Abstract
In the recent years, multiple ways of interaction between the fields of nanotechnology and biology have been opened, mainly in the biomedical research, with the development of tools for diagnosis and controlled delivery of substances.1,2 On the other hand, in the field of plant biology, the interaction between both disciplines has been less frequent. Most of the published work on this field has focus in the environmental impact of nanoparticles on crop growth and development;3,4 and also on the bio production of nanoparticles using plant extracts (reviewed in refs. 5–8). Much less attention has taken other possible aspects of the interrelationship between nanotechnology and plant biology, such as the development of nanodevices for controlled delivery of drugs or different kind of substances,9,10 in a similar way to that already developed in the medical research.
Key words: plant nanobiotechnology, phytosanitaries, nanoparticle uptake, electron microscopy, nanoparticle subcellular localization
Recently, our group has developed an approach for the application of carbon-coated iron nanoparticles to pumpkin plants. The goal of this project was the development of tools for the treatment of pathologies affecting specific areas or organs of the plants. To achieve that, nanoparticles carrying the phyto-remedy will be applied to the affected plants, and magnetic fields will be applied to the organs of the plant affected by the pathology. In this way, the nanoparticles will be retained in the affected area, and the effect of the active compound linked to the nanoparticle will be concentrated in a restricted area.
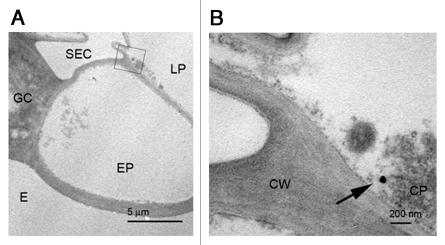
In a previous report, we described the capability of different microscopic methodologies to identify and locate nanoparticles in plant tissue samples, including both electron microscopy and light microscopy approaches.11 Using this knowledge, we used a correlative microscopy approach, identifying first the presence of nanoparticles in sections of resin-embedded tissue by light microscopy (bright field, phase contrast and dark field), followed by a further analysis of consecutive sections from the same block, this time by transmission electron microscopy. This approach allowed us to unveil many different aspects of the behavior of the nanoparticles in the living tissue. First of all, movement of the nanoparticles was detected at different levels: chains of nanoparticle-aggregates carrying cells were apparent close to the application point, when such application was made by ‘injection’ of the nanoparticle suspension into the pith cavity of the stem, suggesting the flux of nanoparticles from one cell to another. Also, after the same kind of application, nanoparticles were detected in an area close to the vascular core, but appearing as isolated particles in the cytoplasm of the cell. Furthermore, after application of the nanoparticle by ‘spray’ (that is, application of a drop of the solution over the surface of the leaf, close to the petiole insertion point), isolated nanoparticles were also detected close to the application point (Fig. 1). This last data is particularly relevant, as this application method attempted to emulate that of breeders and coordinators of phytosanitary control. The fact that the nanoparticles are capable of penetrating through the leaf cuticule and into the cell cytoplasm opens the possibility for the use of this approach in phytosanitary applications.
Figure 1.
Isolated nanoparticles localized close to the epidermis after spray application. (A) Low amplification image of the area were the nanoparticle is (squared area): SEC, sub estomatal cavity; GC, guard cell; E, exterior; LP, lagunar parenchyma; EP, epidermis. (B) Detail of the region squared in (A), showing an isolated nanoparticle (arrow). Bars in (A): 5 µm, (B): 200 nm.
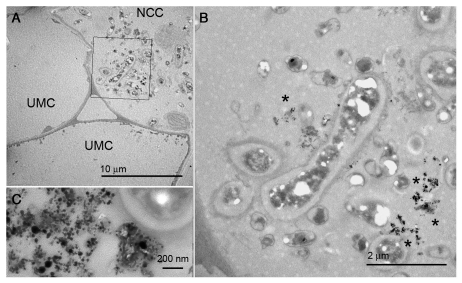
A second question of special interest in our analysis was the differential response to the presence of the nanoparticles shown by the cell cytoplasm when they appear in the form of aggregates when compared with cells carrying non-clustered nanoparticles. In fact, a dense cytoplasm with starch-containing organelles was observed concomitantly with nanoparticle aggregates in the cytosol (Fig. 2), suggesting that plant cells could respond to the presence of a high density of nanoparticles by changing their subcellular organization. On the other hand, no response was observed in those cells in which only isolated nanoparticles were detected (Fig. 1). The change on the cytoplasm of the cells was accompanied by the fact that the cell-to-cell movement of the particles in regions with a high density of aggregates seems to direct them to the exterior of the organism, what points to a physiological response from the plant to the intracellular presence of nanoparticle aggregates.
Figure 2.
Different behavior between cell cytoplasm depending of the content in nanoparticle aggregates. (A) low amplification image showing unmodified cells (UMC) without nanoparticles, next to a nanoparticle carrying cell (NCC), whose cytoplasm is full of organelles. (B) Detail of the area squared in (A), indicating the presence of nanoparticle aggregates (asterisks). (C) Detail of a nanoparticle aggregate. Bars in (A): 10 µm, (B): 2 µm, (C): 200 nm.
Despite the obvious advance that supposes the possibility of the application of nanoparticles on agronomical applications, it is also clear that there are many aspects in the protocols for detection of the nanoparticles and for their infiltration into the plant than can be improved. As shown in our work, the detection of isolated nanoparticles is almost impossible to achieve just by the resolution of the conventional optical microscopy, and their detection by direct scanning of ultrathin sections by electron microscopy is a tedious and time consuming task, especially if there is no clear evidence of the presence or not of nanoparticles in a certain part of the plant. Therefore, it is convenient to develop protocols with auxiliary methodologies to increase the sensibility of the microscopy techniques, that could allow directing the analysis by TEM to samples in which presence of nanoparticles has been previously assessed with certainty. Several methodologies have been employed for the detection of metallic nanoparticles in large tissue samples or in living tissues, with a special development in the field of therapy and diagnosis of diseases in the central nervous system. In this area magnetic resonance imaging,12,13 phototermal interference contrast14 and conventional iron staining13 approaches have been successfully applied, although the resolution level is still low, with ranges between 1 µm and the cell size. A suitable solution could be the optimization of current methods for iron staining (reviewed in ref. 15), provided that lesser equipment requirements are needed than for the other approaches. In this line of action, a protocol should be optimized for their use to detect our particles in plant cells using as a starting point previous reports of iron detection in plant tissues, as the one used by Green16 for detection of inorganic ferric iron in Arabidopsis thaliana. Also, as a suitable system for the detection of global uptake of magnetic nanoparticles into plant organs, a vibrating sample magnetometer has been used successfully to measure the amount of nanoparticles taken by different organs of Cucurbita maxima plants,17 what could be a good option for the selection of samples of interest to be analyzed with microscopy in future experiments. Last, but not least, an even more straight forward approach is the attachment of colored, fluorescent or chemically detectable compounds to the nanoparticles, allowing an enhancement of the capacity of optical microscopy to detect those.18
Our approach has been shown to allow the internalization of nanoparticles into plant cells in vivo, but the system as established initially is far from being functional, and several questions require improvement. First, the distribution of nanoparticles is still very limited, and most of the intracellular translocation of nanoparticles takes place near the application point, and much more efficiently when it is effectuated by injection. The most interesting way of application, the pulverization, has given only very limited results, with presence of intracellular nanoparticles but just in the first cellular layer, the epidermis. Also, long-range transport has still a low efficiency. Observations in samples equivalent to the ones that we have analyzed of fresh vibratome section, in which nanoparticles seemed to be present11 may point to the possibility of loosing some NPs during the processing for EM analysis. Again, this possibility reinforces the need of a pre-scan of the samples to identify the presence of nanoparticles before a further analysis.
Interestingly, it has been described recently the uptake of magnetite nanoparticles through the root system in Cucurbita maxima plants, as well as when applied by spraying, but the first method was tested only in plants growing in liquid media, and in the second only plants growing again in liquid media showed a significant uptake of particles.17 Also, in Arabidopsis and Phalaenopsis plants, it has been possible to perform live imaging of the uptake of other kind of nanoparticles (NaYF4:Yb,Er).19
As stated previously, these experiments support the applicability of magnetic nanoparticles for use in agronomic purposes. But despite of the promising results, there is still a long way to go until they are suited for their use. This commentary has attempted to focus in some of the aspects to improve in the methodology employed.
Acknowledgements
Work supported by the Spanish Ministry of Science and Innovation, MICINN, International project EUI2008-00157 at the Iberian Laboratory of Nanotechnology, and National Projects BFU2008-00203 and AGL2008-04255.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13080
References
- 1.Labhasetwar V. Nanotechnology for drug and gene therapy: the importance of understanding molecular mechanisms of delivery. Currt Opin Biotechnol. 2005;16:674–680. doi: 10.1016/j.copbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8:1112–1120. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 3.Ma X, Geiser-Lee J, Deng Y, Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci Total Environ. 2010 doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 5.Kaushik N, Thakkar MS, Snehit S, Mhatre MS, Rasesh Y, Parikh MS. Biological synthesis of metallic nanoparticles. Nanomedicine: Nanotechnol Biol Med. 2010;6:257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Song JY, Kwon EY, Kim BS. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng. 2010;33:159–164. doi: 10.1007/s00449-009-0373-2. [DOI] [PubMed] [Google Scholar]
- 7.Smithaa SL, Philip D, Gopchandrana KG. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochimica Acta Part A. 2009;74:735–739. doi: 10.1016/j.saa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Jha AK, Prasad K, Kumar V, Prasad K. Biosynthesis of silver nanoparticles using Eclipta leaf. Biotechnol Prog. 2009;25:1476–1479. doi: 10.1002/btpr.233. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-de-Luque A, Rubiales D. Nanotechnology for parasitic plant control. Pest Manag Sci. 2009;65:540–545. doi: 10.1002/ps.1732. [DOI] [PubMed] [Google Scholar]
- 10.Corredor E, Testillano PS, Coronado MJ, González-Melendi P, Fernández-Pacheco R, Marquina C, et al. Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol. 2009;9:45. doi: 10.1186/1471-2229-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González-Melendi P, Fernández-Pacheco R, Coronado MJ, Corredor E, Testillano PS, Risueño MC, et al. Nanoparticles as smart treatment delivery systems in plants: assessment of different techniques of microscopy for their visualisation in plant tissues. Ann Bot. 2008;101:187–195. doi: 10.1093/aob/mcm283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuwelt EA, Várallyay P, Bagó AG, Muldoon LL, Nesbit G, Nixon R. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumors. Neuropath Appl Neurobiol. 2004;30:456–471. doi: 10.1111/j.1365-2990.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 14.Cognet L, Tardin C, Boyer D, Choquet D, Tamarat P, Lounis B. Single metallic nanoparticle imaging for protein detection in cells. Proc Natl Acad Sci USA. 2003;100:11350–11355. doi: 10.1073/pnas.1534635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meguro R, Asano Y, Odagiri S, Li Ch, Iwatsuki H, Shoumura K. Nonheme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Arch Histol Cytol. 2007;70:1–19. doi: 10.1679/aohc.70.1. [DOI] [PubMed] [Google Scholar]
- 16.Green LS, Rogers EE. FRD3 controls iron localization in Arabidopsis. Plant Physiol. 2004;136:2523–2531. doi: 10.1104/pp.104.045633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Han J, Xiao JQ, Jin Y. Uptake, translocation and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit. 2008;10:713–717. doi: 10.1039/b805998e. [DOI] [PubMed] [Google Scholar]
- 18.Kurepa J, Paunesku T, Vogt S, Arora H, Rabatic BM, Lu J, et al. Uptake and distribution of ultrasmall anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana. Nanoletters. 2010;10:2296–2302. doi: 10.1021/nl903518f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hischemöller A, Nordmann J, Ptacek P, Mummenhoff K, Haase M. In-vivo imaging of the uptake of upconversion nanoparticles by plant roots. J Biomed Nanotechnol. 2009;5:278–284. doi: 10.1166/jbn.2009.1032. [DOI] [PubMed] [Google Scholar]