Abstract
Objective
To assess the efficacy of folic acid (FA) supplementation and fortification in preventing neural tube defects (NTDs) in a high prevalence region of the US.
Study design
Active and passive surveillance methods were used to identify all fetuses/infants affected by an NTD in South Carolina. Prevalence rates were compared with FA intake to determine the effects of increased intake on NTD occurrence and recurrence.
Results
From 1992–2009, 916 NTD cases occurred in South Carolina with isolated defects comprising 79% of cases. The NTD rate decreased 58% during this period. There was one NTD-affected pregnancy among 418 subsequent pregnancies (0.2%) in mothers with previous NTD-affected pregnancies who consumed periconceptional FA supplements and four NTDs among 66 pregnancies (6.1%) in which the mother did not take FA supplements. Folic acid supplementation increased from 8% to 35% from 1992–2007 and knowledge of the protective benefits of FA increased from 8% to 65% in women of childbearing age.
Conclusions
Increased periconceptional intake of FA appeared to reduce NTDs in a high prevalence region. The rate of spina bifida and anencephaly in South Carolina is now essentially the same (0.69 cases per 1000 live births and fetal deaths) as the 1998–2005 US rate (0.69).
Keywords: birth defects, folic acid, South Carolina, spina bifida, anencephaly, encephalocele
Neural tube defects (NTDs) are serious forms of craniospinal birth defects that result from the failure of the neural tube to close during the first month of embryonic development.1,2 The three major forms of NTDs are spina bifida, anencephaly, and encephalocele. Spina bifida usually results in paralysis below the level of the spinal lesion and hydrocephaly, whereas anencephaly results in death in utero or death shortly after birth, and the effects and outcome of encephalocele are variable.2,3 Although specific genetic and environmental causes are known for a minority of NTDs, especially those with associated malformations, most isolated NTDs are thought to have a multifactorial basis.1 Recognized predisposing factors that may increase the risk for an NTD include maternal diabetes, maternal obesity, early prenatal exposure to anticonvulsant medication and folic acid (FA) antagonists, early amnion rupture, twin gestation, and prior occurrence of an NTD in a first- to second-degree relative.1,4–7
Following Smithells’ observations (1982) on the protective benefits of FA against NTDs and a decade-long controversy over his findings, two studies were decisive in mounting a public health effort to reduce the risk of NTDs.8–10 The first was the Medical Research Council (1991) case-control study showing 72% reduction in NTDs among high risk pregnancies using 4.0 milligrams (mg) of FA in the periconceptional period.9 The second was the study by Czeizel and Dudas (1992) showing a similar reduction of the occurrence of NTDs using 0.8 mg of FA in the periconceptional period.10
Because South Carolina had been previously identified as a region with a high prevalence of NTDs, having approximately two times the national prevalence rate from 1973–1977,11 the South Carolina NTD Surveillance and Prevention Program was initiated to capitalize on knowledge of the protective effect of FA.12–14 The program followed recommendations from the Centers for Disease Control and Prevention (CDC) that all women of childbearing age should consume 0.4 mg of FA per day and that women with a previous NTD-affected pregnancy consume 4 mg of FA daily in the periconceptional period.15,16 A boost came to the prevention effort in 1996 when the Food and Drug Administration mandated that enriched cereal grain flours be fortified with 0.14 mg FA per 100 grams milled flour.17 The rule, set to take place by January 1998, was accomplished by South Carolina flour producers by the summer of 1996. This report describes the identification and prevention of NTDs in South Carolina from 1992 through 2009.
Methods
Neural tube defects were defined as cases of spina bifida, anencephaly, and encephalocele.1 Midline cranial aplasia cutis congenita and multiple vertebral malformations were included as forme fruste NTDs. Isolated NTDs had no significant anomalies in other organ systems. Hydrocephaly and club feet were considered secondary consequences of NTDs. Abdominal wall defects, diaphragmatic hernia, and holoprosencephaly were considered non-related malformations for purposes of this study.
Both active and passive surveillance methods were used to screen statewide for NTDs.12,13 Active surveillance methods involved monitoring results of maternal α-fetoprotein laboratories, amniocentesis testing, and pregnancy ultrasonography programs. Passive surveillance methods included monthly reviews of medical reports from delivery hospitals and annual reviews of state birth and death certificates. All known live birth and fetal death cases from South Carolina were included. The surveillance period was from October 1 through September 30 of every year from 1992 through 2009. All infants that were conceived in South Carolina, including those delivered out-of-state, were included. Surveillance of NTDs was coordinated by the Greenwood Genetic Center from 1992 through June 2006 and thereafter by the South Carolina Department of Health and Environmental Control.
All cases were classified as spina bifida, anencephaly, or encephalocele. Co-occurring anencephaly and spina bifida (24 cases) were included in the anencephaly category; co-occurring spina bifida and encephalocele (four cases) were designated spina bifida. The two cases of aplasia cutis congenita were designated encephaloceles and the one case of multiple vertebral malformations was designated spina bifida. When possible, cases were examined by geneticists at the Greenwood Genetic Center and chromosome analyses were performed to search for coexisting anomalies and determine causation.
Efforts to decrease the occurrence of NTDs were targeted at health care professionals and the general public. Prevention alerts on FA and annual newsletters were distributed to obstetricians, family physicians, pediatricians, and health department clinics. A FA representative visited physician offices and health clinics to reinforce the FA message and provide patient literature and vitamins.
A public awareness campaign focused on women of childbearing age using billboards, television and radio announcements, newspaper releases, fact sheets, and brochures. The FA prevention message was included in continuing education courses for high school science teachers and in materials distributed at bridal fairs, sports events, and community festivals.
Once notified of an NTD-affected pregnancy, South Carolina Birth Defects Prevention Program personnel obtained permission from the primary physician to contact the mother. With such permission, a letter and a packet explaining recurrence prevention with FA were sent to the mother. The mother was contacted by telephone a week later to see if she was willing to participate in a behavioral risk survey and recurrence prevention program. Folic acid intake of 0.4 mg daily was recommended for women who were not actively attempting to get pregnant and 4.0 mg FA daily when actively trying to get pregnant. Enrolled women were continually monitored for FA supplement use and their reproductive plans. Women who were not actively trying to get pregnant were contacted by telephone every three months. Women who were pregnant or were actively trying to get pregnant were contacted every month and reminded of the recommended amount of FA intake.
During the initial five years of this project, the level of knowledge and use of FA supplementation was determined by interviews of a group of women (n=287) who had pregnancies which were not affected with an NTD. From 1997 to 2007, telephone surveys were made that included approximately 1,000 women each year who were in the childbearing years (ages 15 through 45 years). Among other questions, they were asked if they knew of the health benefits of FA, if they took FA supplements and, if so, how often.
Prevalence rates of NTDs were recorded as the total number of NTD cases per 1,000 live births and fetal deaths. Chi-Square or two-tailed Fisher exact tests were performed using Epi Info™ version 3.4.3 (CDC, Atlanta, GA). Two-sided Cochran-Armitage trend tests were run using SAS Version 9.2 (SAS Institute Inc., Cary, NC).
Results
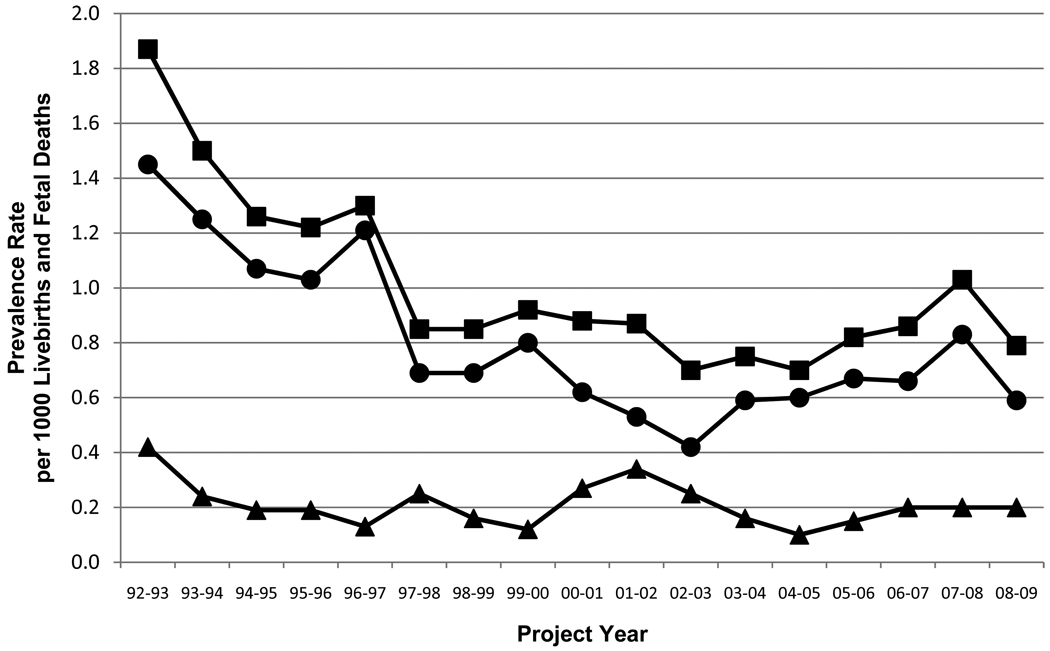
The annual prevalence rates for the 17 years of surveillance are shown in Table I with a graphic representation of the rates of isolated NTDs, NTDs with other anomalies, and total NTDs for years 1–17 (Figure 1). There were statistically significant declines in overall NTDs (p<0.0001) and in isolated NTDs (p<0.0001), but not for NTDs with associated anomalies (p=0.2803) from 1992 through 2009. Table I also shows the rates of spina bifida, anencephaly, and encephalocele for 17 years of surveillance. The rates of spina bifida (p<0.0001) and anencephaly (p<0.0001) declined significantly during this period and the decrease in the rate of encephalocele (p=0.0586) was near significance. The number of NTD cases totalled 916 among the 945,685 livebirths and fetal deaths during the 17 year period.
Table 1.
Neural tube defect (NTD) numbers and rates1 per 1,000 live births and fetal deaths in South Carolina, by project year2 and type, 1992–2009
| TYPE OF NTD | 92–932 | 93–94 | 94–95 | 95–96 | 96–97 | 97–98 | 98–99 | 99–00 | 00–01 | 01–02 | 02–03 | 03–04 | 04–05 | 05–06 | 06–07 | 07–083 | 08–09 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISOLATED SPINA BIFIDA |
14 0.85 |
37 0.69 |
30 0.57 |
23 0.44 |
28 0.54 |
18 0.33 |
16 0.28 |
20 0.34 |
16 0.27 |
16 0.27 |
13 0.22 |
14 0.23 |
19 0.30 |
18 0.29 |
20 0.31 |
18 0.29 |
17 0.29 |
337 0.36 |
| SPINA BIFIDA WITH ANOMALIES |
2 0.12 |
4 0.08 |
2 0.04 |
2 0.04 |
4 0.08 |
2 0.04 |
1 0.02 |
5 0.09 |
8 0.13 |
6 0.10 |
8 0.13 |
5 0.08 |
3 0.05 |
3 0.05 |
8 0.13 |
1 0.02 |
7 0.12 |
71 0.08 |
| TOTAL SPINA BIFIDA |
16 0.97 |
41 0.77 |
32 0.61 |
25 0.48 |
32 0.61 |
20 0.36 |
17 0.30 |
25 0.43 |
24 0.40 |
22 0.37 |
21 0.35 |
19 0.31 |
22 0.35 |
21 0.34 |
28 0.44 |
19 0.31 |
24 0.40 |
408 0.43 |
| ISOLATED ANENCEPHALY |
7 0.42 |
27 0.51 |
17 0.32 |
24 0.46 |
30 0.58 |
13 0.24 |
15 0.26 |
23 0.39 |
17 0.28 |
13 0.22 |
11 0.18 |
19 0.31 |
14 0.22 |
18 0.29 |
15 0.24 |
27 0.44 |
14 0.24 |
304 0.32 |
| ANENCEPHALY WITH ANOMALIES |
4 0.24 |
3 0.06 |
4 0.08 |
4 0.08 |
1 0.02 |
8 0.15 |
6 0.11 |
1 0.02 |
5 0.08 |
9 0.15 |
3 0.05 |
3 0.05 |
0 0.00 |
3 0.05 |
2 0.03 |
8 0.13 |
1 0.02 |
65 0.07 |
| TOTAL ANENCEPHALY |
11 0.66 |
30 0.56 |
21 0.40 |
28 0.54 |
31 0.59 |
21 0.38 |
21 0.37 |
24 0.41 |
22 0.37 |
22 0.37 |
14 0.23 |
22 0.36 |
14 0.22 |
21 0.34 |
17 0.27 |
35 0.57 |
15 0.25 |
369 0.39 |
| ISOLATED ENCEPHALOCELE |
3 0.18 |
3 0.06 |
9 0.17 |
7 0.13 |
5 0.10 |
7 0.13 |
8 0.14 |
4 0.07 |
4 0.07 |
2 0.03 |
3 0.05 |
3 0.05 |
6 0.10 |
5 0.08 |
7 0.11 |
6 0.10 |
4 0.07 |
86 0.09 |
| ENCEPHALOCELE WITH ANOMALIES |
1 0.06 |
6 0.11 |
4 0.08 |
4 0.08 |
2 0.04 |
4 0.07 |
2 0.04 |
1 0.02 |
3 0.05 |
5 0.08 |
4 0.07 |
2 0.03 |
2 0.03 |
3 0.05 |
3 0.05 |
3 0.05 |
4 0.07 |
53 0.06 |
| TOTAL ENCEPHALOCELE |
4 0.24 |
9 0.17 |
13 0.25 |
11 0.21 |
7 0.13 |
11 0.20 |
10 0.18 |
5 0.09 |
7 0.12 |
7 0.12 |
7 0.12 |
5 0.08 |
8 0.13 |
8 0.13 |
10 0.16 |
9 0.15 |
8 0.13 |
139 0.15 |
| ISOLATED NTDS | 24 1.45 |
67 1.26 |
56 1.07 |
54 1.03 |
63 1.21 |
38 0.69 |
39 0.69 |
47 0.80 |
37 0.62 |
31 0.53 |
27 0.45 |
36 0.59 |
39 0.62 |
42 0.69 |
42 0.66 |
51 0.83 |
35 0.59 |
728 0.77 |
| NTDS WITH ANOMALIES |
7 0.42 |
13 0.24 |
10 0.19 |
10 0.19 |
7 0.13 |
14 0.25 |
9 0.16 |
7 0.12 |
16 0.27 |
20 0.34 |
15 0.25 |
10 0.16 |
5 0.08 |
8 0.13 |
13 0.20 |
12 0.20 |
12 0.20 |
188 0.20 |
| TOTAL NTDS | 31 1.87 |
80 1.50 |
66 1.26 |
64 1.22 |
70 1.34 |
52 0.95 |
48 0.85 |
54 0.92 |
53 0.88 |
51 0.87 |
42 0.70 |
46 0.75 |
44 0.70 |
50 0.82 |
55 0.86 |
63 1.03 |
47 0.79 |
916 0.97 |
The decrease in the rate ratio over the 17 years is significant for spina bifida (P<0.0001), for anencephaly (P<0.0009), for isolated NTDs (P<0.0001), and for total NTDs (P<0.0001), but not for encephalocele (P<0.0586) nor NTDs with associated anomalies (P <0.2803).
Project years = October through September, year 1 ending September 1993, year 17 ending September 2009
Anencephaly numbers for this period include 6 terminations in Georgia that we are not able to confirm.
Figure 1.
Rates of total NTDs (squares), isolated NTDs (circles), and NTDs with other anomalies (triangles) per 1000 live births and fetal deaths for 1992 through 2009. Project years run from October to September of the next year. Two-sided Cochrane-Armitage trend test results were significant for total NTDs (p=0.0001) and isolated NTDs (p=0.0001), and the change in rates of NTDs with other anomalies was not significant (p=0.2803).
Ultrasonography was the most frequent means of NTD detection in all years of surveillance, accounting for 55% of identification of cases in the first five years and for 78% in the last five years (Table II; available at www.jpeds.com). Maternal serum screening for alpha fetoprotein became a progressively less frequent source of initial case identification. Only 13% of NTD cases reached delivery without prior detection. Three sources – medical records, genetics clinics/autopsy records, and obstetric offices/clinics – accounted for 76% of case notifications to the surveillance program (Table III; available at www.jpeds.com).
Table 2; online.
Methods of detection of neural tube defects in South Carolina, 1992–2009
| Anencephaly | Encephalocele | Spina Bifida | Total | % of Total | |
|---|---|---|---|---|---|
| Ultrasonography | 270 | 95 | 236 | 601 | 65.6% |
| Maternal serum α- fetoprotein screen1 |
79 | 13 | 78 | 170 | 18.6% |
| Amniotic fluid α- fetoprotein screen |
4 | 6 | 15 | 25 | 2.7% |
| Delivery | 17 | 27 | 76 | 120 | 13.1% |
| Total | 370 | 141 | 405 | 916 | 100% |
This was the first postive screening test that indicated the possibility of an NTD-affected pregnancy in these cases.
Table 3; online.
Methods of notification for the surveillance program from 1992 through 2009
| Methods of Notification | Number (%) |
|---|---|
| Hospital Medical Records Surveillance | 227 (25%) |
| Genetics Clinics/Autopsy | 266 (29%) |
| Obstetric Office or Clinic | 206 (22%) |
| Fetal Boards | 84 (9%) |
| Maternal Serum Screening Program | 70 (8%) |
| Vital Records | 26 (3%) |
| Patient Initiated | 11 (1%) |
| Spina Bifida Clinic | 11 (1%) |
| CDC1 Surveillance Verification | 10 (1%) |
| Health Department Prenatal Clinic | 4 (0.4%) |
| Children's Rehabilitative Services Clinic | 1 (0.1%) |
| Total | 916 (100%) |
CDC = Centers for Disease Control and Prevention
At the outset of this study, spina bifida was the most common of the NTDs, accounting for 52% of the total number. Anencephaly was intermediate in prevalence and encephalocele least common, accounting for 35% and 13%, respectively.
Prevalence rates for spina bifida and anencephaly decreased significantly during the 17 year period with the prevalence rate for anencephaly exceeding the rate for spina bifida in some years (Table I). The prevalence rate for encephaloceles and for NTDs with associated anomalies did not decrease significantly. Among the 916 total NTDs identified, 728 (79%) were isolated defects.
A slight majority (106 of 188 – 56%) of NTDs with other structural anomalies constituted recognizable patterns of malformations (Table IV). Chromosome aberrations were found in 43 cases, with trisomy 18 (21 cases) being most common and triploidy (6 cases) being second most common. Other syndromes included amniotic bands (33 cases), Meckel syndrome (12 cases), OEIS (Omphalocele, Exstrophy of the bladder, Imperforate anus, and Sacral meningomyelocele; 7 cases), NTD-holoprosencephaly (6 cases), limb-body wall complex (2 cases), and Goldenhar syndrome (3 cases).
Table 4.
Neural tube defects (NTDs) with co-occurring anomalies
| SEX1 | RACE2 | |||||||
|---|---|---|---|---|---|---|---|---|
| TYPE OF DEFECT | # OF CASES | M | F | U | W | B | H | O |
| Syndromes | 106 (56.4%) | 49 (26.1%) | 48 (25.5%) | 9 (4.8%) | 62 (33.0%) | 38 (20.2%) | 6 (3.2%) | 0 (0.0%) |
| Chromosome Aberrations | 43 (22.9%) | 20 | 21 | 2 | 27 | 15 | 1 | 0 |
| Amniotic Bands/Cranial Defects | 33 (17.5%) | 17 | 14 | 2 | 15 | 13 | 5 | 0 |
| Meckel Syndrome | 12 (6.3%) | 7 | 3 | 2 | 8 | 4 | 0 | 0 |
| OEIS3 Syndrome | 7 (3.7%) | 2 | 3 | 2 | 4 | 3 | 0 | 0 |
| NTDs/Holoprosencephaly | 6 (3.2%) | 2 | 4 | 0 | 5 | 1 | 0 | 0 |
| Goldenhar Syndrome | 3 (1.6%) | 1 | 2 | 0 | 2 | 1 | 0 | 0 |
| Limb-Body Wall Complex | 2 (1.1%) | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
|
Other associated anomalies not recognized as a syndrome |
82 (43.6%) | 38 (20.2%) | 39 (20.7%) | 5 (2.7%) | 53 (28.2%) | 21 (11.2%) | 4 (2.1%) | 4 (2.1%) |
| TOTAL | 188 (100%) | 87 (46.3%) | 87 (46.3%) | 14 (7.4%) | 115 (61.2%) | 59 (31.4%) | 10 (5.3%) | 4 (2.1%) |
M=male, F=female, U=unknown
W=non-Hispanic white, B=non-Hispanic black, H=Hispanic, O=other
OEIS=Omphalocele, Exstrophy of the bladder, Imperforate anus, and Sacral meningomyelocele
Eighty-two NTD cases with co-occurring anomalies did not appear to constitute recognizable syndromes or there was insufficient documentation to make a syndrome diagnosis (Table V; available at www.jpeds.com). The co-occurring anomalies ranged from mild and trivial (e.g. abnormal palmar creases, bifid scrotum) to severe and lethal (e.g. conjoined twinning, sirenomelia). Certain defects (e.g. diaphragmatic hernia, omphalocele) were placed in this category, although they might also have reasonably been classified as defects secondary to the NTDs.
Table 5; online.
Eighty-two cases of co-occurring anomalies with neural tube defects that do not appear to constitute a recognizable syndrome
| Facial Clefting | |
| Cleft lip/cleft palate | 4 |
| Cleft lip/cleft palate with other anomalies | 8 |
| Cleft lip | 2 |
| Cleft palate | 2 |
| Cleft palate with other anomalies | 3 |
| Facial cleft with other anomalies | 3 |
| Total Facial Clefting | 22 (27%) |
| Cardiac Defects | |
| Cardiac defects | 14 |
| Cardiac defects with other anomalies | 13 |
| Total Cardiac Defects | 27 (33%) |
| Limb Defects | |
| Limb defects | 1 |
| Limb defects with other anomalies | 5 |
| Total Limb Defects | 6 (7%) |
| Gastrointestinal (GI) Defects | |
| GI tract defects with other anomalies | 2 |
| Ventral wall defects | 5 |
| Ventral wall defects with other anomalies | 9 |
| Total GI Tract Defects | 16 (20%) |
| Genitourinary (GU) Defects | |
| Renal agenesis | 1 |
| Renal agenesis with other defects | 9 |
| Other GU tract anomalies | 2 |
| Other GU tract anomalies with other defects | 11 |
| Total GU Tract Defects | 23 (28%) |
| Other Anomalies1 | |
| Diaphragmatic hernia | 9 |
| Situs inversus | 3 |
| Conjoined twins | 2 |
| Microphthalmia | 2 |
| Dandy-Walker | 2 |
| Laryngeal defects | 2 |
| Atelencephaly-aprosencephaly | 1 |
| Craniosynostosis | 1 |
| Cystic lungs | 1 |
| Sirenomelia | 1 |
| Total Other Anomalies | 24 (29%) |
Other findings like single umbilical artery and abnormal palmar creases are not included in the tabulation.
Maternal diabetes mellitus has long been recognized as a risk factor for NTDs.4 We had information on the diabetes status of mothers in 704 pregnancies that resulted in infants born with an NTD. Twenty-eight of these (4.0%) had preexisting diabetes and 27 (3.8%) had gestational diabetes. The percentage of NTD-associated pregnancies with preexisting diabetes (4.0%) was fourfold the background rate of preexisting diabetes in South Carolina (0.9%). The percentage of NTD-associated pregnancies with gestational diabetes (3.8%) was not significantly different from the background rate of gestational diabetes (4.0%).18 After fortification, the rates of NTDs among infants of mothers with preexisting diabetes decreased from 3.5 to 2.7 per 100,000 live births and fetal deaths. The rate of NTDs among infants of mothers with gestational diabetes decreased from 6.4 to 1.4 per 100,000 after fortification.
NTDs (66 spina bifida, 55 anencephaly, and 19 encephalocele) occurred in pregnancies in which the mother reported use of FA during the periconceptional period (Table VI; available at www.jpeds.com). The dose of FA used by the mothers varied from 0.4 mg to 1.8 mg. A slightly higher, but non-significant (chi-square = 0.94, P=0.3321), percentage (23.6%) of these NTD pregnancies had associated anomalies, as compared with the percentage of NTD-associated anomalies (20.0%) born to mothers not using FA.
Table 6; online.
Neural tube defects (NTDs) occurring in pregnancies where mothers who took folic acid
| SEX1 | RACE2 | |||||||
|---|---|---|---|---|---|---|---|---|
| TYPE OF DEFECT | # OF CASES | M | F | U | W | B | H | O |
| Isolated | 107 (76.4%) | 46 (32.9%) | 43 (30.7%) | 18 (12.9%) | 89 (63.6%) | 15 (10.7%) | 2 (1.4%) | 1 (0.7%) |
| Spina bifida | 50 (35.7%) | 26 | 17 | 7 | 40 | 9 | 1 | 0 |
| Anencephaly | 46 (32.9%) | 16 | 22 | 8 | 41 | 4 | 1 | 0 |
| Encephalocele | 11 (7.9%) | 4 | 4 | 3 | 8 | 2 | 0 | 1 |
| Associated Anomalies | 33 (23.6%) | 14 (10.0%) | 16 (11.4%) | 3 (2.1%) | 28 (20.0%) | 5 (3.6%) | 0 (0.0%) | 0 (0.0%) |
| OEIS3 | 3 (2.1%) | 3 | 0 | 0 | 3 | 0 | 0 | 0 |
| Trisomy 18 | 7 (5.0%) | 4 | 3 | 0 | 7 | 0 | 0 | 0 |
| Amniotic bands/cranial defects | 3 (2.1%) | 2 | 1 | 0 | 1 | 2 | 0 | 0 |
| Goldenhar | 1 (0.7%) | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Chromosome aberrations | 5 (3.6%) | 1 | 4 | 0 | 4 | 1 | 0 | 0 |
| Other anomalies | 14 (10.0%) | 4 | 7 | 3 | 12 | 2 | 0 | 0 |
| TOTAL | 140 (100%) | 60 (43.0%) | 59 (42.0%) | 21 (15.0%) | 117 (83.6%) | 20 (14.3%) | 2 (1.4%) | 1 (0.7%) |
M=male, F=female, U=unknown
W=non-Hispanic white, B=non-Hispanic black, H=Hispanic, O=other
OEIS=Omphalocele, Exstrophy of the bladder, Imperforate anus, and Sacral meningomyelocele
Pregnancies (n=484) occurred to women with a prior NTD. Among 418 pregnancies in which the mother used periconceptional FA supplements, there was one recurrence (0.2%), a recurrent anencephaly with a 13q deletion. Among 66 pregnancies in which the mother did not use FA supplements, there were four (6.1%) recurrences (a recurrent Meckel/encephalocele, a recurrent anencephaly, a recurrent encephalocele, and a situation where the first child had spina bifida and the second had craniorachischisis). These proportions are significantly different (p=0.0014) using a two-tailed Fisher exact test. Two of the recurrences (1.2%) occurred in 173 pregnancies prior to fortification, and the other three (1.0%) occurred in 311 post-fortification pregnancies. These proportions are not significantly different (p=1.0000) using a two-tailed Fisher exact test.
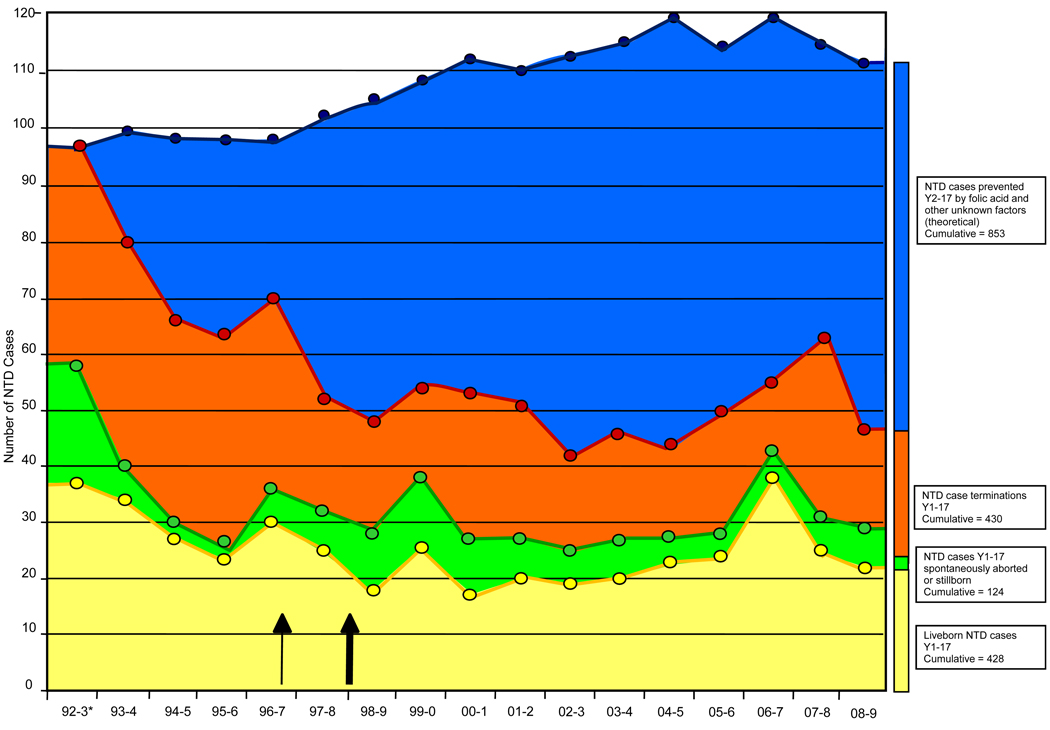
Based on the 1992 prevalence rates of 1.87 cases per 1,000 live births and fetal deaths, approximately 1,738 NTD-affected pregnancies would have been expected over the subsequent 16 years; however, there were only 885 NTD-affected pregnancies during this time period. This represents a reduction of 853 NTD cases prevented by FA and other unknown factors (Table I and Figure 2; available at www.jpeds.com). Since 1992–1993, there has been a decrease in the number of spontaneous abortions with NTDs (67% decrease), terminations of NTD-affected pregnancies (54% decrease), and live born infants with NTDs (54% decrease). Of the total 982 NTD-affected pregnancies over 17 years (Figure 2), 124 (12.6%) were lost to spontaneous abortion and fetal death, 430 (43.8%) were prenatally diagnosed and the pregnancies interrupted, and 428 (43.6%) were live born NTD cases.
Figure 2.
The combined impact of prenatal diagnosis and folic acid utilization on the number of NTDs. The top line of this figure gives an estimate of the neural tube defect cases that would have occurred without folic acid supplementation, fortification, and other unknown factors. The top line was calculated based on the 1992 prevalence rates. *Statewide numbers for this year (1992–1993) extrapolated from surveillance in a 14 county area. All other years are actual surveillance numbers for the entire state. The outcome of three NTD-affected pregnancies is not known. Thin arrow = fortification in South Carolina; thick arrow = mandatory fortification in the US.[C1]
The distribution among males and females with NTDs that had other associated anomalies was equal (87 males, 87 females, 14 with unknown sex), and the sex ratio of isolated NTDs showed significantly more females (47.5%) than males (39.7%, P-value = 0.003). There was a slightly higher prevalence rate of total NTDs among Hispanics (1.2 per 1,000 live births and fetal deaths) than any other racial group (non-Hispanic whites 1.1; non-Hispanic blacks 0.7). Although Hispanics had the highest rate of anencephaly, non-Hispanic whites had the highest rate of encephalocele as well as the highest rate of spina bifida. Hispanics had the highest rate of isolated NTDs, and all races had near equal rates of NTDs with other anomalies.
During the first year of this project (1992), 8% of the women of childbearing age knew of the health benefits of FA and 8% took FA. These percentages increased to 33% and 33% in year 5. These percentages were based on interviews of small numbers (Year 1=38, Year 5=60) of women of childbearing years who completed pregnancies not associated with an NTD infant. From 1997 to 2007, knowledge and use of FA was based on telephone interviews of approximately 1,000 women of childbearing age. The percentage of women having knowledge of FA increased from 32% to 65% and the percentage of women using FA four or more times a week increased from 25% to 35% during this period.
Discussion
A substantial decrease in the prevalence rates of spina bifida, anencephaly, and encephalocele in South Carolina has accompanied the increased consumption of FA by women of childbearing age. Overall, the NTD rate has decreased 58% since 1992, a period during which FA supplement utilization has increased fourfold (from 8% of women of childbearing age to 35%). In addition, many breakfast cereals have been fortified with 400 mcg FA per serving and enriched cereal grain flours have been fortified with 140 mcg FA per 100 g milled flour, the latter occurring between 1996 and 1998.
The latest rate for NTDs from the National Birth Defects Prevention Network covers the period 1998 (fourth quarter) to 2005 (fourth quarter). The “national” rate for spina bifida and anencephaly during this seven year period was 0.69 cases per 1000 live births and fetal deaths. 19,20 The average rate for spina bifida and anencephaly in South Carolina during these same years was 0.69 cases per 1000 live births and fetal deaths. The rate for the most recent year (2009) for South Carolina was 0.65 cases per 1000 live births and fetal deaths, but the national rates for this period are not yet available.
With respect to occurrent NTDs, we also make these observations. First, the birth prevalence in our population has fallen to a level indistinguishable from the national NTD birth prevalence, suggesting that regional differences in NTD occurrence rates are either attributable to FA consumption patterns, or that the variability is confined to folate-responsive etiologic pathways. Second, we note that the reduction in the occurrence rates of spina bifida and anencephaly were more dramatically influenced by folate supplementation than the rate for encephalocele. Given the very high efficacy of FA in recurrence rates for subsequent pregnancies, we infer possible dosage response differences among different types of NTDs. Third, we can infer very little about racial differences in the rates for NTDs, and whether those differences reflect primarily genetic or environmental-cultural differences secondary to actual folate consumption patterns. Finally, we did observe a relatively dramatic reduction in NTD rates that coincided with the introduction of FA into wheat flour spread out over the interval of time from 1996 to 1998 when different sources of wheat flour were consumed by our population of mothers. This was preceded by a decline that mirrors increasing use of folate supplements that were likely attributable to the public health awareness campaign.
At the outset of the study, expectations were that FA supplementation might be approximately 70% efficacious in the prevention of recurrent NTDs.8,9 There has been one recurrence of NTDs in 418 prospectively ascertained subsequent pregnancies (0.2%) to mothers with a prior conception or birth affected with an NTD who used FA in the periconceptional period of the subsequent pregnancy, the recurrence being an encephalocele associated with a 13q deletion. Thus, for isolated NTDs we observed 0.0% recurrence. The four recurrences among the 66 high risk pregnancies (6.1%) in which FA was not used emphasizes the importance of FA in recurrence prevention.8,9 In the absence of folate supplementation, the recurrence rate of NTDs among families with a prior NTD conception or birth is generally stated to be 3–5%, with areas of higher prevalence having recurrence on the high end of that range. 21 For South Carolina, the recurrence risk without folate use would be at the high end, and thus we might have expected 21 or so cases of NTD among the 418 cases receiving folate supplements were folate not efficacious for prevention of NTDs, and approximately three cases among the 66 not receiving folate supplements. The number of pregnancies available for study is still fairly limited. However, we feel that our study and others21 have shown that recurrence prevention is quite effective. Because only approximately one-third of states have or have had NTD recurrence prevention projects,22 more public health agencies need to provide the funding and personnel to prevent the recurrence of NTDs in this very high risk population.
Because FA fortification appears to have been effective in decreasing the NTD rate among infants of mothers with gestational diabetes to the population rate, the NTD rate among infants of mothers with preexisting diabetes remained high after fortification. A greater effort to control preexisting diabetes may be necessary to further reduce the NTD rate in this high risk group. The probability that higher dose FA supplementation might be protective for infants of mothers with preexisting diabetes deserves further study.
Although several of the NTD cases with other anomalies may represent previously reported associations, there were no compelling reasons (known inheritance pattern, specificity of the association, confirmatory laboratory testing) to designate them as such. Among these previously reported associations are facial duplication-anencephaly association,23 anophthalmia-NTD association,24 coloboma-microphthalmia-clefting syndrome,25 laterality syndrome,26 schisis association,27 anterior encephalocele-anophthalmia syndrome,28 and others. Hunter has made an extensive listing of the numerous syndromes and associations of NTDs and other anomalies.2 Specific recurrence risks are available for the NTD syndromes, but not for most of the entities with NTDs and other anomalies that do not constitute a specific syndrome.
Fifteen percent (15%) of NTDs occurred in pregnancies in which the mothers used FA in the periconceptional period. A slightly higher rate of syndromal NTDs was found in this group, suggesting that FA protection is more effective against isolated NTDs.
An increasing number of pregnant mothers receive birth defect screening through ultrasound and blood tests, resulting in an increasing number of NTDs that are diagnosed prenatally (Table III). Consequently, it seems important to point out that although termination of pregnancies affected with NTDs accounts for some fraction of the reduction of NTDs, it is the lower number of NTD-affected pregnancies conceived and not an increasing termination rate that is the driving force in the dramatic reductions we describe (Figure 2 and Table VII).
Table 7.
Outcome of neural tube defect (NTD)-affected pregnancies
| 92–931 | 93–94 | 94–95 | 95–96 | 96–97 | 97–98 | 98–99 | 99–00 | 00–01 | 01–02 | 02–03 | 03–04 | 04–05 | 05–06 | 06–07 | 07–08 | 08–09 | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIVEBIRTHS | 9 | 27 | 20 | 14 | 27 | 21 | 15 | 20 | 12 | 13 | 16 | 15 | 15 | 16 | 26 | 20 | 16 | 302 |
| LIVEBIRTHS (died) | 3 | 7 | 7 | 9 | 3 | 4 | 3 | 6 | 5 | 7 | 3 | 5 | 8 | 8 | 12 | 5 | 6 | 101 |
| TERMINATIONS | 12 | 40 | 36 | 38 | 34 | 20 | 20 | 18 | 26 | 24 | 16 | 18 | 16 | 23 | 11 | 32 | 18 | 402 |
| FETAL DEMISE | 4 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 13 |
| STILLBIRTHS | 2 | 3 | 1 | 0 | 4 | 6 | 10 | 9 | 7 | 3 | 5 | 7 | 5 | 2 | 5 | 5 | 6 | 80 |
| SPONTANEOUS ABORTION |
1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 15 |
| UNKNOWN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| TOTAL NTDs | 31 | 80 | 66 | 64 | 70 | 52 | 48 | 54 | 53 | 51 | 42 | 46 | 44 | 50 | 55 | 63 | 47 | 916 |
Project years = October through September, year 1 ending September 1993, year 17 ending September 2009
Having now passed through the excitement that accompanied the dramatic discovery of the preventive efficacy of FA, we should be concerned about sustaining an awareness of the importance of FA consumption periconceptionally and consider new mechanisms for increasing passive FA intake to maintain the ground we have gained in the fight to eliminate preventable birth defects. The gap between the percentage of women who know about FA (65%) and the percentage who take FA (35%) offers an opportunity to convert knowledge into action through educational means. Our finding that fortification does not appear to reduce recurrent NTDs, calls for public health agencies to focus greater efforts and resources on this identifiable high risk group of pregnancies. Similarly, the failure of fortification to compensate for the influence of preexisting diabetes in the mother, calls for greater attention to diabetes control in the mothers and for a case-control study of the effectiveness of higher dose folate supplementation in this high risk-group. Finally, the possibility of increasing the amount of FA in enriched cereal grain flours, while remote, deserves further examination.
Acknowledgments
We thank the families and physicians, who have provided information for this analysis, and Rene Betros, Carolyn Lovell, Nancy Clary, Sylvia Stidham, Kim Jenkins, Marlena Clary, Debbie Price and Linda Cooley (employees of Greenwood Genetic Center), who reviewed and abstracted medical records. We also thank Sara Sarasua and Dr. Charles Schwartz, who provided constructive reviews of the manuscript.
Supported by the South Carolina (SC) Birth Defects Foundation, SC Department of Disabilities and Special Needs, SC Department of Health and Environmental Control, SC Department of Health and Human Services, the Developmental Disabilities Council of the Governor’s Office, SC Chapter of the March of Dimes, Centers for Disease Control and Prevention (U85/CCU 408774, U50/CCU 421871, and U50/CCU 416008 to R.S.) and the National Institute of Mental Health (MH 57840 to R.S.).
List of Abbreviations
- B
Non-Hispanic Black
- CDC
Centers for Disease Control and Prevention
- F
Female
- FA
Folic Acid
- GI
Gastrointestinal
- GU
Genitourinary
- H
Hispanic
- M
Male
- mg
milligrams
- NTDs
Neural Tube Defects
- OEIS
Omphalocele, Exstrophy of the bladder, Imperforate anus, and Sacral meningomyelocele
- O
Other
- U
Unknown
- W
Non-Hispanic White
- YR
Year
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Rampersaud E, Melvin EC, Speer MC. Nonsyndromic neural tube defects: Genetic basis and genetic investigations. In: Wyszynski DF, editor. Neural Tube Defects, from Origin to Treatment. New York: Oxford Univ Press; 2006. pp. 165–175. [Google Scholar]
- 2.Hunter AGW. Brain and spinal cord. In: Stevenson RE, Hall JG, editors. Human Malformations and Related Anomalies. Ed 2. New York: Oxford Univ Press; 2006. pp. 715–755. [Google Scholar]
- 3.Davis BE, Daley CM, Shurtleff DB, Duguay S, Seidel K, Loeser JD, et al. Long-term survival of individuals with myelomeningocele. Pediatr Neurosurg. 2005;41:186–191. doi: 10.1159/000086559. [DOI] [PubMed] [Google Scholar]
- 4.Locken MR. Current perspectives on the causes of neural tube defects resulting from diabetic pregnancy. Am J Med Genet C. 2005;135C:77–87. doi: 10.1002/ajmg.c.30056. [DOI] [PubMed] [Google Scholar]
- 5.Higginbottom MC, Jones KL, Hall BD, Smith DW. The amniotic band disruption complex: Timing of amniotic rupture and variable spectra of consequent defects. J Pediatr. 1979;95:544–549. doi: 10.1016/s0022-3476(79)80759-3. [DOI] [PubMed] [Google Scholar]
- 6.James WH. Twinning and anencephaly. Ann Hum Biol. 1976;3:401–409. doi: 10.1080/03014467600001661. [DOI] [PubMed] [Google Scholar]
- 7.Greene NDE, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18:R113–R119. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smithells RW. Neural tube defects: prevention by vitamin supplements. Pediatrics. 1982;69:498–499. [PubMed] [Google Scholar]
- 9.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 10.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. New Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg F, James LM, Oakley GP. Estimates of birth prevalence rates of spina bifida in the United States from computer-generated maps. Am J Obstet Gynecol. 1983;145:570–573. doi: 10.1016/0002-9378(83)91198-5. [DOI] [PubMed] [Google Scholar]
- 12.Allen WP, Stevenson RE, Thompson SJ, Dean JH. The impact of prenatal diagnosis on NTD surveillance. Prenat Diag. 1996;16:531–535. doi: 10.1002/(SICI)1097-0223(199606)16:6<531::AID-PD914>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson RE, Allen WP, Pai GS, Best R, Seaver LH, Dean J, et al. Decline in prevalence of neural tube defects in high-risk region of the United States. Pediatrics. 2000;106:677–683. doi: 10.1542/peds.106.4.677. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson RE, Seaver LH, Collins JS, Dean JH. Neural tube defects and associated anomalies in South Carolina. Birth Defects Res A Clin Mol Teratol. 2004;70:554–558. doi: 10.1002/bdra.20062. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Use of folic acid prevention of spina bifida and other neural tube defects – 1983–1991. MMWR. 1991;40:513–516. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR. 1992;41:1–7. [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Federal Register. 1996;61:871. [Google Scholar]
- 18.SC Department of Health and Environmental Control. 2007 Birth Certificate Data. http://scangis.dhec.sc.gov/scan/bdp/tables/birthtable.aspx.
- 19.National Birth Defects Prevention Network. Prevalence of spina bifida and anencephaly before and after folic acid fortification, NBDPN Neural Tube Defect Ascertainment Project; 1995–2005. [updated 2009 July 15; cited 2010 Jan 29]. Available from: http://www.nbdpn.org/current/resources/ntd_fa_info.html.
- 20.Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82:527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 21.Grosse S, Collins JS. Folic acid supplementation and neural tube defect recurrence prevention. Birth Defects Res A Clin Mol Teratol. 2007;79:737–742. doi: 10.1002/bdra.20394. [DOI] [PubMed] [Google Scholar]
- 22.Collins JS, Canfield MA, Pearson K, Kirby RS, Case AP, Mai CT, et al. Public health projects for preventing the recurrence of neural tube defects in the United States. Birth Defects Res A Clin Mol Teratol. 2009;85:935–938. doi: 10.1002/bdra.20619. [DOI] [PubMed] [Google Scholar]
- 23.Andres R, Bixler D. Anencephaly with facial duplications. J Craniofac Genet Dev Biol. 1981;1:245–252. [Google Scholar]
- 24.Rogers RC. SN(GGC-8217) 23 month old white male. Proc Greenwood Genet Center. 1988;7:55. [Google Scholar]
- 25.Ravine D, Ragge NK, Stephens D, Oldrige M, Wilkie AO. Dominant coloboma-microphthalmia syndrome associated with sensorineural hearing loss, hematuria and cleft lip/palate. Am J Med Genet. 1997;72:227–236. doi: 10.1002/(sici)1096-8628(19971017)72:2<227::aid-ajmg19>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Peters HL, Bankier A. Lipomatous meningomyelocele, athyrotic hypothyroidism, and sensorineural deafness: a new form of syndromal deafness? J Med Genet. 1998;35:948–950. doi: 10.1136/jmg.35.11.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Frias ML, Frias JL, Bermejo E, Rodriguez-Pinilla E, Urioste M. Epidemiological analysis of the schisis association in the Spanish registry of congenital malformations. Am J Med Genet. 1997;70:16–23. [PubMed] [Google Scholar]
- 28.al-Gazali LI, al-Shather WA. Anterior encephalocele and anophthalmia. Clin Dysmorphol. 1996;5:81–83. doi: 10.1097/00019605-199601000-00013. [DOI] [PubMed] [Google Scholar]