Abstract
The soil phytopathogen Agrobacterium has the unique ability to introduce single-stranded transferred DNA (T-DNA) from its tumor-inducing (Ti) plasmid into the host cell in a process known as horizontal gene transfer. Following its entry into the host cell cytoplasm, the T-DNA associates with the bacterial virulence (Vir) E2 protein, also exported from Agrobacterium, creating the T-DNA nucleoprotein complex (T-complex), which is then translocated into the nucleus where the DNA is integrated into the host chromatin. VirE2 protects the T-DNA from the host DNase activities, packages it into a helical filament and interacts with the host proteins, one of which, VIP1, facilitates nuclear import of the T-complex and its subsequent targeting to the host chromatin. Although the VirE2 and VIP1 protein components of the T-complex are vital for its intracellular transport, they must be removed to expose the T-DNA for integration. Our recent work demonstrated that this task is aided by an host defense-related F-box protein VBF that is induced by Agrobacterium infection and that recognizes and binds VIP1. VBF destabilizes VirE2 and VIP1 in yeast and plant cells, presumably via SCF-mediated proteasomal degradation. VBF expression in and export from the Agrobacterium cell lead to increased tumorigenesis. Here, we discuss these findings in the context of the “arms race” between Agrobacterium infectivity and plant defense.
Key words: Arabidopsis, defense response, proteasomal degradation, bacterial infection, F-box protein
Agrobacterium infection of plants consists of a chain of events that usually starts in physically wounded tissue which produces Plant defense pathways subverted by Agrobacterium for genetic transformation small phenolic molecules, such as acetosyringone (AS).1 These phenolics serve as chemotactic agents and activating signals for the virulence (vir) gene region of the Ti plasmid.2,3 The vir gene products then process the T-DNA region of the Ti plasmid to a single-stranded DNA molecule that is exported with several Vir proteins into the host cell cytoplasm, in which it forms a the T-DNA nucleoprotein complex (T-complex).4,5 The plant responds to the coming invasion by expressing and activating several defense-related proteins,5 such as VBF6 and VIP1,7 aimed at suppressing the pathogen. However, the Agrobacterium has evolved mechanisms to take advantage of these host defense proteins.8 Some of the unique strategies for achieving this goal include (1) the use of VIP1 to bind the T-complex—via the VIP1 interaction with the T-DNA packaging protein VirE2,9,10—and assist its nuclear import7 and chromatin targeting,11 and (2) the use of VBF to mark VIP1 and its associated VirE2 for proteasomal degradation, presumably for uncoating the T-complex prior to the T-DNA integration into the plant genome.6,12 Here, we examine these subversion strategies in the context of “arms race” between Agrobacterium and plants.
Agrobacterium, VIP1 and MAPK-Mediated Defense Response
While Agrobacterium is aiming to transfer its T-DNA into the host, the plant is trying to suppress this invasion. One of the key host factors in Agrobacterium-mediated genetic transformation is the plant VIP1 protein.7 VIP1 is a defense-related transcription factor involved in the control of the plant pathogenesis-related protein 1 (PR1).13 After the plant recognizes microbes via their pathogen-associated molecular patterns (PAMPs), it responds by activating mitogen-activated protein kinases (MAPK), such as MPK3. VIP1, which is located in the cell cytoplasm, serves as a substrate for MPK3 and VIP1 phosphorylation results in its translocation into the plant cell nucleus14 where it activates the PR1 transcription. Agrobacterium takes advantage of this process by associating VirE2 with VIP1, thereby allowing the T-complex to hitch a ride into the nucleus.7 Since VIP1 is a transcription factor, it has a certain affinity to the plant chromatin. This property is also exploited by Agrobacterium, which uses VIP1 to target the T-complex not only through the nuclear pore, but also to the host chromatin.11 At this point however, the presence of VIP1 as well as its associated VirE2 on the T-DNA becomes a burden because the T-DNA molecule needs to be exposed for integration into the plant genome to occur. Agrobacterium achieves this goal also by subverting a host defense pathway, mediated by the plant F-box protein, VBF.6
Agrobacterium Activates the Host SCFVBF Proteasomal Degradation Pathway
Challenge of plants by bacteria, including Agrobacterium, results in transcriptional activation of an F-box protein, termed VBF. VBF can recognize, interact with and target for degradation VIP1 by the SCFVBF pathway for proteasomal degradation.6 Thus, VBF likely functions in the VIP1/PR1 pathway by reducing the amounts of VIP1, thereby negatively regulating this defense response. But what happens if VIP1 is associated with VirE2? In this case, VBF can destabilize both proteins.6 Agrobacterium takes advantage of this ability of VBF, most likely, using it to destabilize the protein components of the T-complex and uncoat the T-DNA.
If such uncoating takes place after the T-complex has been targeted by VIP1 to the host chromatin, the T-DNA becomes available for integration, which most likely occurs in the naturally occurring double-stranded breaks in the plant genome.15,16 If, however, the uncoating happens before the T-complex reaches the target chromatin, the integration would not occur and the T-DNA most likely will be expressed only transiently and then destroyed by cellular nucleases. Whether or not there exists a mechanism to favor destabilization of VIP1 and, by implication uncoating of the T-complex, at the chromatin remains unknown. Interestingly, however, it is a long-standing observation in Agrobacterium biology that most of the T-DNA molecules are expressed transiently,17 and only a fraction of them become integrated. Potentially, the lack of synchronization between chromatin targeting and proteasomal uncoating of the T-complex may underly this disparity between levels of transient and stable T-DNA expression.
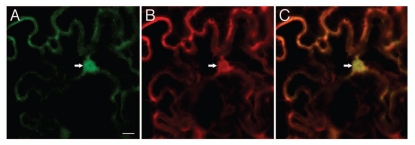
Another interesting aspect of VBF activity is its subcellular localization. Figure 1 shows that GFP-tagged VBF is localized not only in the cell nucleus, but also in the cytoplasm, colocalizing with co-expressed free DsRed2, which is known to label both cellular compartments.18,19 This is in contrast to the localization of the VBF-VIP1 interaction, which is detected almost exclusively in the nucleus.6 Several possible explanations can be offered. VBF may simply play a role in other, cytoplasmic, processes which also include proteasomal degradation; in this case, VBF should have other specific targets in addition to VIP1. Or, by analogy to VIP1, VBF nuclear import may be enhanced during defense response by as yet unknown mechanism.
Figure 1.
Nucleocytoplasmic localization of VBF. GFP-tagged VBF was transiently coexpressed with free DsRed2 in Nicotiana benthamiana epidermis following microbombardment. (A) GFP-VBF. (B) DsRed2. (C) Merged image. GFP signal is in green, DsRed2 signal is in red and overlapping GFP and DsRed2 signals are in yellow. Arrow indicates the cell nucleus. All images are single confocal sections. Bar = 10 µm.
Finally, it is important to note that at least some of the activity of both VIP1 and VBF may have been acquired, probably via convergent evolution,20 by those Agrobacterium Vir effector proteins that are exported into the host cell; specifically, VirE3 functionally mimics VIP1 in the T-complex nuclear import,21 and VirF acts similarly to VBF in the T-complex uncoating.19 Thus, VirE3 and VirF may have evolved as a “backup” system for the pathogen to ensure the completion of the critical stages of the infection process.
A Model for the Roles of Host Defense Factors in Nuclear and Intranuclear Transport and Proteasomal Uncoating of the T-Complex
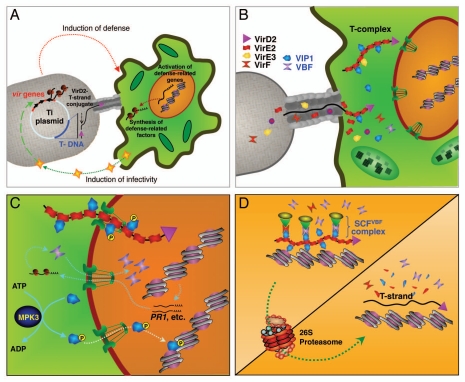
Agrobacterium infection begins with the recognition of the wounded host and attachment of the bacterial cell to the wounded tissue (Fig. 2A). During this first stage, the bacterium and plant cell are sensing each other and responding to each other. In the Agrobacterium cell, this response includes activation of the vir genes and processing the T-DNA for export, i.e., generation of the single-stranded (ss) mobile T-DNA copy (T-strand), mostly by the VirD2 endonuclease, which remains covalently attached to the 5′ end of the T-strand.22 In the plant cell, the pathogen attack triggers different defense responses, such as systemic acquired resistance (SAR)23 (Fig. 2A), which include activation of defense-related genes and synthesis of their protein products, such as VBF (Fig. 2B).
Figure 2.
Interplay between the bacterial virulence and host defense systems during nuclear import, chromatin targeting and proteasomal uncoating of the Agrobacterium T-complex. (A) Activation of infectivity and defense. (B) Transfer of the T-complex and Vir effectors into the host cell. (C) Nuclear import of the T-complex via MPK3-depenent nuclear import of VIP1. (D) Chromatin targeting and proteasomal uncoating of the T-complex by VIP1 and VBF. For details, see text.
Next, the T-strand is transferred into the host cell (Fig. 2B). At the same time and most likely through the same channel, Agrobacterium transfers also several of its Vir proteins, such as VirE2, VirE3 and VirF, into the host cell cytoplasm.21,24 There, VirE2 packages the T-strand into a helical T-complex,9,10 protecting it from cellular nucleases25 and setting stage for it its nuclear import. To this end, VirE2 interacts with the cytoplasmic population of VIP1 (Fig. 2B).
Meanwhile, the activated plant defense employs MPK3 to phosphorylate VIP1, thereby inducing its nuclear import for subsequent induction of expression of the PR1 gene14 as well as other stress genes13 by the nuclear VIP1 (Fig. 2C). Agrobacterium hijacks this pathway and utilizes it to bring the T-complex into the plant cell nucleus (Fig. 2C). Furthermore, following nuclear import, VIP1 is also likely utilized to target the T-complex to the plant chromatin.11,26,27 This is made possible by attaching the T-complex to VIP1 via VirE2 and formation of such VIP1-VirE2-ssDNA and VIP1-VirE2-ssDNA-nucleosome complexes has been demonstrated in vitro.11
As the defense reaction proceeds, there arises a need for its control which likely involves degradation of VIP1 by the 26S proteasome via the SCFVBF pathway.6 Agrobacterium, again, hijacks this pathway to remove VIP1 and VirE2 from the T-complex and expose its T-strand molecule for integration.6 This subversion of plant defense is again based on the interaction between VirE2 and VIP1, such that when VIP1 is recognized by VBF and marked for degradation by the SCFVBF complex, its associated VirE2 is also degraded. That VIP1 associated with VirE2 at the T-complex is situated ectopically, i.e., not at his natural response elements in the plant genome,13 may further enhance or even trigger this degradation.
Acknowledgements
The work our laboratory is supported by grants from NIH, NSF, USDA/NIFA, BARD, and BSF to V.C.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12947
References
- 1.Bhattacharya A, Sood P, Citovsky V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol. 2010;11:705–719. doi: 10.1111/j.1364-3703.2010.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimoda N, Toyoda-Yamamoto A, Nagamine J, Usami S, Katayama M, Sakagami Y, et al. Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proc Nat Acad Sci USA. 1990;87:6684–6688. doi: 10.1073/pnas.87.17.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashby AM, Watson MD, Loake GJ, Shaw CH. Ti plasmid-specified chemotaxis of Agrobacterium tumefaciens C58C1 toward vir-inducing phenolic compounds and soluble factors from monocotyledonous and dicotyledonous plants. J Bacteriol. 1988;170:4181–4187. doi: 10.1128/jb.170.9.4181-4187.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citovsky V, Kozlovsky SV, Lacroix B, Zaltsman A, Dafny-Yelin M, Vyas S, et al. Biological systems of the host cell involved in Agrobacterium infection. Cell Microbiol. 2007;9:9–20. doi: 10.1111/j.1462-5822.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 5.Gelvin SB. Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu Rev Phytopathol. 2010;48:45–68. doi: 10.1146/annurev-phyto-080508-081852. [DOI] [PubMed] [Google Scholar]
- 6.Zaltsman A, Krichevsky A, Loyter A, Citovsky V. Agrobacterium induces expression of a plant host F-box protein required for tumorigenicity. Cell Host Microbe. 2010;7:197–209. doi: 10.1016/j.chom.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzfira T, Vaidya M, Citovsky V. VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J. 2001;20:3596–35607. doi: 10.1093/emboj/20.13.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Kong X, Pan J, Li D. VIP1: linking Agrobacterium-mediated transformation to plant immunity? Plant Cell Rep. 2010;29:805–812. doi: 10.1007/s00299-010-0870-4. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Arish A, Frenkiel-Krispin D, Fricke T, Tzfira T, Citovsky V, Wolf SG, et al. Three-dimensional reconstruction of Agrobacterium VirE2 protein with single-stranded DNA. J Biol Chem. 2004;279:25359–25363. doi: 10.1074/jbc.M401804200. [DOI] [PubMed] [Google Scholar]
- 10.Citovsky V, Guralnick B, Simon MN, Wall JS. The molecular structure of Agrobacterium VirE2-single stranded DNA complexes involved in nuclear import. J Mol Biol. 1997;271:718–727. doi: 10.1006/jmbi.1997.1230. [DOI] [PubMed] [Google Scholar]
- 11.Lacroix B, Loyter A, Citovsky V. Association of the Agrobacterium T-DNA-protein complex with plant nucleosomes. Proc Natl Acad Sci USA. 2008;105:15429–15434. doi: 10.1073/pnas.0805641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citovsky V, Zaltsman A, Kozlovsky SV, Gafni Y, Krichevsky A. Proteasomal degradation in plant-pathogen interactions. Semin Cell Dev Biol. 2009;20:1048–1054. doi: 10.1016/j.semcdb.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Pitzschke A, Djamei A, Teige M, Hirt H. VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc Nat Acad Sci USA. 2009;106:18414–18419. doi: 10.1073/pnas.0905599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djamei A, Pitzschke A, Nakagami H, Rajh I, Hirt H. Trojan horse strategy in Agrobacterium transformation: abusing MAPK defense signaling. Science. 2007;318:453–456. doi: 10.1126/science.1148110. [DOI] [PubMed] [Google Scholar]
- 15.Tzfira T, Frankmen L, Vaidya M, Citovsky V. Site-specific integration of Agrobacterium T-DNA via double-stranded intermediates. Plant Physiol. 2003;133:1011–1023. doi: 10.1104/pp.103.032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chilton MD, Que Q. Targeted integration of T-DNA into the tobacco genome at double-strand breaks: new insights on the mechanism of T-DNA integration. Plant Physiol. 2003;133:956–965. doi: 10.1104/pp.103.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen BJ, Gardner RC. Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol. 1990;14:61–72. doi: 10.1007/BF00015655. [DOI] [PubMed] [Google Scholar]
- 18.Goodin MM, Dietzgen RG, Schichnes D, Ruzin S, Jackson AO. pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 2002;31:375–383. doi: 10.1046/j.1365-313x.2002.01360.x. [DOI] [PubMed] [Google Scholar]
- 19.Tzfira T, Vaidya M, Citovsky V. Involvement of targeted proteolysis in plant genetic transformation by Agrobacterium. Nature. 2004;431:87–92. doi: 10.1038/nature02857. [DOI] [PubMed] [Google Scholar]
- 20.Nagai H, Roy CR. Show me the substrates: modulation of host cell function by type IV secretion systems. Cell Microbiol. 2003;5:373–383. doi: 10.1046/j.1462-5822.2003.00285.x. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix B, Vaidya M, Tzfira T, Citovsky V. The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J. 2005;24:428–437. doi: 10.1038/sj.emboj.7600524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zupan J, Muth TR, Draper O, Zambryski PC. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 2000;23:11–28. doi: 10.1046/j.1365-313x.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 23.He SY. Elicitation of plant hypersensitive response by bacteria. Plant Physiol. 1996;112:865–869. doi: 10.1104/pp.112.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergunst AC, Schrammeijer B, den Dulk-Ras A, de Vlaam CMT, Regensburg-Tuink TJ, Hooykaas PJJ. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science. 2000;290:979–982. doi: 10.1126/science.290.5493.979. [DOI] [PubMed] [Google Scholar]
- 25.Citovsky V, Wong ML, Zambryski PC. Cooperative interaction of Agrobacterium VirE2 protein with single stranded DNA: implications for the T-DNA transfer process. Proc Natl Acad Sci USA. 1989;86:1193–1197. doi: 10.1073/pnas.86.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Krichevsky A, Vaidya M, Tzfira T, Citovsky V. Uncoupling of the functions of the Arabidopsis VIP1 protein in transient and stable plant genetic transformation by Agrobacterium. Proc Natl Acad Sci USA. 2005;102:5733–5738. doi: 10.1073/pnas.0404118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loyter A, Rosenbluh J, Zakai N, Li J, Kozlovsky SV, Tzfira T, et al. The plant VirE2 interacting protein 1. A molecular link between the Agrobacterium T-complex and the host cell chromatin? Plant Physiol. 2005;138:1318–1321. doi: 10.1104/pp.105.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]