2009;75:142–147. doi: 10.1016/j.bioelechem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Jonas H. Oscillations and movements of Mimosa leave due to electric shock. J Interdisciplinary Cycle Research. 1970;1:335–348. [Google Scholar]
- 47.Moran N. Osmoregulation of leaf motor cells. FEBS Lett. 2007;581:2337–2347. doi: 10.1016/j.febslet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Bertholon M. De l'electricite des vegetaux: ouvrage dans lequel on traite de l'electricite de l'atmosphere sur les plantes, de ses effets sur leconomie des vegetaux, de leurs vertus medico. Paris: Didot Jeune PF; 1783. (Fre). [Google Scholar]
- 49.Bose JC. The Nervous Mechanism of Plants. London: Longmans Green; 1926. [Google Scholar]
- 50.Bose JC. The Motor Mechanism of Plants. London: Longmans Green; 1928. [Google Scholar]
- 51.Haberlandt G. Das reisleitende Gewebesystem der Sinnpflanse. Leipzig: Engelmann; 1890. (Ger). [Google Scholar]
- 52.Abe T. Chloride ion efflux during an action potential in the main pulvinus of Mimosa pudica. Bot Mag. 1981;94:379–383. [Google Scholar]
- 53.Stoeckel H, Takeda K. Plasmalemmal, voltage-dependent ionic currents from excitable pulvinar motor cells of Mimosa pudica. J Membr Biol. 1993;131:179–192. doi: 10.1007/BF02260107. [DOI] [PubMed] [Google Scholar]
- 54.Volkov AG, Coopwood KJ, Markin VS. Inhibition of the Dionaea muscipula Ellis trap closure by ion and water channels blockers and uncouplers. Plant Sci. 2008;175:642–649. [Google Scholar]
- 55.Mayer WE, Flash D, Raju MVS, Starrach N, Wiech E. Mechanics of circadian pulvini movements in Phaseolus coccineus L. Shape and arrangement of motor cells, micellation of motor cell walls and bulk moduli of extensibility. Planta. 1985;163:381–390. doi: 10.1007/BF00395147. [DOI] [PubMed] [Google Scholar]
- 56.Tamiya T, Miyasaki T, Ishikawa H, Iruguchi N, Maki T, Matsumoto JJ, Tsuchiya T. Movement of water in conjunction with plant movement visualized by NMR imaging. J Biochem. 1988;104:5–8. doi: 10.1093/oxfordjournals.jbchem.a122421. [DOI] [PubMed] [Google Scholar]
- 57.Fleurat-Lessard P, Bouche-Pillion S, Leloup C, Bonnemain J. Distribution and activity of the plasma membrane H+-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol. 1997;114:827–834. doi: 10.1104/pp.114.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleurat-Lessard P, Roblin G. Comparative histology of the petiole and the main pulvinus in Mimosa pudica L. Annales Botany. 1982;50:83–92. [Google Scholar]
- 59.Ksenzhek OS, Volkov AG. Plant Energetics. San Diego: Academic Press; 1998. [Google Scholar]