Abstract
The role of ABA as the primary long-distance signal produced by water-stressed roots and transported to stomata continues to be challenged. We have recently reported that expression of ABA biosynthetic genes in roots only increases in the later stage of water stress. Our results support the hypothesis that in early water stress, increased levels of ABA in xylem sap are due to leaf biosynthesis and translocation to roots and from there to xylem. If so, other xylem-borne chemicals may be the primary stress signal(s) inducing ABA biosynthesis in leaves. We found that apart from ABA, sulfate was the only xylem-borne chemical that consistently showed higher concentrations from early to later water stress. We also found increased expression of a sulfate transporter gene in roots from early water stress onwards. Moreover, using bioassays we found an interactive effect of ABA and sulfate in decreasing maize transpiration rate, as compared to ABA alone. While ABA is undoubtedly the key mediator of water stress responses such as stomatal closure, it may not be the primary signal produced by roots perceiving water stress.
Key words: abscisic acid, ABA biosynthesis, corn, drought, maize, malate, pH, stomatal conductance, sulfate, Zea mays
Root to Shoot Signaling of Water Stress
Plant roots act as sensors of the soil environment and early warning detectors of rhizosphere stresses such as water deficit. Water stress can have severe implications for plant growth, productivity and survival and therefore root sensing of water availability is an important function of plant roots, particularly for agronomic crops grown in areas with marginal rainfall.1,2 A decrease in leaf stomatal conductance is one of the first physiological responses that occurs when roots sense soil drying. Many studies on plants subjected to water stress have shown that this decrease in leaf conductance precedes changes in leaf water potential,3,4 thus in the early stages of water stress it appears leaves respond to chemical,5 rather than hydraulic,6 signals transported in xylem sap from roots.
Numerous experimental approaches have supported the involvement of the plant hormone abscisic acid (ABA) in chemical root to shoot signaling of water stress.7,8 Nevertheless, the role of ABA as the primary long-distance signal has continually been challenged over the last few decades. For example, the results of a number of studies have shown that ABA can be produced in greater amounts or at an earlier stage in leaves relative to roots in response to water-stress,9–11 and that ABA-induced stomatal closure is not dependent on ABA release from roots.6,12 According to such studies, ABA acting on stomata may be produced in leaves of plants subjected to water stress and if so, other chemical signals must be produced by water-stressed roots and act to induce ABA biosynthesis in leaves.
Early Water Stress does not Increase Expression of ABA Biosynthetic Genes in Roots
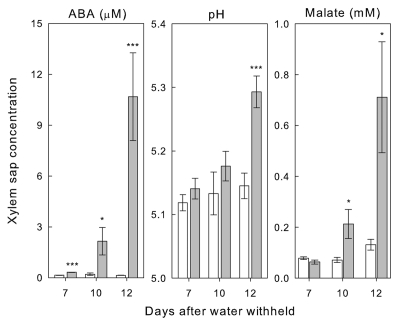
In support of the hypothesis that ABA is not primarily sourced from roots, we have recently shown that gene expression for six key enzymes involved in ABA biosynthesis does not increase in roots of maize plants responding to early water stress with reduced leaf conductance.13 In contrast, at a later stage of water stress when leaf xylem pressure potential also decreased, significant root gene expression increases were detected for five of these enzymes (CME kinase, carotenoid isomerase, vp14/NCED, aldehyde oxidase aao3 and molybdenum cofactor sulfurase). This coincided with a dramatic increase in xylem sap ABA concentration to over 75 times the control, 12 days after water was withheld (Fig. 1). The significant reduction in xylem pressure potential we observed at this later stage of water stress is indicative of hydraulic changes that can manifest as cell volume shrinkage; a phenomenon observed to induce ABA biosynthesis in roots.14,15
Figure 1.
Potential xylem-borne chemical signals: ABA, pH and malate. Xylem sap concentrations for well-watered (open bars) and water-stressed (closed bars) Zea mays plants 7, 10 and 12 days after water was withheld. Values are the mean ± SE of 12 plants for each treatment each day. Significant differences between treatments are indicated as *p < 0.05; ***p < 0.001.
What is the Source of Xylem Sap ABA During Early Water Stress?
We observed a relatively small, but significant increase in xylem sap ABA during early water stress, seven days after water was withheld (Fig. 1). If the initial increase of ABA in the xylem sap of water-stressed plants is not due to de novo ABA biosynthesis in the roots, then it could possibly be due to alkalinization-induced redistribution of existing ABA pools within roots.16–18 Nonetheless, despite a trend of increasing sap pH, we only detected significant alkalinization of the xylem sap 12 days after water stress was imposed (Fig. 1). Alternatively, the increase in xylem sap ABA could be due to catabolism of a glucosyl conjugate form of ABA (ABA-GE) to free ABA by cell wall glucosidases within roots.19 We examined the expression of two β-glucosidase genes in maize roots and did not detect an increase in either in response to early water stress, but did see a transient increase in the expression of one in response to moderate water stress. It should be noted that water stress can induce polymerisation of glucosidase thereby activating the enzyme to release ABA.20 Nevertheless, it has been argued that the amount of ABA-GE in roots is too small to contribute significantly to the overall increase in ABA during water stress.21
Finally, the increased xylem ABA could be due to de novo biosynthesis of ABA in leaves with translocation to roots via phloem where it is then moved to xylem. If the latter is responsible for the increased xylem sap ABA in the early stages of water stress, then what is the primary root-produced signal inducing leaf ABA biosynthesis?
Alternatives to ABA as Primary Signals
A variety of substances have been proposed as alternatives to ABA as xylem-borne stress signals.5,22 Malate is one such sap constituent shown to increase in response to water stress,4,23 and known to be involved in the guard cell signal transduction network.24 Nevertheless, we observed xylem sap malate concentration to increase significantly only in the later stages of water stress (Fig. 1). In addition, the use of malate in a transpiration bioassay had no effect on stomata. In fact, the only xylem sap constituent, other than ABA, that we observed to increase from early water stress onwards was sulfate (Fig. 2). The increase in sap sulfate concentration appeared to be an active process as we also observed some three-fold increase in the expression of a root sulfate transporter gene from seven days after water was withheld (Fig. 2). Interestingly, in Arabidopsis, the high affinity sulfate transporter AtSultr1;2 was expressed in both root cells and also specifically in stomatal guard cells,25,26 suggestive of a particular requirement for sulfate in guard cells.27
Figure 2.
Sulfate transport increases with water stress. (A) Root xylem sap sulfate concentration for well-watered (open bars) and water-stressed (closed bars) Zea mays plants 7, 10 and 12 days after water was withheld. Values are the mean ± SE of 12 plants for each treatment each day. Significant differences between treatments are indicated as ***p < 0.001. (B) Fold change in gene expression of a sulfate transporter in the elongation region (1–6 cm distal to the root tip) of water-stressed maize roots relative to well-watered controls as water stress increases.
We employed bioassays to examine if increased xylem sap sulfate could be involved in water stress signaling, rather than simply present to help maintain sap charge balance.28 We measured transpiration in detached maize leaves placed in artificial xylem sap containing MgSO4, ABA or both at concentrations comparable to that found in xylem sap of intact plants responding to early water stress. The addition of 0.3 µM ABA, but not 2 mM MgSO4, significantly reduced the transpiration rate compared to the control. Remarkably, the subsequent addition of both 0.3 µM ABA and 2 mM MgSO4 resulted in an even greater decrease in transpiration rate, suggesting an interactive effect of ABA and sulfate on stomata. This interactive effect was also evident when ABA concentrations were increased to 1 µM. Similar interactions have recently been observed for ABA and ethylene in controlling responses of plants to water stress.29
The Role of ABA in Chemical Signaling of Water Stress
We present here a working model (Fig. 3) in which initial water stress induces expression of root sulfate transporters resulting in increased sulfate reaching leaves via xylem sap, where it enhances the effect of low concentrations of ABA on stomata. We propose that an alternative chemical signal, such as sulfate, may induce ABA biosynthesis in leaves, with ABA then transported to roots via phloem where it induces water uptake from soil and expression of stress-resistant genes.11 ABA is then cycled back to leaves via xylem to interact with sulfate on stomata (Fig. 3A). As water stress continues, root β-glucosidases are expressed that cleave root ABA-GE to release additional free ABA and initial pH increases facilitate redistribution of root ABA to xylem. The increased ABA reaching stomata interacts with additional sulfate to further depress stomatal conductance at this stage (Fig. 3B). Under prolonged water stress, hydraulic changes in roots induce root biosynthesis of ABA and large pH increases facilitate redistribution of ABA from roots to xylem, resulting in dramatically increased ABA delivery to leaves. β-glucosidase expression may cease at this stage due to exhaustion of root ABA-GE pools. The remarkably high concentrations of ABA transported to leaves via xylem, together with additional sulfate, affect the cessation of stomatal conductance and leaf growth in an attempt to survive prolonged water stress (Fig. 3C).
Figure 3.
Working model for the role of ABA in chemical signaling of water stress. (A) Initial water stress induces root sulfate (SO4) transport, which enhances the effect of ABA on stomata and may induce leaf ABA biosynthesis. Some ABA biosynthesized in leaves is transported to roots in phloem and from there cycled back via xylem to act on stomata. (B) As water stress continues, root β-glucosidases are expressed that cleave ABA-GE to release free ABA and initial pH increases facilitate redistribution of root ABA into the xylem. Additional SO4 delivery and leaf water potential decreases enhance the increased ABA-induced stomatal depression. (C) Prolonged water stress leads to hydraulic changes in roots that induce biosynthesis of root ABA and large pH increases redistribute ABA from roots to xylem. The dramatically increased ABA delivery to leaves interacts with increasing SO4 and decreasing leaf water potential to affect maximum stomatal closure. gs, stomatal conductance to water; Ψ, water potential. Subscripts: X, xylem; L, leaf.
While ABA is undoubtedly the key mediator of water stress responses such as stomatal closure, it may not be the primary signal produced by roots perceiving water stress. Given the intracellular signaling network within guard cells involves a complex array of multiple signaling compounds and interactions,30 it appears likely that long-distance root to shoot signaling will prove similarly complex.
Acknowledgements
This research was funded by NSF-Plant Genome Program Grant (#0211842) to D.P.S. J.Q.D.G. is the recipient of an Australian Research Council Fellowship (Discovery project #DP1094530). D.P.S. thanks the Monsanto Company for supporting completion of this manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13101
References
- 1.Davies WJ, Wilkinson S, Loveys BR. Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol. 2002;153:449–460. doi: 10.1046/j.0028-646X.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 2.Neumann PM. Coping mechanisms for crop plants in drought-prone environments. Ann Bot. 2008;101:901–907. doi: 10.1093/aob/mcn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gollan T, Passioura JB, Munns R. Soil water status affects the stomatal conductance of fully turgid wheat and sunflower leaves. Aust J Plant Physiol. 1986;13:459–464. [Google Scholar]
- 4.Goodger JQD, Sharp RE, Marsh EL, Schachtman DP. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J Exp Bot. 2005;56:2389–2400. doi: 10.1093/jxb/eri231. [DOI] [PubMed] [Google Scholar]
- 5.Schachtman DP, Goodger JQD. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008;13:281–287. doi: 10.1016/j.tplants.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Christmann A, Weiler EW, Steudle E, Grill E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007;52:167–174. doi: 10.1111/j.1365-313X.2007.03234.x. [DOI] [PubMed] [Google Scholar]
- 7.Davies WJ, Kudoyarova G, Hartung W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant's response to drought. J Plant Growth Regul. 2005;24:285–295. [Google Scholar]
- 8.Wilkinson S, Davies WJ. ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ. 2002;25:195–210. doi: 10.1046/j.0016-8025.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Qin X, Zeevaart JAD. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA. 1999;96:15354–15361. doi: 10.1073/pnas.96.26.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christmann A, Hoffmann T, Teplova I, Grill E, Muller A. Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol. 2005;137:209–219. doi: 10.1104/pp.104.053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikegami K, Okamoto M, Seo M, Koshiba T. Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J Plant Res. 2009;122:235–243. doi: 10.1007/s10265-008-0201-9. [DOI] [PubMed] [Google Scholar]
- 12.Holbrook NM, Shashidhar VR, James RA, Munns R. Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot. 2002;53:1503–1514. [PubMed] [Google Scholar]
- 13.Ernst L, Goodger JQD, Alvarez S, Marsh EL, Berla B, Lockhart E, et al. Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J Exp Bot. 2010;61:3395–3405. doi: 10.1093/jxb/erq160. [DOI] [PubMed] [Google Scholar]
- 14.Jia W, Liang J, Zhang J. Initiation and regulation of water deficit-induced abscisic acid accumulation in maize leaves and roots: cellular volume and water relations. J Exp Bot. 2001;52:295–300. [PubMed] [Google Scholar]
- 15.Lü B, Chen F, Gong ZH, Xie H, Zhang JH, Liang JS. Intracellular localization of integrin-like protein and its roles in osmotic stress-induced abscisic acid biosynthesis in Zea mays. Protoplasma. 2007;232:35–43. doi: 10.1007/s00709-007-0278-3. [DOI] [PubMed] [Google Scholar]
- 16.Bacon MA, Wilkinson S, Davies WJ. pH-regulated leaf cell expansion in droughted plants is abscisic acid dependent. Plant Physiol. 1998;118:1507–1515. doi: 10.1104/pp.118.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson S, Corlett JE, Oger L. Effects of xylem pH on transpiration from wild-type and flacca tomato leaves: a vital role for abscisic acid in preventing excessive water loss even from well-watered plants. Plant Physiol. 1998;117:703–709. doi: 10.1104/pp.117.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson S, Davies WJ. Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol. 1997;113:559–573. doi: 10.1104/pp.113.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauter A, Hartung W. The contribution of internode and mesocotyl tissues to root-to-shoot signalling of abscisic acid. J Exp Bot. 2002;53:297–302. doi: 10.1093/jexbot/53.367.297. [DOI] [PubMed] [Google Scholar]
- 20.Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, et al. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell. 2006;126:1109–1120. doi: 10.1016/j.cell.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 21.Priest DM, Ambrose SJ, Vaistij FE, Elias L, Higgins GS, Ross AR, et al. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 2006;46:492–502. doi: 10.1111/j.1365-313X.2006.02701.x. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez S, Goodger JQD, Marsh EL, Chen SX, Asirvatham VS, Schachtman DP. Characterization of the maize xylem sap proteome. J Proteome Res. 2006;5:963–972. doi: 10.1021/pr050471q. [DOI] [PubMed] [Google Scholar]
- 23.Patonnier M, Peltier J, Marigo G. Drought-induced increase in xylem malate and mannitol concentrations and closure of Fraxinus excelsior L. stomata. J Exp Bot. 1999;50:1223–1231. [Google Scholar]
- 24.Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2 and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yomeyama T, et al. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 2002;29:475–486. doi: 10.1046/j.0960-7412.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002;29:465–473. doi: 10.1046/j.0960-7412.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- 27.Hawkesford MJ. Transporter gene families in plants: the sulphate transporter gene family—redundancy or specialization? Physiol Plant. 2003;117:155–163. [Google Scholar]
- 28.Goodger JQD, Schachtman DP. Nitrogen source influences root to shoot signaling under drought. In: Pareek A, Sopory SK, Bohnert HJ, Govindjee, editors. Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation. Dordrecht: Springer Science; 2010. pp. 165–173. [Google Scholar]
- 29.Wilkinson S, Davies WJ. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 2010;33:510–525. doi: 10.1111/j.1365-3040.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Assmann SM, Albert R. Predicting essential components of signal transduction networks: A dynamic model of guard cell abscisic acid signaling. PLoS Biol. 2006;4:312. doi: 10.1371/journal.pbio.0040312. [DOI] [PMC free article] [PubMed] [Google Scholar]