Abstract
Local, efflux-dependent auxin gradients and maxima mediate organ and tissue development in plants. The auxin-efflux pattern is regulated by dynamic expression and asymmetric subcellular localization of PIN auxin-efflux proteins during plant organogenesis. Thus, the question of how the expression and subcellular localization of PIN proteins are controlled goes to the heart of plant development. It has been shown that PIN expression and polarity are established not only through a self-organizing auxin-mediated polarization mechanism, but also through other means such as cell-fate determination. We found that the Arabidopsis NO VEIN (NOV) gene, encoding a novel, plant-specific nuclear factor, is required for leaf vascular development, cellular patterning and stem-cell maintenance in the root meristem and cotyledon outgrowth and separation. NOV function underlies cell-fate decisions associated with auxin gradients and maxima, thereby establishing cell type-specific PIN expression and polarity. We propose that NOV mediates cell acquisition of the competence to undergo auxin-dependent coordinated cell specification and patterning, thereby educing context-dependent auxin-mediated developmental responses.
Key words: Arabidopsis, auxin, PIN, organ development, vascular development, stem-cell maintenance, NO VEIN
In plants, local auxin gradients associated with auxin maxima mediate coordinated cell specification and patterning in the root,1–3 lateral organ,4–6 embryo7–9 and vascular tissue.10–13 A central factor in the formation of auxin concentration gradients and maxima is polar auxin transport, which is defined by cell type-specific expression and asymmetric subcellular localization of the PIN family of auxin-efflux proteins.14,15 Reciprocally, auxin can induce changes in PIN localization16,17 and expression18,19 under the influence of cell fate. Therefore, PIN expression and polarity are established not only through the self-organizing auxin-mediated feedback mechanism, but also through cell-fate determination. However, the molecular mechanism regulating PIN expression and polarity remains largely unknown.
As a model system to study auxin-mediated polarized development, we have genetically analyzed vascular development in Arabidopsis. The no vein-1 (nov-1) mutant was identified among Arabidopsis mutants defective in leaf vascular development. We found that the Arabidopsis NOV gene is required for leaf vascular development, cellular patterning and stem-cell maintenance in the root meristem and cotyledon outgrowth and separation.20 nov mutations affect many aspects of auxin-dependent development without directly affecting auxin perception. NOV encodes a novel, plant-specific nuclear factor expressed in developing embryos, leaf primordia, lateral-root primordia and the meristematic regions of shoots and roots.20 Here we present additional data on cell specification defects in nov-1 roots, further supporting that NOV function underlies cell-fate decisions associated with auxin gradients and maxima, thus establishing cell-type-specific PIN expression and polarity.
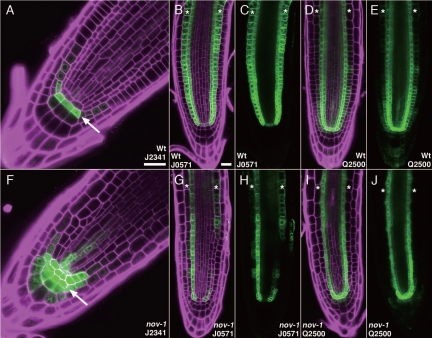
In wild type, PIN3, PIN4 and PIN7 proteins exhibit differential expression patterns in the columella root-cap cells. The first tier of columella cells (columella stem cells) express PIN4, the second tier of columella cells expresses PIN3, PIN4 and PIN7 and the third tier expresses PIN3 and PIN7.2,3,20,21 In nov-1 columella cells, while expression of PIN3 and PIN7 was decreased or almost absent, PIN4 expression was expanded to the third tier of columella cells,20 suggesting that the differential expression of the PIN proteins is disrupted in nov-1. Cell specification defects are also seen in columella cells of nov mutants. In nov-1 and nov-3, the first tier columella stem cells contain starch granules, which in wild type are usually absent, suggesting that fate of the first tier columella stem cells is not maintained in nov-1 and nov-3.20 On the other hand, the columella stem-cell marker J2341 is ectopically expressed in the second and third tiers of nov-1 columella cells (cf. Fig. 1F with A), suggesting that these cells adopt at least some traits of first tier columella cells. Loss of PIN3 and PIN7 expression in the second and third tiers of columella cells and expansion of PIN4 expression to the third tier in nov-1 fit well with the idea that the second and third tiers of columella cells adopt the first tier traits in nov-1. These data suggest that the first to third tiers of nov-1 columella cells adopt mixed cell fates and that NOV is required for establishing both cell fate and PIN expression pattern in columella root cap cells.
Figure 1.
Cell-marker expression in wild-type and nov-1 root tips. (A and F) Expression of the columella initials marker J2341. In wild-type roots (A), J2341 is expressed strongly in the first tier of columella cells and very weakly in the quiescent center and other initials. In nov-1 roots (F), J2341 expression encompasses the first to third tiers of columella cells, the quiescent center and cortex/endodermis initial cells. (B, C, G and H) Expression of the ground-tissue marker J0571. In wild-type roots (B and C), J0571 is expressed in the cortex and endodermis and weakly in the quiescent center and cortex/endodermis initials. In nov-1 roots (G and H), J0571 expression is disrupted in the cortex and occasionally perturbed in the endodermis. (D, E, I and J) Expression of Q2500. In wild-type roots (D and E), Q2500 is expressed mainly in the endodermis, weakly in the pericycle and in the epidermis and cortex closer to the root stem-cell niche. In nov-1 roots (I and J), Q2500 expression is also disrupted in the epidermis and cortex. Seedlings used were vertically grown on the surface of 1.5% agar plates for 5 (B, C, G and H) and 7 (A, D–F, I and J) days. The reporter GFP expression (green) is shown with (magenta; A, B, D, F, G and I) and without (C, E, H and J) propidium iodide staining for cell boundary. Arrows in (A) and (F) indicate positions of the first tiers of columella cells. Asterisks in (B–E) and (G–J) mark positions of the cortex cell files. Scale bars = 20 µm [equal scale in (A and B) and in (B–E and G–J), respectively].
In wild-type root tips, PIN2 is polarized apically in the epidermis and basally in the cortex.22 In nov-1, PIN2 polarity in the cortex was not basal, but either apical or non-polar.20 In nov mutants, root cortex cells also have cell specification defects. In seedlings of nov-1 and embryos of nov-2, -3, -4 and -5, cortex/endodermis stem cells often undergo premature periclinal division without prior anticlinal division and are thus not maintained as stem cells.20 In nov-1 roots, expression of the ground-tissue marker J0571 is disrupted in the cortex and occasionally perturbed in the endodermis (cf. Fig. 1G and H with 1B and C) and Q2500 expression is also disrupted in the cortex (cf. Fig. 1I and J with 1D and E), suggesting that nov-1 root cortex cells lose some traits of the cortex. These indicate that NOV is required for establishing both cell fate and PIN2 polarity in root cortex cells.
Collectively, our data suggest that the NOV indirectly regulates expression and polarity of PIN proteins through mechanisms that include the determination and/or stabilization of cell fate in the root meristem.20 We have also shown that NOV is required for provascular PIN1 expression and region-specific expression of PIN7 in leaf primordia, that NOV helps cells to acquire and maintain their ability to differentiate into vascular cells in response to auxin, that NOV is required for normal cellular organization and stem-cell maintenance in the root stem-cell niche, that NOV has an important role in auxin-mediated embryonic development, and that NOV encodes a previously undescribed plant-specific nuclear factor specifically expressed in developing organs and tissues.20 Together with the data presented in this report, we suggest that NOV function underlies cell-fate decisions associated with auxin gradients and maxima, thus establishing PIN expression and polarity and auxin-mediated development. We propose that NOV is a novel competence factor mediating cell acquisition of competence to undergo auxin-dependent coordinated cell specification and patterning, thereby educing context-dependent developmental responses. Future studies on NOV may shed new light on the fundamental mechanisms by which auxin regulates the formation of plant organs and tissues, regardless of their fate and origin.
Acknowledgements
We thank Jim Haseloff for making GFP marker lines J0571, J2341 and Q2500 available in the Arabidopsis Biological Resource Center, William Teale for critically reading the manuscript and Shiho Terada for technical assistance. This work was supported in part by Grant-in-Aid for Scientific Research (21657014 to R.T.) from the Japan Society for the Promotion of Science, by Grant-in-Aid for Creative Scientific Research (19GS0315 to K.O. and R.T.) and Grant-in-Aid for Scientific Research on Priority Areas (19060004 to K.O.) from the Ministry of Education, Culture, Sports, Science and Technology, by the Core Research for Evolutional Science and Technology program of the Japan Science and Technology Agency to K.O.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12948
References
- 1.Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 2.Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 3.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová E, Jürgens G, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 6.Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 7.Liu Cm, Xu Zh, Chua NH. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell. 1993;5:621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, et al. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- 9.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 10.Sachs T. Cell polarity and tissue patterning in plants. Development. 1991;1:83–93. [Google Scholar]
- 11.Mattsson J, Sung ZR, Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- 12.Sieburth LE. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol. 1999;121:1179–1190. doi: 10.1104/pp.121.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattsson J, Ckurshumova W, Berleth T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003;131:1327–1339. doi: 10.1104/pp.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 15.Wisniewska J, Xu J, Seifertová D, Brewer PB, Ruzicka K, Blilou I, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 16.Paciorek T, Zazímalová E, Ruthardt N, Petrásek J, Stierhof YD, Kleine-Vehn J, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 17.Sauer M, Balla J, Luschnig C, Wisniewska J, Reinöhl V, Friml J, et al. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006;20:2902–2911. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieten A, Vanneste S, Wisniewska J, Benková E, Benjamins R, Beeckman T, et al. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;132:4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Hofhuis H, Heidstra R, Sauer M, Friml J, Scheres B. A molecular framework for plant regeneration. Science. 2006;311:385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]
- 20.Tsugeki R, Ditengou FA, Sumi Y, Teale W, Palme K, Okada K. NO VEIN mediates auxin-dependent specification and patterning in the Arabidopsis embryo, shoot and root. Plant Cell. 2009;21:3133–3151. doi: 10.1105/tpc.109.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci. 2005;10:170–177. doi: 10.1016/j.tplants.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz P, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]