Abstract
Acyl hydrolases remodel biological membranes and release signaling molecules in response to a variety of biotic and abiotic stresses. After wounding or pathogen treatment lipases are necessary to release fatty acids as substrate for jasmonate biosynthesis. In osmotic stressed tissue they maintain integrity and functionality of membranes and during senescence lipases destroy and recycle membranes. Recently the role of several acyl hydrolases including DEFECTIVE IN ANTHER DEHISCENCE1 (DAD1) and DAD1-like lipase, e.g., DONGLE (DGL) and the phospholipase A (PLA) PLA-Iγ1 in jasmonate biosynthesis after wounding were investigated and functional redundancy within this family has been stated. Here we report necessity of diverse DAD1-like lipases in response to salt and sorbitol treatment. The lipase PLA-Iγ1 and PLA-Iβ2, which were both impaired in wound response, were also affected in response to osmotic stress in seed germination assays. Based on our observations and interpretations of transcription analyses generated by AtGenExpress project we speculate about more general roles of the DAD1-like lipase in diverse biological processes.
Key words: osmotic stress, salt stress, lipases, DAD1, Arabidopsis
Introduction
Osmotic stress, caused by drought, freezing or high salt affects integrity and functionality of membranes. To survive and adapt to osmotic stress plants use a range of biochemical and developmental changes including remodeling of lipid composition and activation of a variety of phospholipid-based signaling pathways.1 Wounding and pathogen attack destroys membranes and free fatty acids were released. Unsaturated acids, namely linolenic acid derived from chloroplast membranes, act as precursor for bioactive oxylipins including Jasmonic acid (JA). JA is an important signaling compound that regulates developmental processes including root growth, pollen development, abscission and senescence. In addition, JA also mediates the response to wounding, pathogen attack and UV irradiation.2 All those processes include action of one or more galactolipases or phospholipases A (PLA) to generate lysolipids and free fatty acids, which both have been reported to act as signaling molecules by themselves.3–5
Recently, we described different Arabidopsis mutant lines of PLA with respect to changes in oxylipin biosynthesis after wounding and treatment with the avirulent Pseudomonas strain DC3000(avrRPM1). Using six single and one quadruple knockout mutants it was shown that the lipases PLA-Iα1 (DGL, At1g05800), PLA-Iβ1 (DAD1, At2g44810), PLA-Iβ2 (At4g16820), PLA-Iγ1 (At1g06800), PLA-Iγ2 (At2g305509) and PLA-Iγ3 (At1g51440) act in a functional redundant manner for basal JA biosynthesis as well as after wounding. And to make the picture more complex, still unidentified lipolytic proteins seem to be necessary in addition.6 After inoculation of leaves with Pseudomonas strain DC3000(avrRPM1) only minor differences in JA levels between pla-Iα1 knock down mutant lines and wild type and no differences in bacterial growth between the mutant and wild type could be detected. Nevertheless, it was found that the development of infection symptoms and the production of reactive oxygen species were significant retarded.
Results and Discussion
In addition to wounding and pathogen treatment, results of transcription analyses generated using the microarray data from the AtGenExpress project7,8 (summarized in Table 1, for more details please see www.weigelworld.org/resources/microarray/AtGenExpress) suggested that transcription levels of DAD-1-like lipases change also after osmotic stress and during senescence. The role of PLA-Iα2 in senescence was recently reported.9 To test necessity of the lipases PLA-Iα1 (DGL), PLA-Iβ2, PLA-Iγ1, PLA-Iγ2 and PLA-Iγ3 in response to osmotic stress semiquantitative transcription analyses and germination studies of mutant lines were performed.
Table 1.
Summary of transcription analyses generated using the microarray data from the AtGenExpress project
| Abiotic stress | PLA-Iα1 (At1g05800) 261278_at | PLA-Iα2 (At2g31690) 263451_at | PLA-Iβ2 (At4g16820) 245447_at | PLA-Iγ1 (At1g06800) 260833_at | PLA-Iγ2 (At2g30550) 267496_at | PLA-Iγ3 (At1g51440) 260491_at | |
| 150 mM NaCl | root | ++ | ++ | ++ | + | + | + |
| shoot | ○ | + | + | + | + | −− | |
| 300 mM Mannitol | root | + | + | + | + | + | + |
| shoot | + | − | + | ++ | ++ | − | |
| Wounding 30 min | root | ○ | + | ○ | ○ | ○ | ○ |
| shoot | ○ | − | ○ | ○ | ○ | ○ | |
| Biotic stress | |||||||
| B. cinerea 48 hpi | + | + | + | + | ++ | − | |
| Pst (DC3000) 24 hpi | ++ | ○ | ○ | ○ | ++ | − | |
| Pst (avrRpm1) 24 hpi | ++ | + | + | + | ++ | − | |
| P. infestans 24 hpi | ○ | − | ++ | ++ | + | − | |
| Organ | |||||||
| senescent leaf | + | + | ○ | ○ | ++ | ○ | |
| flower stage 15 | − | ○ | ○ | ○ | ++ | ++ | |
More than two-fold induction is indicated by ++, 0.5- to 2-fold induction by +, no significant changes with ○, 0.5- to 2-fold repression with − and more than two fold repression with −−. For description of microarray experiments see: www.weigelworld.org/resources/microarray/AtGenExpress.
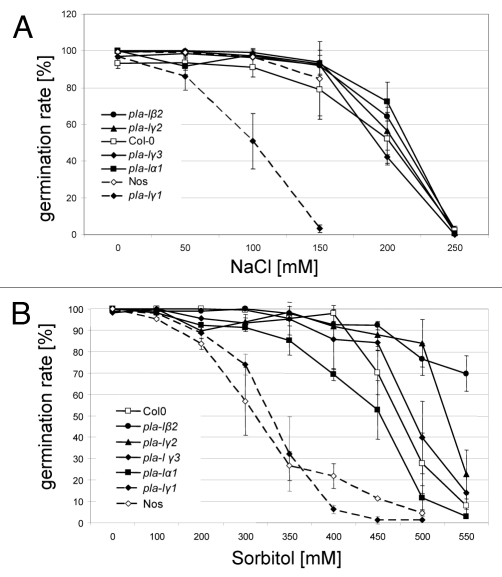
In contrast to the results of AtGenExpress, no rise in transcript level one or two hours after 150 mM sodium chloride (NaCl) treatment of two-week old seedlings could be detected when using semiquantitative reverse transcription-PCR experiments. After treatment with 350 mM Sorbitol an induction in transcript levels could be confirmed for genes PLA-Iα1 (DGL), PLA-Iβ2, PLA-Iγ1 and PLA-Iγ2 (data not shown). We used Sorbitol as osmolyt to avoid secondary effects due to toxicity of high Mannitol concentrations.10 For germination studies sterile seeds of the four knock out mutant lines pla-Iβ2, pla-Iγ1, pla-Iγ2 and pla-Iγ3 and the knock down line pla-Iα1 8-1 (for detailed description of mutant lines please see Ellinger et al.6) were transferred to 1/2-MS media supplemented either with different concentrations of NaCl or Sorbitol. Col-0 and also Nossens ecotype, background of the mutant line pla-Iγ1, serve as controls. Germination rates were determined three (Fig. 1) and five days (data not shown) after stratification.
Figure 1.
Germination rates of lipase mutant line seedlings germinated on NaCl or Sorbitol supplemented media. Sterile seedlings were plated on ½-MS media supplemented with indicated amount of NaCl or Sorbitol. Germination rates were determined on the third day after stratification. Data represent the mean of five biological replicates ± SD.
On media supplemented with rising concentration of NaCl the germination rate of mutant line pla-Iγ1 was strongly reduced. Further tested lines germinated as their corresponding wild type. Three days after stratification germination rate for mutant line pla-Iγ1 decreases to less than 50% at 100 mM NaCl while both wild types Nos and Col-0 show still above 90% germination. On media supplemented with Sorbitol mutant lines pla-Iβ2 and pla-Iγ2 germinated even better than wild type. At a concentration of 500 mM Sorbitol only 28% of Col-0 but about 78% of mutant lines pla-Iβ2 and pla-Iγ2 seeds germinated. At a concentration of 550 mM Sorbitol mutant line pla-Iβ2 had still a good germination rate of 70% when Col-0 was highly impaired (8% germination). In contrast to its different behavior under salt stress mutant line pla-Iγ1 displayed no changes to wild type, when stressed by sorbitol. This shows not only a role for pla-ly1 in coping with osmotic stress but more with salt homeostasis. Additionally growth and development of all osmotic stressed seedlings from NaCl and Sorbitol supplemented media were monitored for two more weeks but without any significant phenotypic differences between mutant and wild-type lines.
Osmotic stress response includes a variety of signaling pathways.11 Next to nutritional disorder, water-deficient stress caused by Sorbitol and salt stimulates lipolytic and peroxidative activities and inhibits lipid biosynthesis.12,13 Increase of lipolytic activity and occurrence of lipid derived signals take part in signal transduction after wounding,14 pathogen treatment15,16 or during senescence,17 too. Since maintaining the structural integrity of membranes also includes mobilization of fatty acids additional experiments are necessary to ensure exclusive role in JA biosynthesis. Therefore, it would be noteworthy to analyze the role of those five lipases using single and multiple knock out lines during senescence or infections with the pathogens listed in Table 1.
The results presented here together with those of Ellinger et al.6 show that different DAD1-like lipases are part of the various signaling pathway after wounding, pathogen stress or osmotic stress. Namely, the lipase PLA-Iγ1, plays a role after wounding as wells as during salt stress, although its role is not understood and studied in detail.
Acknowledgements
This work was supported by grants from SFB480/A-3 and the Ruhr-Universitat Research School funded by the DFG in the framework of the Excellence Initiative.
Abbreviations
- PLA
phospholipase A
- JA
Jasmonic acid
- DAD1
DEFECTIVE IN ANTHER DEHISCENCE1
- DGL
DONGLE
- NaCl
sodium chloride
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13012
References
- 1.Munnik T, Meijer HJG. Osmotic stress activates distinct lipid and MAPK signaling pathways in plants. FEBS Lett. 2001;498:172–178. doi: 10.1016/s0014-5793(01)02492-9. [DOI] [PubMed] [Google Scholar]
- 2.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HY, Bahn SC, Kang YM, Lee KH, Kim HJ, Noh EK, et al. Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell. 2003;15:1990–2002. doi: 10.1105/tpc.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaeno T, Matsuda O, Iba K. Role of chloroplast trienoic fatty acids in plant disease defence responses. Plant Journal. 2004;40:931–941. doi: 10.1111/j.1365-313X.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- 5.Yi H. In vivo evidence for the involvement of Phospholipase A and protein kinase in the signal transduction pathway for Auxin-induced corn coleoptile elongation. Physiologia Plantarum. 1996;96:359–368. [Google Scholar]
- 6.Ellinger D, Stingl N, Kubigsteltig II, Bals T, Juenger M, Pollmann S, et al. DONGLE and DEFECTIVE IN ANTHER DEHISCENCE1 lipases are not essential for wound- and pathogen-induced jasmonate biosynthesis: redundant lipases contribute to jasmonate formation. Plant Physiol. 2010;153:114–127. doi: 10.1104/pp.110.155093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, et al. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007;50:347–363. doi: 10.1111/j.1365-313X.2007.03052.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, et al. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 9.Padham AK, Hopkins MT, Wang TW, McNamara LM, Lo M, Richardson LGL, et al. Characterization of a plastid triacylglycerol lipase from Arabidopsis. Plant Physiol. 2007;143:1372–1384. doi: 10.1104/pp.106.090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todd R, Tague BW. Phosphomannose isomerase: A versatile selectable marker for Arabidopsis thaliana germ-line transformation. Plant Molecular Biology Reporter. 2001;19:307–319. [Google Scholar]
- 11.Zhu JK. Salt and Drought Stress Signal Transduction in Plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matos AR, d'Arcy-Lameta A, França M, Pêtres S, Edelman L, Kader J, et al. A novel Patatin-like gene stimulated by drought stress encodes a galactolipid acyl hydrolase. FEBS Lett. 2001;491:188–192. doi: 10.1016/s0014-5793(01)02194-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 14.Christmann A, Moes D, Himmelbach A, Yang Y, Tang Y, Grill E. Integration of abscisic acid signaling into plant responses. Plant Biol. 2006;8:314–325. doi: 10.1055/s-2006-924120. [DOI] [PubMed] [Google Scholar]
- 15.Katagiri F, Thilmony R, He SY. The Arabidopsis thaliana-Pseudomonas syringae interaction. In: Meyerowitz EM, Somerville CR, editors. The Arabidopsis Book. Rockville: American Society of Plant Biologists; 2002. pp. 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;12:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Wingler A, Roitsch T. Metabolic regulation of leaf senescence: interactions of sugar signaling with biotic and abiotic stress responses. Plant Biol. 2008;10:50–62. doi: 10.1111/j.1438-8677.2008.00086.x. [DOI] [PubMed] [Google Scholar]