Abstract
Background & Aims
Metabolic effects of dietary fat quality in people with type 2 diabetes are not well-understood. The study objective was to evaluate effects of conjugated linoleic acid (CLA) and safflower (SAF) oils on glycemia, blood lipids, and inflammation. The hypothesis we tested is SAF oil improves glycemic and inflammatory markers in a time dependent way that follows accumulation of linoleic acid and CLA isomers in serum of subjects supplemented with dietary oils.
Methods
Fifty-five post-menopausal, obese women with type 2 diabetes enrolled, and 35 completed this randomized, double-masked crossover study. Treatments were eight grams daily of CLA and SAF for 16 weeks each. We used a multiple testing procedure with predetermined step analysis to determine when the earliest time to effect was significant.
Results
CLA did not alter measured metabolic parameters. SAF decreased HbA1c (−0.64±0.18%, p<0.0007) and C-reactive protein (−13.6±8.2mg/L, p<0.0472), increased QUICKI (0.0077±0.0035, p<0.0146) with a minimum time to effect observed 16 weeks after treatment. SAF increased HDL cholesterol (0.12±0.05mmol/L, p<0.0228) with the minimum time to detect an effect of SAF at 12 weeks. The minimum time to detect an increase of c9t11-CLA, t10c12-CLA and linoleic acid in serum of women supplemented CLA or SAF respectively was four weeks.
Conclusions
We conclude that eight grams of SAF daily improved glycemia, inflammation, and blood lipids indicating that small changes in dietary fat quality may augment diabetes treatments to improve risk factors for diabetes-related complications.
Keywords: type 2 diabetes, safflower oil, conjugated linoleic acid, n-6 PUFA
Introduction
Obesity is present in 80% or more of type 2 diabetes cases1. Because of the known benefits for overweight individuals with type 2 diabetes, weight loss is recommended by the American Diabetes Association for optimal diabetes management2. Weight loss resulting in decreased visceral adipose mass may be particularly beneficial, as excess visceral adipose tissue is more strongly associated with markers of metabolic risk than subcutaneous adipose tissue3. A modest 5% loss of body weight can improve glycemia, insulin sensitivity, and blood lipids4. Improvement in glycemia decreases risk for diabetes-related micro- and macrovascular complications5.
Achieving and maintaining weight loss requires significant lifestyle and behavior modification6. To aid in weight loss, an estimated 33.9% of people trying to lose weight report using non-prescription dietary supplements7. A number of dietary supplements are marketed for weight loss, one of which is conjugated linoleic acid (CLA).
CLA refers to a group of positional and stereoisomers of linoleic acid. CLA is present in small quantities in ruminant meat and dairy products, primarily as the c9t11 isomer. Dietary supplements typically provide substantially higher quantities of CLA than is consumed from foods. Most supplements contain approximately equal amounts of the c9t11 and the t10c12 isomers, with t10c12-CLA being the more important isomer with regard to weight loss8. In a meta-analysis, dietary CLA supplementation was shown to have a modest effect in reducing fat mass in humans9. However, there are few studies specifically reporting the effects of CLA supplementation in people with type 2 diabetes.
In a study in our laboratory, we found that CLA reduced body weight by 1.1% and total adipose mass by 3.2% in obese, postmenopausal women with type 2 diabetes10. Despite a significant decrease in both body and adipose masses, the 5% weight loss suggested for improved glycemia was not achieved within the 16 weeks of intervention.
Safflower oil was chosen as a comparison treatment to CLA in this study. Safflower oil is available in U.S. grocery stores and is rich in the essential n-6 polyunsaturated fatty acid (PUFA) linoleic acid. Numerous health organizations have recommendations for dietary linoleic acid intake, generally falling within the range of 3–10% of total energy consumption11. The average daily intake of linoleic acid for women ages 51–70 in the U.S. is 12.6 g, which equates to 5.7% of energy in a 2,000 kilocalorie diet12.
To our knowledge, safflower oil had not previously been implicated in body composition changes in humans, but in our study we unexpectedly found a 6.3% decrease in fat mass of the trunk region, and a 1.6% increase in total body lean mass after safflower oil supplementation10.
The unexpected outcomes of this study led us to conduct secondary analyses to address the following: 1) the effect of these oils on metabolic parameters specifically related to glycemia, blood lipids, and inflammation; and 2) the time course of metabolic changes and its relationship to the time course of supplemented fatty acid appearance in the serum. The hypotheses were 1) safflower oil, and not CLA, would improve markers of glycemia, blood lipids, and inflammation, and 2) treatment effects would not become apparent until after an increase in supplemented fatty acids was detected in the serum.
Materials and Methods
All clinical procedures were conducted at the Clinical Research Center at The Ohio State University Medical Center (Columbus, Ohio). The study was approved by The Ohio State University Institutional Review Board and the Clinical Research Center Advisory Committee. The study was conducted in agreement with the Declaration of Helsinki, and in accordance with International Harmonization/Good Clinical Practice Guidelines.
Subjects
Fifty-five post-menopausal, obese women with type 2 diabetes were recruited from Columbus, Ohio and surrounding communities. All women gave written informed consent before being screened for study participation. Inclusion criteria were as follows: female; ≤ 70 years of age; post-menopausal (absence of menses ≥ 1 yr); obese (BMI ≥30 kg/m2); and HbA1c of 6.5–14%. Exclusion criteria were tobacco use; drug or alcohol abuse; impaired cognitive function; renal, liver or gastrointestinal diseases; current use of exogenous insulin therapy; and current or previous (within the past six months) use of hormone replacement therapy. Of 55 subjects enrolled in the study, 35 completed the entire study. The number of subjects in the study at weeks 0, 4, 8, 12, and 16 of Supplementation Period 1 were 22, 17, 17, 17, and 16 for the CLA treatment, and 33, 31, 28, 27, and 27 for the SAF treatment. The number of subjects in the study at weeks 0, 4, 8, 12, and 16 of Supplementation Period 2 were 27, 27, 24, 24, and 22 for CLA treatment, and 16, 14, 14, 13 and 13 for SAF treatment. Reasons for withdrawal did not vary between groups, and completers were not different than non-completers with regard to demographic variables, BMI, diabetes medications, or years since diagnosis of diabetes. Number and type of adverse events were also not different between treatment groups10.
Study Design
The study was a double-masked, randomized, cross-over design. Subjects were block-randomized by BMI and HbA1c to one of two treatment groups. One group received conjugated linoleic acid (CLA) supplementation for 16 weeks, followed by a 4-week washout period and safflower oil (SAF) supplementation for 16 weeks (CLA to SAF). The other group received treatments in the opposite order with SAF supplementation first, followed by CLA (SAF to CLA).
Treatments
Both treatments provided 8 grams of oil daily, which was consumed in 1-gram capsules provided by Cognis Corporation (Cincinnati, Ohio). The CLA treatment contained a total of 6.4 grams of mixed isomer CLA (50% each of c9t11 and t10c12-CLA) out of 8 grams of total oil. The SAF treatment contained a total of 8 grams of safflower oil. Subjects were instructed to take two capsules each at breakfast, lunch, dinner, and a snack. Fatty acid content of the capsules was verified by gas chromatography analysis10.
CLA supplementation decreased BMI, body weight, and body fat mass. Body weight loss averaged 1.1 kg, and loss of fat mass averaged 1.3 kg. SAF supplementation did not alter body weight, BMI or total fat mass. SAF supplementation increased total lean mass by 1.0 kg and decreased trunk fat mass by 1.6 kg. Details of these analyses are published elsewhere10.
Study Visits
Study visits were held every four weeks in the morning after a 10–12 hour fast. Subjects refrained from taking morning diabetes medications and study supplements until the end of each visit. At all visits, vital signs, body weight and a blood sample were collected. At the beginning and end of each supplementation period (weeks 0 and 16 of both CLA and SAF treatments), subjects underwent an oral glucose tolerance test and measurement of HbA1c.
Oral Glucose Tolerance Test
For the three days prior to the test, subjects were instructed to consume ≥150 grams of carbohydrate per day. Carbohydrate intake was verified from food records. Venous blood was collected at −10, −5, and 0 minutes prior to the start of the test. After the 0 minute blood collection, subjects consumed a 75 gram glucose beverage (NERL Diagnostics, Baltimore, MD) within 10 minutes. Blood samples were collected at 15, 30, 45, 60, 90, 120, 150, and 180 minutes after the start of the test. Serum was analyzed for glucose, insulin, and C-peptide. Area under the curve for glucose, insulin, and C-peptide was calculated using the method of Wolever13.
Biochemical Analyses
Glucose (enzymatic assay), insulin (RIA), C-peptide (RIA), interleukin-6 (IL-6; ELISA) and non-esterified fatty acids (enzymatic assay) were analyzed by the Clinical Research Center Core Laboratory. Glycated hemoglobin (HbA1c) was analyzed with the DCA 2000 (Bayer Co., Indianapolis, IN) with a drop of whole blood obtained from a finger stick. C-reactive protein (CRP; ELISA), total cholesterol (enzymatic assay), HDL cholesterol (immunoinhibition assay), LDL cholesterol (calculated using the Friedwald equation), and triglycerides (enzymatic assay) were analyzed by The Ohio State University Medical Center.
Insulin sensitivity was estimated with the QUICKI equation, which utilizes fasting serum glucose and insulin concentrations14.
Serum fatty acid composition was analyzed for weeks 0, 4, 8, 12, and 16 of each supplementation period as previously described15. Data are reported as percentage of total fatty acids detected for t10c12-CLA, c9t11-CLA and linoleic acid.
Assessment of Adherence
Adequate compliance was defined as the consumption of ≥70% of supplements each month and was assessed through daily logbooks kept by subjects, counting returned pills, and accumulation of t10c12-CLA, c9t11-CLA, and linoleic acid in the serum.
Statistics
To examine the treatment effect and week effect, a mixed effects linear model was used to fit the data. In this model, treatment, week, and interaction between week and treatment were considered as fixed effects, while subject was considered as a random effect. PROC MIXED in SAS 9.2 (SAS Institute, Cary, NC) was applied to analyze the data. The Proc Mixed procedure with subject as a random effect takes into account correlation of the observations between weeks within each subject. A compound symmetry variance-covariance structure was used to estimate the variances and covariances of error terms within subjects. Values are reported as least square estimates and their SE from the mixed effects model. Measurements of serum t10c12-CLA were not normally distributed, with 138 measurements below detection limit of gas chromatography. Therefore, the sign test, a nonparametric method, was used to assess the treatment effect and compute the confidence interval. For each treatment and each endpoint, to determine the earliest time point for which there is a significant change from baseline, a multiple testing procedure with pre-determined steps16 was applied. All statistical testing was at a 5% significance level.
Results
Markers of glucose tolerance and insulin sensitivity
We had previously found a decrease in fasting glucose with SAF and no effect of CLA when looking at the change from the beginning to the end of the study10. To determine the time course of this change, pre-determined steps analysis was employed. This analysis revealed that 16 weeks was the minimum time of supplementation required for SAF to decrease fasting glucose (Figure 1A).
Figure 1.
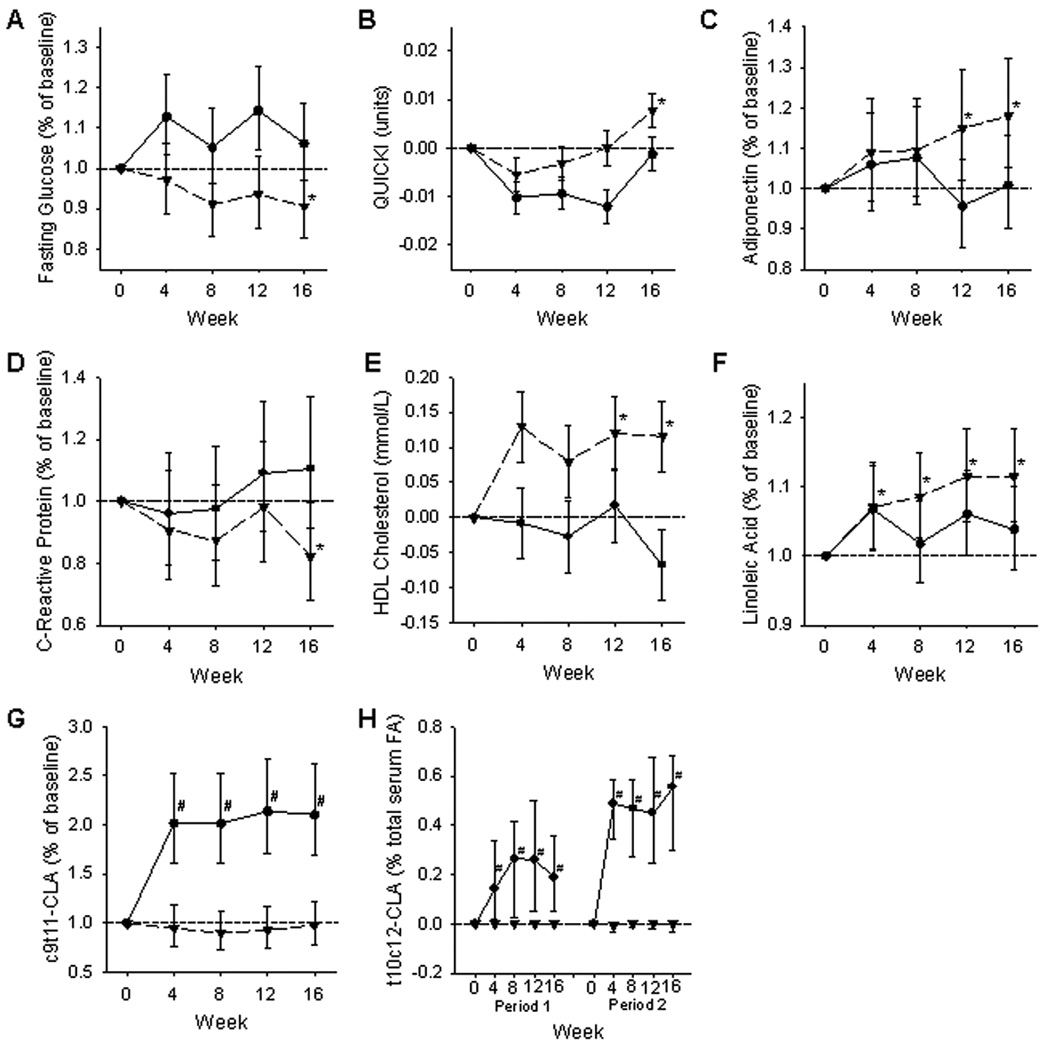
Pre-determined steps analysis (16) was utilized to determine the earliest time point at which a significant change from baseline was detectable for each of the two treatments, CLA and SAF. Values at individual time points were calculated as ½(Δ period 1) + ½(Δ period 2) for all but the t10c12-CLA endpoint. QUICKI and HDL-C are pictured as actual mean change (LSM ± SE) from baseline (y = 0). Data for fasting serum glucose, adiponectin, CRP, linoleic acid, and c9t11-CLA were analyzed after log-transformation to achieve normal distribution. Data are presented as estimated percentage change from baseline (y = 1) with 95% confidence intervals. Data for t10c12-CLA were analyzed with the Sign test and are pictured as median change from baseline (y = 0) with 95% confidence intervals for each of the two supplementation periods. CLA (circle with solid line); SAF (inverted triangle with dashed line). *SAF is significantly different from baseline, #CLA is significantly different from baseline.
QUICKI, a surrogate marker of insulin sensitivity, increased with SAF supplementation while CLA had no effect after 16 weeks. Changes in QUICKI from SAF and CLA were not different when compared to each other (Table 1). Using pre-determined steps analysis, 16 weeks was the minimum time of supplementation required to see an effect of SAF on QUICKI (Figure 1B).
Table 1.
Markers of glycemia with CLA and SAF supplementation
| Supplementation Period 1 | Supplementation Period 2 | Trend within Treatment* |
Treatment Comparison† |
||||
|---|---|---|---|---|---|---|---|
| Baseline (Week 0) |
Δ (week 16 - week 0) |
Baseline (Week 0) |
Δ (week 16 - week 0) |
½ (Δ period 1) + ½(Δ period 2) |
P-Value | P-Value | |
| HbA1c (%) | 0.0028 | ||||||
| SAF | 7.9±0.3 | −0.7±0.2 | 8.0±0.4 | −0.6±0.3 | −0.6±0.2 | 0.0007 | |
| CLA | 8.4±0.3 | −0.2±0.3 | 7.1±0.3 | 0.5±0.2 | 0.1±0.2 | 0.4434 | |
| QUICKI | 0.0893 | ||||||
| SAF | 0.298±0.005 | 0.010±0.004 | 0.311±0.006 | 0.005±0.006 | 0.008±0.004 | 0.0146 | |
| CLA | 0.305±0.006 | 0.003±0.005 | 0.306±0.005 | −0.004±0.004 | −0.001±0.003 | 0.8648 | |
| Glucose AUC (mmol*min*L−1) | 0.1700 | ||||||
| SAF | 55.8±2.4 | 0.5±3.2 | 59.6±3.3 | −3.4±3.2 | −1.4±2.0 | 0.9232 | |
| CLA | 58.6±2.94 | 4.1±2.9 | 57.1±2.6 | 5.0±2.50 | 4.6±1.93 | 0.0630 | |
| 2-hour post-prandial glucose (mmol/L) | 0.0503 | ||||||
| SAF | 7.6±0.4 | 0.3±0.4 | 8.4±0.6 | −1.3±0.8 | −0.5±0.5 | 0.2460 | |
| CLA | 8.1±0.5 | 0.4±0.6 | 7.5±0.5 | 1.0±0.6 | 0.7±0.4 | 0.0994 | |
| Insulin AUC (pmol*min*L−1) | 0.2309 | ||||||
| SAF | 37162±4600 | 28±3000 | 22678±5970 | −841±4221 | −407±2589 | 0.6672 | |
| CLA | 25485±5668 | −984±3969 | 38062±4790 | −8157±3312 | −4570±2585 | 0.2310 | |
| C-peptide AUC (nmol*min*L−1) | 0.3632 | ||||||
| SAF | 271±23 | 30±16 | 223±30 | −20±23 | 5±14 | 0.3790 | |
| CLA | 222±28 | 23±21 | 297±24 | −43±18 | −10±14 | 0.6864 | |
| HOMA1 β-cell (%) | 0.3173 | ||||||
| SAF | 144.5±26.3 | 25.1±26.10 | 59.5±36.1 | −1.2±36.6 | 11.8±22.5 | 0.4533 | |
| CLA | 88.9±32.3 | 18.8±32.8 | 116.3±29.2 | −8.1±28.9 | 5.3±21.8 | 0.5065 | |
Values are LSM ± SE, pooled across SAF and CLA groups. Obese, post-menopausal women with type 2 diabetes were treated with either SAF or CLA for 16 weeks (Supplementation Period 1), and then crossed over to the other treatment for 16 weeks (Supplementation Period 2). Supplementation Periods were separated by a four-week washout. Results were derived from tests for orthogonal contrasts used in the mixed-effects model.
Within-treatment effect of supplementation periods (final – initial value).
Between-treatment effect (SAF compared with CLA).
Analysis of area under the curves for serum glucose, insulin and C-peptide revealed no differences within or between the two supplements. The change in serum glucose from baseline to two hours was also not altered with either SAF or CLA supplementation. There was a non-significant trend (p = 0.0503) indicative of an increased change in 2-hour serum glucose for CLA when compared to SAF (Table 1).
HbA1c was measured at the beginning and end of each supplementation period. While CLA did not change HbA1c, SAF supplementation caused a significant decrease in HbA1c after 16 weeks. The change in HbA1c with SAF supplementation was significantly different compared to the change in HbA1c with CLA supplementation (Table 1).
Previously, we found an increase in adiponectin with SAF and no effect with CLA when comparing the change from the beginning to the end of the study10. Using pre-determined steps analysis to evaluate the time course of supplementation on serum adiponectin, we found that 12 weeks was the minimum time of supplementation required to see an effect of SAF on adiponectin (Figure 1C).
Markers of Inflammation
SAF supplementation for 16 weeks decreased CRP, but there was no difference in CRP with CLA supplementation. CRP was also decreased with SAF when compared to CLA (Table 2). Using pre-determined steps analysis, 16 weeks was the minimum time of supplementation required to see a lowering effect of SAF on CRP (Figure 1D). No differences were detected within or between treatments for serum IL-6 (Table 2).
Table 2.
Blood lipids and inflammatory markers with CLA and SAF supplementation
| Supplementation Period 1 | Supplementation Period 2 | Trend within Treatment* |
Treatment Comparison† |
||||
|---|---|---|---|---|---|---|---|
| Baseline (Week 0) |
Δ (week 16 - week 0) |
Baseline (Week 0) |
Δ (week 16 - week 0) |
½(Δ period 1) + ½ (Δ period 2) |
P-Value | P-Value | |
| Total Cholesterol (mmol/L) | 0.8940 | ||||||
| SAF | 4.2±0.2 | −0.2±0.3 | 4.8±0.3 | 0.2±0.4 | 0.0±0.2 | 0.8950 | |
| CLA | 4.4±0.3 | 0.0±0.4 | 4.4±0.3 | 0.0±0.3 | 0.0±0.2 | 0.9546 | |
| HDL-C (mmol/L) | 0.0111 | ||||||
| SAF | 0.7±0.1 | 0.1±0.1 | 0.9±0.1 | 0.1±0.1 | 0.1±0.1 | 0.0228 | |
| CLA | 0.9±0.1 | −0.1±0.1 | 0.9±0.1 | −0.1±0.1 | −0.1±0.1 | 0.1835 | |
| LDL-C (mmol/L) | 0.8386 | ||||||
| SAF | 2.6±0.2 | −0.1±0.2 | 2.8±0.3 | 0.2±0.3 | 0.1±0.2 | 0.6837 | |
| CLA | 2.5±0.2 | 0.1±0.3 | 2.8±0.2 | 0.0±0.2 | 0.0±0.2 | 0.9032 | |
| TG (mmol/L) | 0.1947 | ||||||
| SAF | 4.1±0.6 | −0.5±0.4 | 5.9±0.7 | −1.5±0.6 | −1.0±0.4 | 0.2111 | |
| CLA | 4.8±0.7 | 0.2±0.6 | 3.7±0.6 | 0.6±0.5 | 0.4±0.4 | 0.5542 | |
| NEFA (g/L) | 0.3476 | ||||||
| SAF | 6.7±0.5 | 0.6±0.5 | 6.0±0.6 | 0.3±0.7 | 0.5±0.4 | 0.2757 | |
| CLA | 6.4±0.6 | 0.4±0.6 | 7.3±0.5 | −0.6±0.56 | −0.1±0.4 | 0.8363 | |
| CRP (mg/L) | 0.0325 | ||||||
| SAF | 54.5±15.3 | 3.4±9.9 | 79.8±18.7 | −30.5±13.2 | −13.6±8.2 | 0.0472 | |
| CLA | 70.1±19.0 | 19.3±12.9 | 68.0±15.1 | −4.0±10.1 | 7.7±8.2 | 0.3017 | |
| IL-6 (pg/ml) | 0.8854 | ||||||
| SAF | 2.1±0.4 | 0.3±0.3 | 2.4±0.4 | 0.4±0.3 | 0.4±0.2 | 0.1460 | |
| CLA | 2.6±0.4 | 0.7±0.3 | 2.5±0.3 | 0.1±0.3 | 0.4±0.2 | 0.0949 | |
Values are LSM ± SE, pooled across SAF and CLA groups. Obese, post-menopausal women with type 2 diabetes were treated with either SAF or CLA for 16 weeks (Supplementation Period 1), and then crossed over to the other treatment for 16 weeks (Supplementation Period 2). Supplementation Periods were separated by a four-week washout. Results were derived from tests for orthogonal contrasts used in the mixed-effects model.
Within-treatment effect of supplementation periods (final – initial value).
Between-treatment effect (SAF compared with CLA).
Serum Lipids
There were no differences in total cholesterol, LDL cholesterol, triglycerides, or non-esterified fatty acids within or between treatments. HDL cholesterol increased after 16 weeks of SAF supplementation and did not change with CLA. Change in HDL was also increased with SAF when compared to the change in HDL from CLA (Table 2). Using pre-determined steps analysis, 12 weeks was the minimum time of supplementation required to see an effect of SAF on HDL (Figure 1E).
Serum Fatty Acid Composition
Serum linoleic acid was increased with SAF, and there was no difference with CLA. Change in serum linoleic acid was not different between the SAF and CLA groups (Table 3). Using pre-determined steps analysis, four weeks was the minimum time of supplementation required to see an effect of SAF on serum linoleic acid (Figure 1F).
Table 3.
Serum levels of linoleic acid and conjugated linoleic acid with SAF and CLA supplementation
| Supplementation Period 1 | Supplementation Period 2 | Trend within Treatment* |
Comparison of Treatments† |
||||
|---|---|---|---|---|---|---|---|
| Baseline (Week 0) |
Δ (week 16 - week 0) |
Baseline (Week 0) |
Δ (week 16 - week 0) |
½(Δ period 1) + ½(Δ period 2) |
P-Value | P-Value | |
| Linoleic Acid (% of total fatty acids) | 0.0889 | ||||||
| SAF | 24.2±0.9 | 2.7±0.9 | 24.2±1.1 | 2.9±1.3 | 2.8±0.8 | 0.0004 | |
| CLA | 23.1±1.2 | 2.9±1.2 | 25.6±0.9 | −1.1±0.9 | 0.9±0.8 | 0.2320 | |
| c9t11-CLA (% of total fatty acids) | < 0.0001 | ||||||
| SAF | 0.8±0.2 | 0.09±0.2 | 0.7±0.2 | −0.02±0.3 | 0.03±0.2 | 0.8387 | |
| CLA | 0.9±0.2 | 0.3±0.3 | 0.6±0.1 | 1.0±0.2 | 0.6±0.2 | < 0.0001 | |
| t10c12-CLA (% of total fatty acids) | |||||||
| SAF | 0.0 | 0.0 | 0.0 | 0.0 | |||
| (0.0, 0.0) | (0.0, 0.0) | (0.0, 0.0) | (0.0, 0.0) | ||||
| CLA | 0.0 | 0.2 | 0.0 | 0.6 | |||
| (0.0, 0.0) | (0.1, 0.4) | (0.0, 0.0) | (0.3, 0.7) | ||||
Subjects were treated with either SAF or CLA for 16 weeks (Supplementation Period 1), and then crossed over to the other treatment for 16 weeks (Supplementation Period 2). Results were derived from tests for orthogonal contrasts used in the mixed-effects model. Values are LSM ± SE, pooled across SAF and CLA groups for linoleic acid and c9t11-CLA. Analysis for t10c12-CLA was done using the Sign test, and data are presented as median (95% CI). Values are expressed as a percentage of total fatty acid content of the serum for each subject.
Within-treatment effect of supplementation periods (final – initial value).
Between-treatment effect (SAF compared with CLA).
Serum levels of both the c9t11- and t10c12-CLA isomers were increased in subjects taking CLA, and no change was detected with SAF supplementation. The change in serum CLA was significantly larger in the CLA group compared to the SAF group for both isomers (Table 3). Using pre-determined steps analysis, four weeks of CLA supplementation was the minimum time required to see an effect of CLA on serum c9t11-CLA (Figure 1G) and t10c12-CLA (Figure 1H).
Discussion
We previously reported that safflower oil supplementation for 16 weeks decreased fat mass in the trunk region, and CLA decreased total body fat mass and body weight10. As a result of these findings, in the present study we hypothesized that safflower oil would improve measures of glycemic control, blood lipids and inflammatory markers, and CLA would have no effect on these parameters. Additionally, we hypothesized that any metabolic effects of safflower oil and CLA would become evident after there was an increase in serum linoleic acid and CLA, respectively.
Although CLA caused a reduction in both body weight and fat mass10, CLA did not improve markers of glycemic control, insulin sensitivity, inflammation or blood lipids. This may be due to an inadequate loss of body weight or body fat in the 16 week treatment period. CLA induced a 1.1 kg, or 1.1% body weight loss and a 1.3 kg, or 3.2% fat mass reduction2, which is smaller than the 5% body weight loss recommended for improved glycemic control4. In another study which resulted in no detectable changes in body composition, supplementation with 3.0 grams of mixed-isomer CLA for 8 weeks worsened markers of insulin sensitivity in people with type 2 diabetes17. This difference of results with our study may be due to the length of supplementation. The pre-determined steps statistical model utilized in our study first tests whether a difference exists between weeks 0 and 16 of supplementation. If there is no difference, earlier weeks are not analyzed. However, it is possible that fasting glucose and QUICKI were worsened with CLA supplementation at earlier time points (weeks 4, 8, and 12) and normalized by 16 weeks.
Contrary to CLA, SAF supplementation improved numerous metabolic endpoints including HbA1c, fasting glucose, insulin sensitivity estimated by QUICKI, HDL cholesterol, CRP, and adiponectin. The dramatic physiological effect of SAF was unexpected because the intake of linoleic acid typically consumed in the American diet is adequate when compared to dietary recommendations. Women in our study reported an average linoleic acid intake of 6.8% (or ~12.6 g/d) of total energy. Adding linoleic acid consumed from the supplements, average intake increased to 9.8% of energy. The Dietary Reference Intake Report has set the acceptable macronutrient distribution range at 5–10% of energy from n-6 PUFA11. Accordingly, subjects in our study were not consuming a sub-optimal amount of linoleic acid before supplementation, nor did supplementation increase linoleic acid consumption beyond what is recommended for a healthy diet. The SAF treatment, consisting primarily of linoleic acid (18:2n6), was a minor amount of added oil (8 g or ~1 2/3 tsp), making this addition to the diet achievable on a daily basis. These results provide evidence that a small change in dietary behavior to alter the fatty acid content of the diet may improve metabolic parameters in people already consuming what is currently considered to be an adequate amount of dietary linoleic acid.
The health effects of n-6 PUFA intakes in the range of 5–10% of energy include decreased risk for heart disease through the improvement of multiple risk factors such as blood lipid profile and inflammatory status11. However, it is difficult to determine from intervention studies whether increased PUFA alone is responsible for these effects, because increasing dietary PUFA is typically accomplished by decreasing intake of another macronutrient such as saturated fat or carbohydrate11. In the present study, subjects consumed 8 g of oil containing 72 kcal per day. Although subjects were aware of the energy content of the supplements, they were not given specific guidelines for reducing energy intake to avoid weight gain. Consequently, there were no significant differences in the macronutrient content of the diet throughout the study10, and the supplement likely changed macronutrient distribution primarily by increasing total polyunsaturated fat intake. The evidence from our study supports an independent effect of increasing intake of a linoleic acid-rich oil on the endpoints measured in this study.
In the pre-determined steps statistical analysis, it was determined that significant effects of SAF treatment became evident after either 12 or 16 weeks of supplementation. The minimum time at which an increase in serum linoleic acid could be detected was after four weeks of supplementation. It can be inferred then, that the biological activity of linoleic acid may depend on a certain level of its accumulation in the body. However, from this study we cannot determine mechanistically how linoleic acid or other components of safflower oil are acting to induce biological effects. In addition, we cannot determine if safflower oil as a whole or linoleic acid specifically is inducing the effects seen in this study. In particular, tocopherals are naturally present in safflower oil. Although both the CLA and SAF treatments contained tocopherals, the distribution of isomers and the total tocopherol content was different. Future studies with safflower oil should measure serum tocopherol accumulation.
Previously, we reported that SAF supplementation reduced trunk fat by 6.3%10, which may explain some of the beneficial metabolic effects detected in the current analyses. Metabolic syndrome5, insulin resistance18, and elevations in CRP19 are positively correlated with visceral adipose tissue, and some of these factors appear to have even stronger associations in women than men5. Although not a marker for visceral fat mass, adiponectin, an insulin sensitizing adipocytokine that was increased with SAF supplementation, has a weak negative association with visceral adipose mass20. We analyzed body composition with DEXA, which is unable to differentiate between the loss of subcutaneous and visceral fat in the trunk region. However, it is likely that some of the trunk fat lost can be attributed to decreased visceral fat because of the association between visceral fat and several metabolic markers that were improved with SAF. In support of this, abdominal subcutaneous fat has not been found to have consistent deleterious effects as has visceral fat21.
Subjects in this study were post-menopausal, obese women with type 2 diabetes who had low HDL cholesterol and elevated CRP and HbA1c. This metabolic profile is associated with an increased risk of cardiovascular disease22. HDL cholesterol was less than the 1.3 mmol/L value recommended by the American Heart Association as the minimum desirable level. SAF treatment for 16 weeks increased HDL by an average of 0.12 mmol/L, bringing HDL levels to 0.82 mmol/L in Supplementation Period 1 and 1.0 mmol/L in Supplementation Period 2. Although this increase did not raise HDL to 1.3 mmol/L, it has been estimated that a 0.026 mmol/L increase in HDL is associated with a 1.9%–2.9% decrease in cardiovascular disease risk23, making the increase seen in our study clinically meaningful. CRP is an independent risk factor for cardiovascular disease24 and in this study the average baseline CRP levels fell into the “high risk” category (> 3 mg/L) as defined by the American Heart Association. Therefore the average decrease of CRP by 17.5% observed after 16 weeks of SAF supplementation is also a clinically relevant change. The average decrease in HbA1c was 0.64% after 16 weeks of SAF supplementation. Additionally, fasting glucose and QUICKI were improved. In a meta-analysis, intensive treatment of type 2 diabetes over 5 years resulted in a 0.9% decrease in HbA1c. This corresponded to a 17% decrease in non-fatal myocardial infarction and a 15% decrease in events of coronary heart disease5. From the present study we are unable to determine long-term effects of SAF supplementation, but it is possible that risk of cardiovascular outcomes may be decreased in this high-risk group if the changes seen in our study are maintained.
In conclusion, daily supplementation with 6.4 g of mixed-isomer CLA for 16 weeks did not improve markers of glycemic control, inflammation, or blood lipids in obese, post-menopausal women with type 2 diabetes. On the contrary, daily supplementation of 8 grams of safflower oil for 16 weeks improved fasting glucose, HbA1c, QUICKI, CRP, HDL cholesterol, and adiponectin,. Inclusion of a small amount of readily available and inexpensive safflower oil into the diet may have meaningful effects on clinically important risk factors in the management of diabetes and prevention of diabetes-related complications.
Acknowledgements
The authors would like to thank Yi Liu for her assistance with the statistical analyses, Julia Richardson for editorial assistance with the manuscript, the staff of the Clinical Research Center for their help with data collection, and the study participants for their time and dedication to being a part of the study. This study was funded by an unrestricted gift from Cognis Corporation (Monheim, Germany, and Cincinnati, Ohio), the National Center for Research Resources (UL1RR025755), the Clinical Research Center at The Ohio State University (grant M01-RR00034 from the NIH), and the NIH (R21 AT003520-03S1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. Funding organizations were not involved in the study design, data collection and analysis, or manuscript preparation.
Abbreviations
- CLA
conjugated linoleic acid
- SAF
safflower oil
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation of some parts of this work was made at Experimental Biology 2008, San Diego, CA, U.S.A.
Clinical Trials Registration Number - NCT01121471
Conflict of Interest Statement
There are no conflicts of interest declared by any of the authors.
Statement of Authorship
The contribution of each author is as follows: M. Asp oversaw clinical visits, data collection, data analyses, primary writer and author; A. Collene- oversaw clinical visits, data collection, and was a contributing author and editor of final drafts; L. Norris oversaw clinical visits, data collection, and was a contributing author and editor of final drafts; R. Cole conducted fatty acid analyses of serum samples, participated in data collection, data analyses, and was a participating author and editor of final drafts; M. Stout conducted fatty acid analyses of serum samples, data collection, data analyses, and was a contributing author; S. Tang conducted statistical model design, data analyses, and was a contributing author; J. Hsu designed statistical model, conducted data analyses, and was an author and editor of final drafts; M. Belury designed original clinical intervention and oversaw all revisions to the protocol, procured funding, oversaw clinical visits, data collection and analyses, and is the senior, corresponding author and editor of final drafts.
References
- 1.Caterson ID, Hubbard V, Bray GA, Grunstein R, Hansen BC, Hong Y, Labarthe D, Seidell JC, Smith SC., Jr Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group III: worldwide comorbidities of obesity. Circulation. 2004;110:e476–e483. doi: 10.1161/01.CIR.0000140114.83145.59. [DOI] [PubMed] [Google Scholar]
- 2.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 Suppl 1:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 5.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 6.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 7.Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity (Silver Spring) 2008;16:790–796. doi: 10.1038/oby.2007.136. [DOI] [PubMed] [Google Scholar]
- 8.Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- 9.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–1211. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 10.Norris LE, Collene AL, Asp ML, Hsu JC, Liu LF, Richardson JR, Li D, Bell D, Osei K, Jackson RD, Belury MA. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am J Clin Nutr. 2009;90:468–476. doi: 10.3945/ajcn.2008.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 12.Moshfegh A, Goldman J, Cleveland L. What we eat in America, NHANES 2001–2002: Usual nutrient intakes from food compared to dietary reference intakes. U.S. Department of Agriculture, Agricultural Research Service; 2005. [Google Scholar]
- 13.Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54:846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 14.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 15.Belury MA, Kempa-Steczko A. Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids. 1997;32:199–204. doi: 10.1007/s11745-997-0025-0. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JC, Berger RL. Stepwise confidence intervals without multiplicity adjustment for dose-response and toxicity studies. Journal of the American Statistical Association. 1999;94:468–482. [Google Scholar]
- 17.Moloney F, Yeow TP, Mullen A, Nolan JJ, Roche HM. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2004;80:887–895. doi: 10.1093/ajcn/80.4.887. [DOI] [PubMed] [Google Scholar]
- 18.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D’Agostino RB, O’Donnell CJ, Fox CS. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity. 2010 doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemieux I, Pascot A, Prud’homme D, Alméras N, Bogaty P, Nadeau A, Bergeron J, Després JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 20.Jain SH, Massaro JM, Hoffmann U, Rosito GA, Vasan RS, Raji A, O’Donnell CJ, Beigs JB, Fox CS. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes Care. 2009;32:903–908. doi: 10.2337/dc08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue : a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114:413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 23.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]


