Abstract
Background: Insulin-dependent diabetes mellitus are at high risk for vascular disorders as hypertension and nephropathy. Ginseng is one of the most widely used herbal medicines and is reported to have a wide range of therapeutic and pharmacological applications for antioxidant and vasorelaxation although the mechanism is not clear. This study, aimed to evaluate hypoglycemic, antioxidant and vasodilator effects of Panax quinquefolium aqueous ginseng extract (AGE) against streptozotocin (STZ)-induced diabetes in male rats. Furthermore explore the role of AGE in C-peptide and nitric oxide (NO) and their relation in STZ induced diabetic rats. Methods: Thirty White male Sprague daw-ley rats weighing 150-200 gm, about 4 month old were equally divided into the following: a control group (normal, nondiabetic), a diabetic group induced by intraperitoneal (I/P) injection of STZ (non-AGE-treated) and an AGE-treated diabetic group (STZ+AGE) (for 8 days). Serum level of urea, creatinine, glucose, insulin, C-peptide and NO were analyzed. Activities of hepatic glucose-6-phosphatase (G6Pase), hepatic glycogen phosphorylase and the renal antioxidant enzyme, catalase were analyzed. Also renal oxidative stress marker malondialdehyde (MDA) was measured. Results: Data showed that STZ treated rats produced a significant increased level of serum urea, creatinine, glucose, NO and renal MDA. Also, induced significantly higher activities of hepatic G6Pase and glycogen phosphorylase compared with controls, while give significant lowered serum insulin, C-peptide level and renal catalase activity. Whereas treatment with AGE led to a significant amelioration in the hyperglycemia (lower the activity of G6Pase and glycogen phosphorylase), hyperinsulinemia and oxidative stress markers. Besides declining the higher level of renal function test and NO. Conclusions: STZ induced-diabetes (DM) associated with renal function disturbances, hypoinsulinemia, defective antioxidant stability and increased (NO) this may have implications for the progress of DM and its related problems. Treatment with AGE improved DM and its associated metabolic problems in different degrees. Furthermore it has insulin sensitizing, hypoglycemic, antioxidant and vasodilator effects. Communally AGE is a potential way to surmount the diabetic state and it has vasodilator effects.
Keywords: Panax quinquefolium, diabetes, kidney function, oxidative stress, NO, C-peptide
Introduction
Diabetes mellitus disorders are of multiple etiologies characterized by chronic hyperglycemia with disturbances of carbohydrate, fat and lipid metabolism resulting from defect in insulin secretion, insulin action or both. The effects of diabetes mellitus include long term damage, dysfunction and failure of various organs [1].
The disease symptoms which are polyuria, poly-phagia and polydypsia are not severe or may be absent and consequently hyperglycemia sufficient to cause pathological and functional changes may be present for a longtime before the diagnosis is made. The long term effects of diabetes mellitus include progressive development of the specific complications of hypertension and nephropathy that may lead to renal and cardiac malfunction [2].
Diabetic nephropathy is the leading cause of end-stage renal disease and endothelial dysfunction is the central pathophysiologic denominator for all cardiovascular complications of diabetes including nephropathy. Abnormalities of NO production modulate renal structure and function in diabetes [3] but, despite the vast literature, major gaps exist in our understanding in this field because the published studies mostly are confusing and contradictory.
American ginseng (Panax quinque folius L.) is slow-growing perennial plants with fleshy roots, belonging to the Panax genus in the family Arali-aceae. The genus name of Panax ginseng was coined by the Russian botanist, C.A. Meyer, and is derived from the Greek terms ‘pan', meaning all and ‘axos’ meaning cure. The specie name was derived from the Chinese word ‘jensheng', implying the herb whose roots resemble the human body. Thus, Panax ginseng means ‘the all-healing man. Ginseng has been used as a general tonic for thousands of years in Asian countries, and has become a popular herbal medicine all over the world [4].
From the pervious study it appears that the antidiabetic effect of American ginseng may not be linked to its antioxidant actions. The mechanisms of American ginseng's effects on reducing high blood glucose levels and body weight remain to be investigated in future experiments.
The root of Panax quinquefolium L. used in treating the majority of ageing-associated diseases as well as sexual dysfunction in men and the effects include adaptive, aphrodisiac and nourishing stimulant, and the root is most often available in dried form, either whole or sliced.
In oriental medicine, ginseng is extracted with boiling water and used for medicinal purposes. Aqueous extracts of ginseng are composed of a glycosides mixture, ginsenosides, trace minerals and a variety of complex carbohydrates as well as proteins, peptides and amino acids. The root of ginseng contains several saponins (ginsenosides) which are biologically active compounds. Individual ginsenosides suppress tumor cell growth, induce cell differentiation, regulate apoptosis and inhibit metastasis formation [5].
In addition to ginsenosides, some glycans and peptides isolated from ginseng root may also have hypoglycemic effect in mouse models of diabetes. In terms of stability, ginsenosides are better than the glycans and peptides. Ginsenosides can be administrated orally or through injection. However, the bioactivities in glycans and peptides are inactivated in the stomach or intestine if taken orally. When ginseng is taken orally, ginsenosides should be the major active compounds in the blood. Ginsenosides are expensive since the price of ginseng root is much higher than many other herbs. As a result, the crude extract of ginseng root is often used instead of ginsenosides in many laboratory studies.
The main pharmacologically active constituents of ginseng are believed to be ginsenosides, derivatives of the triterpene dammarane structure. According to the Electron Spin Resonance (ESR) data, the extraction temperature had no effect on their free radical scavenging activity. The most abundant ginsenosides of American ginseng are neutral ginsenosides, Rb1, Rb2, Rc, Rd, Re and Rg1[6].
The mechanisms of American ginseng root in the treatment of diabetes remains an ambiguity. This significantly limits the use of American ginseng to assist diabetic treatment.
During diabetes mellitus, endogenous hepatic glucose production increases as a result of impaired activities of the key enzymes of carbohydrate metabolism, which leads to the condition known as hyperglycemia.
Diabetes mellitus is a major challenge for health care systems worldwide because it is strongly associated with several major health risk factors. Currently marketed drugs are insufficient to achieve long-term control of blood glucose levels and induce considerable side effects. The development of more effective drugs for metabolic diseases is still of urgent interest.
C-Peptide is produced in beta-cells in the pancreas, and secreted into the blood stream. For a long time, C-peptide was considered as an important component in the biosynthesis of insulin, but otherwise believed to possess minimal biological activity [7]. C-Peptide is an excellent parameter for evaluating pancreatic ß-cells function. It has equimolar secretion with insulin, longer half life, and negligible hepatic clearance. Some researchers prefer C-Peptide concentrations to insulin concentrations in detecting changes in the ß-cell secretion of insulin.
From the previous background, prevention of the incidence and development of diabetic nephropathy has become a very important issue. Hence, great effort has focused on herbal medication without side effects to find a novel therapeutic agent for diabetic nephropathy. Therefore, we investigated its biological mechanisms of action related to metabolic enzymes, hormones, NO, renal function and oxidative stress.
Material and methods
Diet
Composition of the experimental diet (g/kg diet) was according to the formula of Kim et al. [8]. It included the normal diet for control rats (fat 5%, carbohydrates 65%, proteins, 20.3% fiber 5%, salt mixture and 3.7% vitamin mixture 1%). Diets were purchased from El-Gomhoria Company, Cairo, Egypt.
Experimental animals
30 white male rats (Sprague dawley strain) weighing 150-200 gm, about 4 month old were used for this study. Adult male rats were used, since male in generally are more susceptible to diabetes than females and young ones have a higher resistance to the diabetogenic effect of streptozotocin than adult.
Rats were purchased from the National Research Center, Cairo, Egypt. All animals were housed in stainless steel cages contain barriers for each rat for individual housing and the cage contain 5 rats and each rat had a tag number. They kept under standard environmentally controlled, clean-air room with temperature 24±5° C, illumination (12 h light/12 h dark cycles), a relative humidity of 60±4%, and water and rodent chow were available ad libitum throughout the period of the investigation. They were housed for two weeks after their arrival in the laboratory for accommodation.
Our work was carried out in accordance with the guidelines of Beni Suef University for animal use and approved by Ethics and Animal Care Committee. These animals were used for induction of Diabetes mellitus.
Plant material preparation of AGE
Herbs were purchased from local Mohey El-Attar Company in El-Minia city. Identification and extractions of medicinal plants were completed in department of Pharmacognacy, faculty of pharmacy, El Minia University.
The plant roots were soaked in cold water for 2 h, and then cut into small pieces less than 2mm in diameter. These pieces were mixed with hot water about 95°C for 1 h, and then filtered. The filtrates were evaporated and lyophilized. The dried powders were suspended in bath solution. The suspension was centrifuged for 10 min at 3000rpm and the supernatant was used for the experiment [9].
After isolation by several column chroma-tographic steps from the extract and characterization by spectroscopic methods, the main compounds identified and analysed were, ginse-nosides which are a special group of triterpe-noid saponins that can be classified into two groups by the skeleton of their aglycones, namely dammarane glycosides - and oleanane-type.
The biological activity of AGE is the result of saponins, believed to be the main active constituents in American ginseng. In this diverse group of saponins, a representative ginse-nosides, as constituents in AGE root were used to test for this study [5].
Drug administration
AGE 300 mg/kg BW of rats [6]. Animals received daily intraperitoneal (IP) injections of AGE for 8 consecutive days. The rout, dose and period of administration were novel in comparison with other studies to decrease the time of treatment [8].
At the end of the experiment, all groups were bled by vein puncture; at once samples of blood, liver were collected. In mice, the LD50for ginseng ranges from 10 to 30 g/kg [10].
Experimental design and animal grouping:
A total 30 Sprague dawley strain rats were used for this experiment and diabetes was induced in 20 rats by a double-intraperitoneal injection of STZ (Sigma Chemical Co). It was dissolved in citrate buffer (0.05 mM, pH 4.5) and given in dose 30 mg/kg for each rat two days with total 60 mg /kg/rat. After ten days from STZ injection, the glucose level of blood from the tail vein was determined.
Rats that demonstrate serum glucose level over 180 mg/dl were selected as diabetic for this experiment. Normal rats were injected with vehicle (citrate buffer).
Rats were divided into 3 groups each of 10 rats as follow: Group 1, consider as normal, group 2, diabetic control and group 3 as diabetic group supplemented with AGE (300 mg/kg body weight (BW) of rats) after 10 days of induction into diabetes.
Our goal is to achieve diabetic model followed by treatment. This model provided us reliable method and resembles the clinical cases of diabetes and its treatments; also the period of treatment is safe and recommended in previous research.
Sampling and tissue preparation
Blood Sampling: By the end of the experimental periods, venous blood samples were collected from the orbital sinus of normal, diabetic control and diabetic treated with STZ rats via glass capillaries in a fasting state. The blood samples were collected in dry glass centrifuge tubes, allowed to coagulate at room temperature and centrifuged at 3500 rpm for 15 minutes at room temperature for separation of serum. The clear, non-haemolysed supernatant sera were separated using clean dry disposable plastic syringes and stored at -20°C for subsequent biochemical measurements of, urea, creatinine, glucose, insulin, C-peptide and NO levels in serum.
Tissue sampling: Rats were sacrificed by decapitation and an abdominal incision was immediately done for separation of the kidney and liver that was immediately excised, weighed (g.) and underwent homogenization for oxidative stress markers, MDAand catalase evaluation in kidney while, the activities of both glycogen phosphory-lase and G6Pase were measured in liver.
Biochemical analysis of serum and tissue:
Urea and creatinine were determined in serum by the method of Fawcett and Scott [11] and Henry [12] respectively. Serum glucose was measured by the glucose oxidase colorimetric method using Stanbio Laboratory USA Kits. Serum insulin was assayed in the Radioactive Isotopes Unit, Central Department of Scientific Analysis and Test, National Research Center (Dokki, Giza) using radioimmunoassay kits (Diagnostic Products Corporation, Los Angeles, USA) [Coat-A-Count]. Serum C-peptide was assayed in the Radioactive Isotopes Unit, Central Department of Scientific Analysis and Test, National Research Center (Dokki, Giza) using radioimmunoassay kits (Diagnostic Products Corporation, Los Angeles, USA) [Double-Antibody] according to the method of Bonser and Garcia-Webb [13]. Hepatic G-6-Pase was measured according to the method of Begum et al., [14] and glycogen phosphorylase was measured according to the method of Stalmans and Hers [15].
Renal lipid peroxidation was measured through malondealdehyde (MDA) levels, according to the methods of Mihara and Uchiyama [16]. Renal catalase (CAT) activity was determined according to the procedure of Cohen etal. [17].
NO was determined in serum as nitrite concentration after reduction of nitrate to nitrite. The concentration of NO was determined using sodium nitrite as standard method modified by Van Bezooijen etal. [18].
Statistical analysis
Statistical analysis was carried out using Graph Pad Instat software (version 3, ISS-Rome, Italy). Unless otherwise specified, groups of data were compared with an unpaired t-test one-way analysis of variance (ANOVA) followed by Tukey-Kramer (TK) multiple comparisons post-test. Values of P <0.05 were regarded as significant. Data were expressed in tables and figures as mean ±SEM.
Results and discussion
Serum urea and creatinine levels were significantly elevated in diabetic in comparison with normal group and giving AGE normalized these effects (Table 1)
Table 1.
Effect of AGE on biochemical parameters in normal, diabetic control and diabetic+AGE groups in serum of rat
| Parameter | Normal | Diabetic control | Diabetic+AGE |
|---|---|---|---|
| Urea (mg/dl) | 35.60±0.94 | 50.11±1.33**a | 39.86±0.65b |
| Creatinine (mg/dl) | 0.6±0.05 | 1.31±0.06*a | 1.03±0.03b |
| Glucose (mg/dl) | 68.166±2.63 | 201±3.028**a | 175.41±2.54b |
| C-peptide (ng/ml) | 0.63±0.05 | 0.49±0.02*a | 0.41±0.06a |
| No (μmol/L) | 7.2±0.54 | 19.4±0.9 **a | 11.2±0.46b |
Data are expressed as mean ± SEM;
Values significantly different compared to normal group P*<0.05
Values high significantly different compared to normal group P**<0.01;
Means not sharing common letter are significantly different (p<0.05) compared with diabetic control rats.
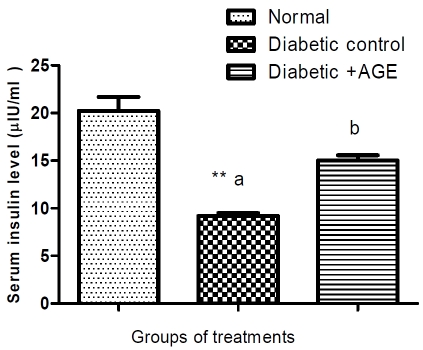
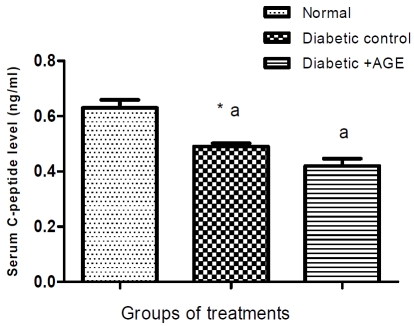
Serum glucose level was increased significantly, while insulin and C-peptide decreased in STZ-induced diabetic rats compared with normal group and treatments with AGE significantly ameliorated the induced hyperglycemia (Table 1) and hypoinsulinemia without changes in Cpeptide level compared to diabetic group during the treatment period (Figure 1 and 2).
Figure 1.
Effect of AGE on serum insulin level (μIU/ml) in normal, diabetic control and diabetic+AGE groups in rats. **Values significantly different compared to normal group (P*<0.05) and high significantly different at (P**<0.01); aMeans not sharing the same letter are significantly different (p<0.05) compared with diabetic control. (Each group includes 10 rats); bAn insulin level was decreased in STZ-diabetic rats compared with normal group and treatments with AGE significantly ameliorated the produced hypoinsulinemia compared to diabetic group.
Figure 2.
Effect of AGE on serum C-peptide level (ng/ml) in normal, diabetic control and diabetic+AGE groups in rat. *Values significantly different compared to normal group (P<0.05) and **high significantly different at (P<0.01). Means not sharing the same letter are significantly different (p<0.05) compared with diabetic control. (Each group includes 10 rats); aThe data indicated that C-peptide level reduced significantly in STZ-diabetic compared with normal rats group, while there was non significant change with AGE administration.
Activity of renal catalase decreased significantly in the (STZ) group compared with normal and the treatments with AGE significantly elevated its activity during the treatment period (Table 2). Serum NO and renal MDA levels were significantly (P<0.01) elevated in the STZ compared to the normal. Additional administration of AGE resulted in a significant improvement in these changes (Table 1 and 2).
Table 2.
Effect of AGE on renal oxidative stress markers in normal, diabetic control and diabetic+AGE groups
| Parameter | Normal | Diabetic control | Diabetic +AGE |
|---|---|---|---|
| Renal M.D.A (n mol /g/hr) | 4.29±0.06 | 9.0±0.08**a | 7.44 ± 0.08b |
| Renal Catalase (K X 10-2) | 50.50±0.93 | 21.0±0.63**a | 32.22 ± 1.06b |
Data are expressed as mean ± SEM; *Values significantly different compared to normal group P<0.05; **Values high significantly different compared to normal group P<0.01;
Means not sharing common letter are significantly different (p<0.05) compared with diabetic control rats.
Activity of hepatic G6Pase and glycogen phos-phorylase increased significantly (P<0.01) in the (STZ) group compared with normal and treatments with AGE significantly ameliorated these changes (Table 3).
Table 3.
Effect of AGE on hepatic glycogen phosphorylase and hepatic glucose -6-phosphatase in normal, diabetic control and diabetic+AGE groups
| Parameter | Normal | Diabetic control | Diabetic+AGE |
|---|---|---|---|
| Glycogen phosphorylase (mU/g tissue) | 1.23±0.14 | 5.62±0.69**a | 3.99±0.34b |
| G6Pase (mU/g tissue) | 1.025±0.06 | 7.21±0.56**a | 3.41±0.36b |
Data are expressed as mean ± SEM; *Values significantly different compared to normal group P<0.05; **Values high significantly different compared with normal group P<0.01;
Means not sharing the same letter are signifi-
Diabetes mellitus is a syndrome initially characterized by a loss of glucose homeostasis. The disease is progressive and associated with a high risk of arthrosclerosis, heart disease, stroke and peripheral vascular disease.
Ginseng is a well-known medicinal plant used in traditional oriental medicine. Ginseng has been estimated to be the second top-selling herbal supplement. In recent decades, ginseng root has gained popularity as a dietary supplement in the United States. Ginseng, a complex system composed of several compounds, has a broad range of pharmacological activities, including immunomodulation, improvement of physical strength and stimulation of appetite and has valuable effects on learning, memory [19].
Effect of STZ and or AGE on glucose homeostasis
The present study revealed that intra peritonea l injection of streptozotocin induced biochemical changes including hyperglycemia and hypoinsu-linemia with an obvious evidence of diabeto-genesis (Table 1) and (Figure 1).
The possible mechanisms for β-cells destruction by Streptozotocin was reported to induce generation of some types of oxygen free radicals and alteration of endogenous scavengers of these reactive species, fragmentation of DNA and the subsequent increase in the activity of poly-ADP ribose synthase (an enzyme known to deplete nicotinamide adenine dinucleotide in β-cells), inhibition of ATP synthesis and islet mitochondria l respiratory enzymes [20].
STZ diabetic rats show a highly significant increase in serum glucose level as compared to normal rats. The diabetogenic agent streptozotocin selectively destruct β-cells of the islets of Langerhans in the pancreas result in inhibition of insulin synthesis and elevation of blood glucose level, firstly due to reduction in entry of glucose to peripheral tissues, muscle and adipose tissue. Secondly, Increased glycogen breakdown and increased gluconeogenesis and hepatic glucose production [21].
This finding was confirmed by our results of diabetic rats which indicate a marked increase of the detected gluconeogenic enzyme, G6Pase and liver glycogenolytic enzyme, liver glycogen phosphorylase activities as compared with that of the non diabetic group (Table 3).
According to our data, inhibiting the hepatic G6Pase and glycogen phosphorylase is emerging as a novel mechanism for combating diabetic hyperglycemia, preventing high blood glucose and saving liver glycogen.
Our study presents a novel evidence for a method to regulate metabolic enzymes that may merit the attention of diabetes investigators and community. The mechanisms responsible for the antidiabetic activity of ginseng radix and rootlet could be through improvement of hyperglycemia by blocking intestinal glucose absorption and inhibiting hepatic G6Pase [22].
The marked increase in G6Pase in STZ diabetes markedly increased mRNA levels of the catalytic subunit of G-6-Pase in the liver and to the same extent in the kidney [23]. Insulin plays an important role in the regulation of G-6-Pase activity in both liver and kidney in vivo. A multicomponent insulin responsive sequence identified in the promoter region of the G-6-Pase gene appears to be regulated via P13-K and downstream PKB-dependentand independent pathways [24].
In addition to the increasing effect on these gluconeogenic enzymes, the serum glucose oxi-dative shunt enzyme, glucose-6-phosphate de-hydrogenase activity, exhibited a marked decrease in the diabetic rats, as in the present study, which results in a decreased metabolism of glucose via oxidative shunt process and accumulation of glucose -6-phosphate in the liver.
Our results revealed that fasting serum insulin level showed a significant decrease in the STZ diabetic rats as compared with the non diabetic ones (Figure 1) and AGE administration significantly declines the resulted hyperglycemia and sensitizes insulin by improving its low level in diabetic rats in agreement with those of Luo and Luo [25]. The mechanism of American ginseng roots in the treatment of diabetes occurs through increasing insulin production and reduces cell death in pancreatic beta-cells. This greatly maximizes the effective utilization of American ginseng in facilitating diabetic therapy. Ginseng-specific saponins (ginsenosides) were considered as the major bioactive compounds for the metabolic activities of ginseng.
Hypoglycemic activity of AGE may be attributed to the enhancement of aerobic glycolysis through stimulation of beta-adrenoceptor and increase of various rate-limiting enzyme activities related to tricarboxylic acid cycle (T.C.A).
This hypoglycemic mechanism may be related to inhibition of mitochondrial function, activation of AMPK pathway, suppression of adipogenesis and induction of low-density lipoprotein (LDL) receptor expression [26].
Moreover plasma glucose-lowering action of AGE may be due to the ability of ginsenoside Rh2 [10], the major principles contained in P. ginseng root to, increase insulin secretion by ß-cells as a result of the release of acetylcholine from nerve terminals that then stimulates mus-carinic M3 receptors in pancreatic cells
The hypoglycemic effect may be mediated in part through increased NO production, a neuro-transmitter and in pancreatic nitrergic nerves, that may be implicated in the parasympathetic modulation of insulin secretion [27]. Also, AGE regulates Uncoupling Protein-2 (UCP-2), which resulted in a decrease in apoptosis and an increase of ATP production and thus insulin production [28].
AGE was found to increase peroxisome prolif-erator-activated receptors (PPAR-γ) transactivation activity and dose-dependently significantly increased the expression of glucose transporter 4 (GLU4) which may lead to enhancement of insulin sensitivity and glucose metabolism [29]. Hence, American ginseng attenuates hyperglycemia and may present itself as a supplement to diabetes therapy.
In Summary, administration of STZ led to a significant hyperglycemia and a decrease in insulin level, through significant increase (p <0.01) in the activity of Phosphorylase and G6Pase in the liver resulting in glycogenolysis and conversion of glucose 6-P into circulating glucose.
Ginseng restored the altered G6Pase and glyco-gen phosphorylase levels to near normal. The results of this experimental study indicated that Ginseng possesses hypoglycemic, and insulin mimetic activities and a positive role in carbohydrate metabolism and hence it could be used for treating diabetes. AGE affect both gluconeogenic and glycogenolytic pathways and demonstrate a potential for a beneficial effect on diabetic nephropathy.
Effect of STZ and AGE on renal oxidative stress
There was a significant increase (P<0.01) in renal MDA level, an indicator of free radical generation, which increases at the end of lipid per-oxidation, while there was a significant decrease in catalase activity in the kidney of STZ-induced diabetic rats compared with normal groups. These findings agree with that of Shim, etal., [30].
Administration of AGE to diabetic rats significantly reduced the levels of lipid peroxidation (MDA) and significantly increased the activities of antioxidant catalase enzymes (Table 2). Our results are the first for renal oxidative stress markers MDA and catalase using short term dose of AGE, where Fu, and Ji [31] studied the effect of Panax quinquefolium on oxidative stress using high dose for 4 month.
The multifold bioactive medicinal properties of ginseng have been closely linked to its antioxidant ability, which is related to its ginsenoside content that could induce the antioxidant enzymes which are important for maintaining cell viability by lowering the level of oxygen radical generated from intracellular metabolism [32]. AGE, also has the ability to intercalate into the cell membrane, changing its fluidity and inhibit lipid peroxidation by chelating transition metals and scavenging ROS [33].
The present results suggested that the AGE enhanced the antioxidant defence against reactive oxygen species produced under hyperglycaemic conditions, hence protecting the kidney cells. Thus AGE beneficial for the treatment of diabetic associated with renal function disturbance via the inhibition of oxidative stress.
Effect AGE on nitric oxide in diabetic rat
NO is formed enzymatically from L-arginine in the presence of NO synthase. NO is produced constitutively in endothelial cells in stress and blood-borne substances. It also generated in nerve cells and act as a neurotransmitter and neuromodulator in nerve endings. Furthermore, NO can be formed via enzyme induction in many tissues in the presence of cytokines. Being a free radical with vasodilator properties NO exerts dual effects on tissues and cells in various biological systems. At low levels NO can dilate the blood vessels and improve the circulation, but at high levels it can cause circulatory shock and induce cell death. Therefore, diseases can arise in the presence of the extreme ends of the physiological levels of NO [34].
STZ diabetic rats showed significant increase in level of NO as compared with in normal rats (Table 1) in accordance with Napoli, and Ignarro [35].
At pathological concentrations, NO can cause irreversible alterations in respiratory function and can also interact with ROS to form reactive nitrogen species, which may further impair mito-chondrial respiration and can even lead to opening of the mitochondrial permeability transition pore and cell death.
Diabetes and heart failure have all been associated with altered ROS generation, which can alter the delicate regulatory balance of effects of NO in the mitochondria [36].
These observations offer further light on alterations of NO not only in the course of vascular complications but in the pathogenesis of diabetes itself.
Based on the current data, it is reasonable to conclude that early renal function interruption in diabetes is associated with increased NO production mediated primarily by constitutively released NO (endothelial NO synthase and neu-ronal NO synthase). The enhanced NO production may contribute to hyperfiltration and micro-albuminuria that characterizes early diabetic associated renal function disorders.
On the other hand, some of the studies indicated that advanced nephropathy leading to severe proteinuria, declining renal function, and hypertension is associated with a state of progressive NO deficiency. Some factors including hyperglycemia, increased oxidant stress, as well as activation of protein kinase C contribute to decrease NO production and/or availability. Finally, genetic polymorphisms of the nitric oxide synthetase (NOs) enzyme also may play a role in the NO abnormalities that contribute to the development and progression of diabetic nephropathy [3].
The present study has suggested that an early increase in NO production or activity mediates pathophysiologic and biochemical changes in diabetic associated biochemical disturbances. While, the decreased NOS activity during the late phase of diabetes found in other studies was partially associated with a decrease in protein expression in kidney macula densa [37].
AGE treatment resulted in a significant decrease in NO level as compared to diabetic rats (Table 1) in accordance with Luo and Luo [21].
Saponins from ginseng, ginsenosides, have effects on the NO signaling pathway as it has been shown to relax blood vessels [38].
AGE may stabilize the membrane system of mitochondria and increase activity of mitochon-drial cytochrome c oxidase which is the major route by which NO is removed from mitochondria-rich cells resulting into removal of NO from mitochondria-rich cells [39].
Effect ofSTZand or AGE on renal function
The identification and management of early-stage diabetic kidney disease are important, but the majority of people exhibit no symptoms until the disease is more advanced [40]. Therefore, we have investigated therapeutic agents for the prevention of early-stage diabetic renal damage using short-term streptozotocin induced diabetic rats.
STZ diabetic rats showed significant increase in levels of serum urea and creatinine as compared to normal ones (Table 1). These findings are in agreement with Kim, et al., [8]. Advanced glycation end products and oxidative stress have close association with diabetic renal damage.
Additionally, administration of AGE showed significant decrease in serum urea and creatinine levels when compared with diabetic rats in agreement with Hyun et al., [41].
AGE resulted in activation of renal N-methyl-D-aspartate (NMDA) receptor that leads to retaining calcium (Ca2+) homeostasis, the decrease in intracellular Ca2+ and inactivated NOs; resulting in decreases (NO) production, so bring about amelioration of the renal dysfunction of diabetic rats.
Ginsenoside Re, a major ingredient of Panax ginseng, enhances the slowly activating component of the delayed rectifier K+ current (IKs) and suppresses the L-type Ca2+ current (I (Ca, L)). In addition, ginsenoside releases NO via membrane sex steroid receptors, resulting in Ca (2+)activated K+ channels [42].
Recently, the increased insulin secretion was recorded to activate ATP-sensitive potassium channels K (ATP) channel, Hence, membrane hyperpolarization closes voltage-dependent Ca2+ channels, which leads to a reduction in intracellular Ca2+ and vasodilatation that ameliorate renal function [37].
Communally streptozotocin induces diabetes mellitus in experimental animals and the heat-processed American ginseng has protective effect against diabetic renal damage in rats.
Association among C-peptide, Nitric oxide and vascular blood flow in STZ and AGE administered rat
C-peptide is a product of pro-insulin cleavage; numerous studies have demonstrated that C-peptide, although not influencing blood glucose control, may play a role in preventing and potentially reversing some of the chronic complications of type 1 diabetes [43]. This study presents novel functions of C-peptide, focusing on its role in NO generation.
The current data indicated that C-peptide level was reduced significantly in STZ diabetic compared with normal rats group (Figure 2). These findings coincide with the results of Cong and Chen [44].
Recently, the role of C-peptide, released from the pancreatic beta cell, in regulating microvascular blood flow, has received increasing attention. In type 1 diabetic patients, intravenous application of C-peptide in physiological concentrations, which resemble the normal groups in our experiment, was shown to increase mi-crovascular blood flow and to improve microvascular endothelial function.
Increasing evidence suggests that declining ß-cell function in diabetes and the lack of C-peptide secretion might play a putative role in the development of microvascular blood flow abnormalities, which go beyond the effects of declining insulin secretion or increased blood glucose levels in DMT1. The underlying mechanisms involve the activation of endothelial NO synthase and the activation of Na+K+ ATPase, which was shown to be calcium-dependent and ouabain sensitive [45]. The biological effects of C-peptide are, at least in part, mediated through the modulation of endothelial function and microvascular blood flow. In several tissues, an increase in microvascular and nutritional blood flow could be observed during substitution of physiological amounts of C-peptide [13].
We suggested that the lack in C-peptide in the diabetic group responsible for the progress of renal disorder which may be due to disturbance in the maintaining of normal NO level in DMT1 leading to the development of microvascular complications such as nephropathy.
Also, we agree in our in vivo experimental trial that normal level of C-peptide maintain normal NO level which is important for endothelial function and microvascular blood flow, while its decrease disturbed No level which was elevated due to STZ and develop renal function impairment. There is still a substantial need for the further investigation of the molecular effects of C-peptide on cellular level and effect of AGE.
C-peptide-induced nitrites generation and increase in calcium was observed in freshly isolated primary peritoneal macrophages. This nitrite generation suggests that C-peptide may elicit immune modulatory function via modulation of the calcium/JAK-STAT pathway [43]. Microvascular blood flow after C-peptide supplementation in DMT1 encourages the claim for further prospective interventional trials to establish the clinical relevance for C-peptide supplementation. Administration of AGE reduces NO level independent on C-peptide in comparison with STZ group while there was no effect on C-peptide level.
Conclusion
It could be concluded that STZ induced DMT1 associated with low values of insulin and C-peptide, defective antioxidant stability and increased NO levels which may have implications for the progress of microvascular complications as diabetes associated renal disturbances. Also treatment with (AGE) improved DM and its associated nephropathy.
Furthermore AGE has hypoglycemic, insulin sensitizing, antioxidant, vasodilator effects and consider as a way to surmount and indicate its usefulness as potential treatment in diabetes and its associated renal function disturbance. The results suggest that alterations in the activities of key metabolic enzymes of carbohydrate metabolism could be one of the biochemical rationale by which AGE attenuates the hyperglycemic and oxidative stress effects in diabetic rats.
Additionally, AGE may be useful in delaying the complicated effects of diabetes as renal function disturbances due to imbalance between free radicals and antioxidant systems, while have no effect on C-peptide, despite its ability to normalize NO levels.
Acknowledgments
This study was supported by Beni-Suef University. We appreciate the assistance and advice of Prof. Dr Bastawy M., Vice Dean of College of Science, Beni-Suef Univ.
References
- 1.Brunton S. Beyond glycemic control: treating the entire type 2 diabetes disorder. Postgrad Med. 2009;121:68–81. doi: 10.3810/pgm.2009.09.2054. [DOI] [PubMed] [Google Scholar]
- 2.Stitt-Cavanagh E, MacLeod L, Kennedy C. The podocyte in diabetic kidney disease. Scientific-World J. 2009;14:1127–1139. doi: 10.1100/tsw.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004;2(4):333–344. doi: 10.1016/j.semnephrol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. American J Chinese Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varjas T, Nowrasteh G, Budán F, Nadasi E, Horváth G, Makai S, Gracza T, Cseh J, Ember I. Chemopreventive effect of Panax ginseng. Phytother Res. 2009;23:1399–1403. doi: 10.1002/ptr.2786. [DOI] [PubMed] [Google Scholar]
- 6.Beveridge TH, Li TS, Drover JC. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;13:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 7.Forst T, Hach T, Kunt T, Weber MM, Pfützner A. Molecular effects of C-Peptide in microvascular blood flow regulation. Rev DiabetStud. 2009;6(3):159–167. doi: 10.1900/RDS.2009.6.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HY, Kang KS, Yamabe N, Nagai R, Yokozawa T. Protective effect of heat-processed American ginseng against diabetic renal damage in rats. J Agric Food Chem. 2007;17(21):8491–8497. doi: 10.1021/jf071770y. [DOI] [PubMed] [Google Scholar]
- 9.Yuan CS, Wu JA, Lowell T, Gu M. Gut and brain effects of American Ginseng root on brainstem neuronal activities in rat. Am J Chin Med. 1998;26:1–9. doi: 10.1142/S0192415X98000075. [DOI] [PubMed] [Google Scholar]
- 10.Kitts D D, Hu C. Efficacy and safety of ginseng. Public Health Nutr. 2000;(4A):473–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 11.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Path. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry RJ. New York: Harper & Row Publishers; 1964. Clinical Chemistry; p. 181. [Google Scholar]
- 13.Bonser AM, Garcia-Webb P. C-peptide measurement: methods and clinical utility. Crit Rev Clin Lab Sci. 1984;19(4):297–352. doi: 10.3109/10408368409165766. [DOI] [PubMed] [Google Scholar]
- 14.Begum N, Sam GPK, Shanmugasundaram KR. Serum enzymes I human and experimental diabetes mellitus. Indian J Med Res. 1978;68:774–784. [PubMed] [Google Scholar]
- 15.Stalmans W, Hers HG. The stimulation of liver glycogen phosphorylase b by AMP, fluoride and sulfate. A technical note of the specific determination of the a and b forms of liver glycogen phosphorylase. Eur j Biochem. 1975;54:341–350. doi: 10.1111/j.1432-1033.1975.tb04144.x. [DOI] [PubMed] [Google Scholar]
- 16.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissue by thiobarbi-turic acid test. Anal Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 17.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Analytical Biochem. 1970;34(1):30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 18.Van Bezooijen RL, Que I, Ederveen AG, Kloosterboer HJ, Papapoulos SE, Löwik CW. Plasma nitrate+nitrite levels are regulated by ovarian steroids but do not correlate with trabecular bone mineral density in rats. J Endocrinol. 1998;159(1):27–34. doi: 10.1677/joe.0.1590027. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Zhang J, Fang Y, Zhao C, Zhu Y. Ginse-noside Rg1 delays tert-butyl hydroperoxide-induced premature senescence in human WI-38 diploid fibroblast cells. J Gerontol A Biol Sci Med Sci. 2008;63(3):253–264. doi: 10.1093/gerona/63.3.253. [DOI] [PubMed] [Google Scholar]
- 20.Ohkuwa T, Sato Y, Naoi M. Hydroxyl radical formation in diabetogenic rats induced by streptozotocin. Life Sci. 1995;56:1789–1795. doi: 10.1016/0024-3205(95)00150-5. [DOI] [PubMed] [Google Scholar]
- 21.Daisy P, Balasubramanian K, Rajalakshmi M, Eliza J, Selvaraj J. Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine. 2010;17(1):28–36. doi: 10.1016/j.phymed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Chung SH, Choi CG, Park SH. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch Pharm Res. 2001;24(3):214–218. doi: 10.1007/BF02978260. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Mechin MC, van de Werve G. Diabetes affects similarity the catalytic subunit and putative glucose-6-phosphate translocase of glucose-6-phosphatase. J Biol Chem. 1999;274:33866–33868. doi: 10.1074/jbc.274.48.33866. [DOI] [PubMed] [Google Scholar]
- 24.Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of gucose-6-phosphase gene expression by protein kinase Bα and the fork head transcription factor FKHR. Evidence for insulin response unit dependent and independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 25.Luo JZ, Luo L. Ginseng on hyperglycemia: effects and mechanisms. Evid. Based Complement Alternat Med. 2009;6(4):423–427. doi: 10.1093/ecam/nem178. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wang BX, Zhou QL, Yang M, Wang Y, Cui ZY, Liu YQ, Ikejima T. Hypoglycemic mechanism of ginseng glycopeptide. Acta . Pharmacol. Sin. 2003;24(1):61–66. [PubMed] [Google Scholar]
- 27.Scott GI, Colligan PB, Ren BH, Ren J. Ginse-nosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134(6):1159–1165. doi: 10.1038/sj.bjp.0704377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo JZ, Luo L. American Ginseng Stimulates Insulin Production and Prevents Apoptosis through Regulation of Uncoupling Protein-2 in Cultured beta Cells. Evid Based Complement Alternat Med. 2006;3(3):365–372. doi: 10.1093/ecam/nel026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han KL, Jung MH, Sohn TH, Hwang JK. Ginse-noside 20(S)-Protopanaxatriol (PPT) activates peroxisome proliferator-activated receptor γ (PPAR γ) in 3T3-L1 adipocytes. Biol Pharm Bull. 2006;29(1):110–113. doi: 10.1248/bpb.29.110. [DOI] [PubMed] [Google Scholar]
- 30.Shim JY, Kim MH, Kim HD, Ahn JY, Yun YS, Song JY. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicol Appl Pharmacol. 2010;242(3):318–325. doi: 10.1016/j.taap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y, Ji LL. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr. 2003;133(11):3603–3609. doi: 10.1093/jn/133.11.3603. [DOI] [PubMed] [Google Scholar]
- 32.Lee TK, O'Brien KF, Wang W, Sheng C, Wang T, Johnke RM, Allison RR. American Ginseng Modifies Cs-Induced DNA Damage and Oxidative Stress in Human Lymphocyte. Open Nucl Med J. 2009;1(1):1–8. doi: 10.2174/1876388X00901010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Molec. Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 34.Achike FI, Kwan CY. Nitric oxide, human diseases and the herbal products that affect the nitric oxide signalling pathway. Clin. Exp Pharmacol Physiol. 2003;30(9):605–615. doi: 10.1046/j.1440-1681.2003.03885.x. [DOI] [PubMed] [Google Scholar]
- 35.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32(8):1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 36.Davidson SM, Duchen MR. Effects of NO on mitochondrial function in cardiomyocytes: Pathophysiological relevance. Cardiovasc Res. 2006;71(1):10–12. doi: 10.1016/j.cardiores.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Khamaisi M, Keynan S, Bursztyn M, Dahan R, Reinhartz E, Ovadia H, Raz I. Role of renal nitric oxide synthase in diabetic kidney disease during the chronic phase of diabetes. Nephron Physiol. 2006;102(3-4):72–80. doi: 10.1159/000089946. [DOI] [PubMed] [Google Scholar]
- 38.Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. Systematic review of the effects of ginseng on cardiovascular risk factors. Ann Pharmacother. 2006;40(1):83–95. doi: 10.1345/aph.1G216. [DOI] [PubMed] [Google Scholar]
- 39.Pearce LL, Kanai AJ, Birder LA, Pitt BR, Peterson J. The catabolic fate of nitric oxide: the nitric oxide oxidase and peroxynitrite reductase activities of cytochrome oxidase. J Biol Chem. 2002;277(16):13556–13562. doi: 10.1074/jbc.M109838200. [DOI] [PubMed] [Google Scholar]
- 40.Figarola JL, Scott S, Loera S, Xi B, Synold T, Weiss L, Rahbar S. Prevention of early renal disease, dyslipidaemia and lipid peroxidation in STZ-diabetic rats by LR- 9 and LR-74, novel AGE inhibitors. Diabetes Metab Res Rev. 2005;21:533–544. doi: 10.1002/dmrr.550. [DOI] [PubMed] [Google Scholar]
- 41.Hyun YK, Ki SK, Yamabe N, Nagai R, Yokozawa T. Protective effect of heat-processed American ginseng against diabetic renal damage in rats. Agricu. and Food Chem. 2007;55(21):8491–8497. doi: 10.1021/jf071770y. [DOI] [PubMed] [Google Scholar]
- 42.Nakaya Y, Mawatari K, Takahashi A, Harada N, Hata A, Yasui S. The phytoestrogen ginsensoside Re activates potassium channels of vascular smooth muscle cells through PI3K/Akt and nitric oxide pathways. J Med Invest. 2007;54:381–384. doi: 10.2152/jmi.54.381. [DOI] [PubMed] [Google Scholar]
- 43.Lee SK, Lee JO, Kim JH, Jung JH, You GY, Park SH, Kim HS. C-peptide stimulates nitrites generation via the calcium-JAK2/STAT1 pathway in murine macrophage Raw264.7 cells. Life Sci. 2010;86(23-24):863–868. doi: 10.1016/j.lfs.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Cong L, Chen J. Effects of exercise on leptin in streptozotocin-induced diabetic rats. Wei Sheng Yan Jiu. 2001;30(3):158–159. [PubMed] [Google Scholar]
- 45.Forst T, Kunt T, Wilhelm B, Weber MM, Pfützner A. Role of C-Peptide in the regulation of microvascular blood flow. Exp Diabetes Res. 2008:176245. doi: 10.1155/2008/176245. [DOI] [PMC free article] [PubMed] [Google Scholar]