Abstract
Background
Transfusion of ABO non-identical plasma, platelets and cryoprecipitate is routine practice even though adverse effects can occur.
Methods and Materials
Our hospital changed transfusion practice in 2005 and adopted a policy of providing ABO identical blood components to all patients when feasible. We retrospectively compared the transfusion requirements, length of stay, and in-hospital mortality in relation to ABO blood group in surgical patients who received platelet transfusions before and after this change to determine if it resulted in any benefit.
Results
Prior to the change in practice both group B and AB patients received more ABO non-identical platelet transfusion (p = 0.0004), required significantly greater numbers of red cell transfusions (p = 0.04), and had 50% longer hospital stays (p = 0.039) than group O and A patients. Following the policy change, there was a trend for fewer red cell transfusions (p = 0.17) and length of stay in group B and AB patients than group O or A patients. Overall, the mortality rate per red cell transfusion decreased from 15.2 per 1000 to 11.0 per 1000 (p = 0.013).
Conclusions
These results, in the context of previous findings, suggest that providing ABO identical platelets and cryoprecipitate might be associated with reduction in transfusion requirements and improve outcomes in surgical patients.
Keywords: Transfusion, Transfusion reaction, Platelet transfusion, ABO-identical, Transfusion requirements, Blood products ratio
INTRODUCTION
It is common practice to transfuse ABO non-identical platelets, fresh frozen plasma (FFP) and cryoprecipitate. The guiding principle has been to minimize the risk of hemolytic transfusion reactions by transfusing only ABO antigen compatible red blood cells, and less so, by transfusing only ABO antibody compatible plasma (e.g., avoiding minor side incompatibility). Whereas transfusion of group O FFP (about 250 ml in volume per donor unit) to non-O patients is strictly avoided, it is widely accepted practice to transfuse group O platelets to group A or B recipients for logistic reasons [1]. Infrequent reports of fatal [2] or near fatal [3] hemolysis due to ABO non-identical (minor incompatible) platelet transfusions continue to appear.
Several observations [4-8] led us to question whether infusion of ABO non-identical platelets and cryoprecipitate might actually impair, rather than improve hemostasis. First, exposure of platelets to immune complexes or platelet specific antibody can interfere with platelet function in vitro [4, 5]. Second, in cardiac surgery patients receiving similar numbers of platelet transfusions, those receiving ABO non-identical platelets require 50% more red cell transfusions and have increased mortality [6]. Finally, patients with acute leukemia receiving prophylactic platelet transfusions largely without regard to ABO blood group have serious bleeding at a rate of 15-20% [7]. In contrast, the bleeding rate in patients receiving only ABO identical platelets is below 5% [8]. Recently, transfusion of group AB plasma to O patients has been associated with a significant 10% increase in mortality in a large national cohort study [9].
In April 2005, we implemented a policy of routinely transfusing, whenever feasible, only ABO identical transfusions to all patients. To determine if this practice change impacted outcomes, we compared red cell transfusion utilization by surgical patients receiving at least one platelet transfusion before and after its implementation.
METHODS
Because of reports of worse outcomes in patients receiving ABO mismatched platelets transfusions, we implemented a change in transfusion practice in April 2005. Prior to the change, a policy for transfusion of only ABO identical FFP was already in place, whereas afterward this policy expanded to include platelets and cryoprecipitate. To evaluate the effect of this change we compared transfusion needs and outcomes before and after implementation. We chose to closely evaluate surgical patients who needed at least one platelet transfusion during or after surgery. We retrospectively reviewed transfusion service and hospital demographic records of these patients and recorded platelet transfusion, red blood cell transfusion, length of stay, and mortality during the same hospital admission prior to discharge. The end point of this analysis was red blood cell utilization, discharge, and/or mortality.
We analyzed the data with trauma patients included or excluded, as these patients occasionally receive small numbers of ABO non-identical red cells in many instances, through use of “universal donor group O” units prior to the establishment of their ABO blood group. As the results did not differ we report the results on all patients, including those cared for by our trauma service. The current study was conducted as an element of transfusion service quality improvement activities and thus did not require institutional human subjects review.
Statistical analysis
The statistical significance between means among blood groups was determined by use of the 2-tailed Student t test. Deaths in hospital for each blood group were assessed using Chi Square analysis. Deaths per total red blood cell and per total blood components transfusions were analyzed by Fisher’s Exact Test. A P value of less than 0.05 was considered statistically significant. No adjustment for multiple comparisons was performed.
RESULTS
2002-2003—No policy restricting transfusion of ABO non-identical platelets and cryoprecipitate to surgical patients
Patient Characteristics and Transfusions
Table 1 shows the demographics, transfusion, use, and outcomes of all 335 surgical patients who received at least one platelet transfusion during this period. All, but 2 patients, received red blood cell transfusion(s) as well. As expected, patients from groups B (n = 39) and AB (n = 15) were more likely to receive ABO non-identical transfusions (mostly group O). Blood group O and A patients required fewer red cells transfusions as compared with blood groups B and AB patients (p =0.04). Total units of pooled platelets transfused during or after surgery in all groups were not significantly different (p = 0.802). However, O and A patients received fewer ABO non-identical platelets than B and AB patients (p = 0.0004). No significant differences were seen in ABO identical platelet transfusions between all groups (p = 0.527).
Table 1.
Characteristics of Surgical Patients by ABO Group from 2002 to 2003
| Blood Group | O | A | B | AB | |
|---|---|---|---|---|---|
| Mean± SD (n=153) |
Mean± SD (n=128) |
Mean ± SD (n=39) |
Mean± SD (n=15) |
P value* | |
| Age | 59.2 ± 1.6 | 59.4 ± 1.7 | 53.8 ± 3.1 | 54.0 ± 5.0 | 0.318 |
| Gender (M/F) | 89 / 64 | 89 / 39 | 28 / 11 | 3 / 12 | 0.083 |
| Red Cells Transfused (Units) |
13.4 ± 1.2 | 12.0 ± 1.4 | 20.1 ± 2.5 | 15.5 ± 4.0 | 0.04 |
| Total Platelets Transfused (Units) |
13.8 ± 1.5 | 14.7 ± 1.6 | 17.1 ± 3.0 | 13.9 ± 4.8 | 0.802 |
| ABO Non-identical Platelets Transfused (Units) |
2.7 ± 0.6 | 4.0 ± 0.6 | 6.9 ± 1.2 | 9.2 ± 1.9 | 0.0004 |
| ABO Identical Platelets Transfused (Units) |
11.1 ± 1.3 | 10.6 ± 1.4 | 10.2 ± 2.5 | 4.7 ±4.1 | 0.527 |
| FFP Transfused (Units) | 10.0 ± 1.3 | 6.9 ± 1.4 | 11.0 ± 2.6 | 5.1 ± 4.2 | 0.256 |
| Total Cryoprecipitate Transfused (Units) |
5.2 ± 0.7 | 4.4 ± 0.7 | 5.6 ± 1.3 | 3.9 ± 2.1 | 0.754 |
| ABO Non-identical Cryo-precipitate Transfused (Units) |
2.4 ± 0.4 | 2.2 ± 0.5 | 4.4 ± 0.9 | 3.6 ± 1.4 | 0.129 |
| Post-Op Length of Stay (Days) |
23.4 ± 3.0 | 20.3 ± 3.3 | 29.1 ± 6.0 | 45.1 ± 9.7 | 0.083 |
| Deaths in Hospital | 30 (20%) | 31 (24%) | 5 (13%) | 4 (27%) | 0.39 |
| Death/1,000 Red Cell | 15 | 20 | 6.4 | 17 | 0.07 |
| Death/1,000 Components |
4.5 | 6 | 2.3 | 5.7 | 0.089 |
Categorical data were compared by Chi square or Fisher’s exact test as appropriate and continuous data by two-sided t tests.
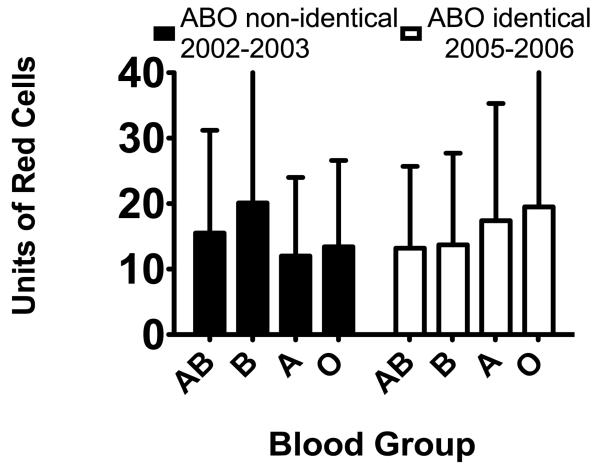
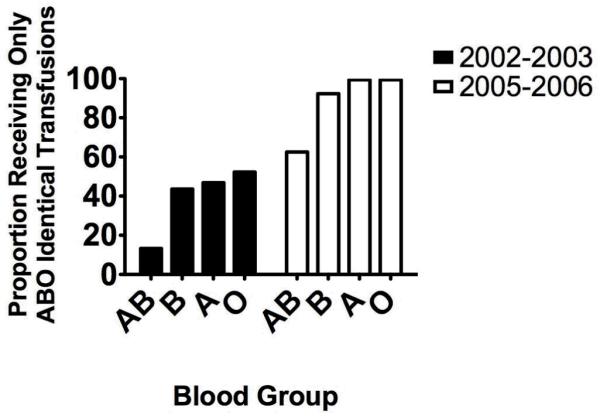
Red cell utilization by ABO blood group is shown in the left panel of figure 1. The majority of patients (176 of 335; 52.5%) received at least one ABO non-identical transfusion of cryoprecipitate or platelets. Of these 176, 135 received at least one ABO non-identical platelet transfusion and 88 received at least one non-identical cryoprecipitate transfusion. The proportion of patients receiving only ABO identical platelets and cryoprecipitate is shown by blood group in left panel of figure 2.
Figure 1.
The mean ± SD number of red cell transfusions is shown by ABO blood group of the patient, grouped by the time period and ABO transfusion policy applicable to those patients. N = 15 (AB), 39 (B), 128 (A), 153 (O) for 2002-2003 and 24(AB), 52 (B), 158 (A), 175 (O) for 2005-2006 The overall ANOVA for the 2002-2003 data is significant (p=0.040). Employing Fisher’s PLSD post-hoc test, the differences between blood groups A and B (p=0.0047) and O and B (p=0.016) are statistically significant. The overall ANOVA for the 2005-2006 data is not significant (p=0.19).
Figure 2.
The proportion of patients receiving only ABO identical transfusions is shown by blood group for the two time periods of the study (see Figure 1 for the N in each group, and here as well). For both periods, the proportion of patients in blood group AB who received only ABO identical components is statistically significantly smaller than for the other blood groups (p = 0.034 for 2002-2003; <0.0001 for 2005-2006 by Chi square). However the ratio of patients receiving only ABO identical transfusions in blood groups B, A and O to patients receiving only ABO identical transfusions in blood group AB is 3.3-4.0 fold in 2002-2003 as compared with 1.5 fold in 2005-2006.
Length of Stay and In-Hospital Mortality
Group O/A patients had shorter lengths of stay as compared with B/AB patients (p = 0.039) (Table 2). In-hospital mortality did not differ between the two group (p = 0.40). Mortality per red cell transfused overall was 15.2 per 1,000, with significantly higher mortality among the O/A patients (p = 0.021). Mortality per total components transfused was 4.8 per 1,000 and was also higher in O/A patients (p = 0.05) (Table 2).
Table 2.
Characteristics of Surgical Patients by O/A and B/AB groups for both periods (2002-2003 and 2005-2006)
| 2002-2003 (n=335) | 2005-2006 (n=410) | |||||
|---|---|---|---|---|---|---|
| Blood Groups O and A |
Blood Groups B and AB |
Blood Groups O and A |
Blood Groups B and AB |
|||
| Mean± SD (n=281) |
Mean± SD (n=54) |
P value* | Mean ± SD (n=334) |
Mean± SD (n=76) |
P value* | |
| Age | 59.3 ± 19.0 | 53.9 ± 20.4 | 0.06 | 55.0 ± 20.5 | 56.4 ± 20.8 | 0.59 |
| Gender (M/F) | 178 / 103 | 40 / 14 | 0.13 | 211 / 122 | 44 / 32 | 0.79 |
| Red Cells Transfused (Units) |
12.8 ±12.7 | 18.8±25.1 | 0.0086 | 18.5 ± 20.9 | 13.6 ± 13.5 | 0.051 |
| Total Platelets Transfused (Units) |
14.2 ±18.9 | 16.2±16.1 | 0.47 | 15.6 ± 25.1 | 9.5 ± 7.7 | 0.036 |
| ABO Non-identical Platelets Transfused (Units) |
3.3 ± 6.2 | 7.5 ± 11 | 0.0001 | 0.0 ± 0.0 | 1.0 ±2.8 | 0.0001 |
| ABO Identical Platelets Transfused (Units) |
11 ± 16 | 9 ± 12 | 0.35 | 15.6 ± 25.1 | 9.5 ±7.7 | 0.036 |
| FFP Transfused (Units) | 8.6 ±16.9 | 9.4±13.5 | 0.75 | 13.3 ± 19.3 | 7.9 ± 9.5 | 0.017 |
| Total Cryoprecipitate Transfused (Units) |
6.0 ± 8.0 | 8.0±9.2 | 0.79 | 7.3 ± 11.9 | 4.3 ±7.7 | 0.04 |
| ABO Non-identical Cryo-precipitate Transfused (Units) |
2.3 ± 3.1 | 4.2 ± 5.1 | 0.02 | 0.06 ± 0.77 | 0.53 ± 3.6 | 0.03 |
| Post-Op Length of Stay (Days) |
22.0 ±32.1 | 33.5±57.8 | 0.039 | 22.8 ± 33.6 | 23.8 ± 33.8 | 0.81 |
| Deaths in Hospital | 61 (22%) | 9 (17%) | 0.40 | 66 (20%) | 12 (16%) | 0.33 |
| Death/1,000 Red Cells | 17 | 9 | 0.021 | 11 | 11.6 | 0.12 |
| Death/1,000 Components |
5.3 | 3.3 | 0.05 | 3.6 | 4.4 | 0.11 |
Categorical data were compared by Chi square or Fisher’s exact test as appropriate and continuous data by two-sided t tests.
2005-2006—After institution of a policy to transfuse only ABO identical platelets and cryoprecipitate to surgical patients
Patient Characteristics and Transfusions
Table 3 shows the demographics, transfusion use, and outcomes of the 410 patients who required at least one platelet transfusion in the cohort receiving exclusively ABO identical blood components in so far as was logistically possible. Red cell utilization by ABO blood group is shown in the right panel of figure 1. The proportion of patients receiving only ABO identical blood platelets and cryoprecipitate is shown in right panel of figure 2. In contrast to the period when ABO non-identical platelets and cryoprecipitate were routinely transfused, blood groups O and A surgical patients now required more red cell transfusions than blood group B or AB patients (p = 0.051). The pattern of hemostatic component transfusion seen in the previous cohort receiving ABO non-identical platelets and cryoprecipitate routinely is the reverse of that seen in this cohort receiving primarily ABO identical platelets and cryoprecipitate. Group O/A patients in this later cohort received significantly more platelets, FFP and cryoprecipitate than did B/AB patients (Table 2). When calculations were made for each of the four blood groups separately, the results for were largely identical to the grouped trends (Table 3).
Table 3.
Characteristics of Surgical Patients by ABO Group from 2005 to 2006
| Blood Group | O | A | B | AB | |
|---|---|---|---|---|---|
| Mean± SD (n=175) |
Mean± SD (n=159) |
Mean ± SD (n=52) |
Mean± SD (n=24) |
P value* | |
| Age | 53.5 ± 1.6 | 56.3 ± 1.6 | 58.0 ± 2.9 | 52.8 ± 4.2 | 0.414 |
| Gender (M/F) | 110 / 65 | 102 / 57 | 30 / 22 | 14 / 10 | 0.831 |
| Red Cells Transfused (Units) |
19.5 ± 1.5 | 16.8 ± 1.5 | 13.7 ± 2.7 | 13.2 ± 4.0 | 0.17 |
| Total Platelets Transfused (Units) |
17.4 ± 1.7 | 13.2 ± 1.8 | 9.2 ± 3.2 | 10.0 ± 4.7 | 0.068 |
| ABO Non-identical Platelets Transfused (Units) |
0.0 ± 0.1 | 0.0 ± 0.1 | 0.4 ± 0.2 | 2.5 ± 0.2 | 0.0001 |
| ABO Identical Platelets Transfused (Units) |
17.4 ± 1.7 | 13.2 ± 1.8 | 8.8 ± 3.2 | 7.5 ± 4.6 | 0.033 |
| FFP Transfused (Units) | 14.3 ± 1.4 | 12.2 ± 1.4 | 8.6 ± 2.5 | 6.4 ± 3.7 | 0.067 |
| Total Cryoprecipitate Transfused (Units) |
7.8 ± 0.9 | 6.7 ± 0.9 | 5.0 ± 1.6 | 2.9 ± 2.3 | 0.14 |
| ABO Non-identical Cryo-precipitate Transfused (Units) |
0.06 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.2 | 1.7 ± 0.3 | 0.0001 |
| Post-Op Length of Stay (Days) |
21.8 ± 2.1 | 19.5 ± 2.2 | 23.9 ± 3.9 | 23.4 ± 5.7 | 0.736 |
| Deaths in Hospital | 40 (23%) | 26 (16%) | 9 (17%) | 3 (13%) | 0.36 |
| Death/1,000 Red Cells | 11.8 | 9.8 | 12.6 | 9.5 | 0.07 |
| Death/1,000 Components |
3.7 | 3 | 4.5 | 3.4 | 0.089 |
Categorical data were compared by Chi square or Fisher’s exact test as appropriate and continuous data by two-sided t tests.
All but 15 of 410 patients in this cohort received only ABO identical platelets and cryoprecipitate, and all patients received only ABO identical FFP (Figure 2).
Length of Stay and In-Hospital Mortality
Group O/A patients receiving ABO identical blood components had lengths of stay that were not significantly different from B/AB patients (p = 0.81) (Table 2). Also, in-hospital mortality did not differ between the two groups (p = 0.33). Mortality overall was 78 deaths per 7,089 red cells transfused (11.0 per 1,000), which is 28% lower than the cohort receiving many ABO non-identical platelet and cryoprecipitate transfusions prior to the change (15.2 per 1,000), (p = 0.013). Mortality per total components transfused yielded similar results (4.8 versus 3.6 per 1000, before and after the change, respectively, p = 0.089). In an exploratory subgroup analysis of massively transfused patients receiving 10-30 units of red cells (approximately 1-3 blood volumes), the mortality rates before and after the change were 25 of 137 (18.2%), compared with 18 of 161 (11.8%), (p = 0.084).
DISCUSSION
These results confirm previous observations from multiple investigators that exposure to ABO non-identical platelet or plasma transfusions is associated with increased red cell transfusion needs and morbidity in surgical patients [6][9,18-21]. When platelet and cryoprecipitate transfusions were given without regard to ABO blood group in the 2002-2003 cohort, group B/AB patients, who receive more non-identical transfusions, required significantly more red cell transfusions. This is despite the fact that group B/AB patients have markedly higher levels of von Willebrand factor, and are more likely to experience thrombosis in some treatment settings [11]. In contrast, group O/A patients required more red cell, platelet, FFP and cryoprecipitate transfusions than did group B/AB patients after the change in practice. This reversal of the pattern of red cell transfusion needs after implementation of a policy of ABO identical transfusions was striking and statistically significant. This observation benefits from the power of Mendelian randomization [12] as we examined red cell use, length of stay and mortality as a function of ABO blood group, not the non-random factor of whether ABO non-identical transfusions were given. ABO blood group itself is unrelated to transfusion needs in previous studies [13, 14]. The finding that red cell utilization was strongly associated with ABO blood group, which itself determines the likelihood of ABO non-identical transfusions, is more robust because of the Mendelian randomization approach. A preliminary report from our center restricted to trauma patients from this study, containing more comprehensive clinical information, demonstrated increased multi-organ failure and red cell transfusion requirements in patients receiving ABO non-identical red cells and platelets [10].
Most importantly, after implementation of a rigorous ABO identical transfusion policy, the mortality rate per red cell or blood component transfused decreased by 28% and 25%, respectively, despite a greater acuity of illness (as measured by numbers of components transfused). Transfusion dose is a powerful predictor of clinical outcomes [15], as it is a surrogate measure of clinical acuity, and may have immunomodulatory/inflammatory/pro-thrombotic effects [16]. The finding of an unfavorable association of ABO non-identical transfusions with mortality is consonant with a recent large epidemiologic study demonstrating a 10% mortality increase when ABO non-identical plasma is transfused [9]. Virtually all studies demonstrated a dose response association between number of red cells or total blood components transfused and mortality in surgical patients [17]. Thus a 28% drop in mortality rate per red cell transfused is notable and unlikely to be explained by confounding factors such as changes in case mix. Despite increased acuity of patient illness (as measured by blood transfusion needs), surgical mortality decreased by 2% overall, and 28% on a per transfusion basis in the cohort treated with ABO identical platelet and cryoprecipitate transfusions. Although the overall mortality results were not statistically significant, the per transfusion decrease in mortality rate approaches significance (p = 0.084), and this is consistent with previous reports of decreased morbidity, bleeding and mortality with use of ABO identical components [6, 9, 18-20].
One potential mechanism for increased red cell transfusion needs in patients receiving ABO non-identical platelets and cryoprecipitate is that platelet and/or endothelial dysfunction due to ABO antibody or immune complexes interferes with hemostasis. Similar findings regarding bleeding and red cell transfusion needs in patients receiving ABO non-identical platelet transfusions have been described in patients with acute leukemia [18] and during cardiac surgery [6]. ABO non-identical transfusions also have been associated with increases in mortality [19] and veno-occlusive disease [20] in stem cell transplantation, as well as in all patients in a national database [9]. In a recent report, Inaba et al., showed significant increase in complications rate among trauma patients who received ABO-compatible nonidentical plasma (n = 284) versus patients who received ABO-identical plasma (n = 230), (53.5% vs 40.5%, P = .002, respectively). These complications tend to increase in patients receiving more than 6 units of ABO-compatible plasma. Higher incidences of acute respiratory distress syndrome (ARDS) and sepsis were also observed in ABO-compatible plasma recipient group (19.4% vs 9.2%, P = .001, and 38.0% vs 28.9%, P = .02, respectively). A four-fold increase in ARDS was seen in patients receiving more than 6 units of ABO-compatible plasma. No statistically significant effect on mortality was detected between the groups (35.2% vs 33.5%, P = .66). [21]
We speculate that transfusion of ABO non-identical platelets, FFP and/or cryoprecipitate may exacerbate bleeding, hemolysis (minor side incompatible transfusions only), and red cell transfusion needs, rather than correcting defects in hemostasis. Thus, a policy of transfusing only ABO identical blood components could reduce red cell transfusion needs and mortality in surgical patients. The use of ABO identical transfusions is primarily a logistical problem, but the cost and technical issues are comparatively minor. ABO non-identical minor side incompatible plasma, cryoprecipitate and platelet transfusions are well known to cause serious [3] or even fatal [2] hemolysis in rare instances. Developing procedures so that only ABO identical transfusions are administered, except in emergencies, has much to recommend it as a standard of practice, given findings previously reported by multiple groups, and these current data. We suggest that physicians carefully consider in each case the benefit to risk ratio of giving ABO non-identical transfusions versus withholding transfusion until ABO identical components are available.
The retrospective analysis of this data is one of the serious limitations in this study. The use of historical controls is another critical limitation. In addition, patients’ clinical condition before surgery and the presence of other existing risk factors for bleeding and mortality were not evaluated. Nonetheless, the altered pattern of red cell utilization according to ABO blood group observed is an indication of a potentially causal association, taken in the context of previously published work by several groups. [6,9,18-21] A multi-center prospective randomized trial is needed to definitively evaluate these findings .
Acknowledgments
We are grateful to the surgical team at Strong memorial Hospital for their assistance and support. This work was supported in part by NIH grants HL078603, HL086367, HL095467, HL100051, and HL093129.
Footnotes
Conflict-of-interest disclosure: M. A. R. receives research support from CSL Behring. All other authors declare no competing financial interests.
References
- 1.Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma: a survey of 3156 North American laboratories. Arch Pathol Lab Med. 2007;131:909–16. doi: 10.5858/2007-131-909-TOPCAP. [DOI] [PubMed] [Google Scholar]
- 2.Sadani DT, Urbaniak SJ, Bruce M, Tighe JE. Repeat ABO-incompatible platelet transfusions leading to haemolytic transfusion reaction. Transfus Med. 2006;16:375–9. doi: 10.1111/j.1365-3148.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Harris SB, Josephson CD, Kost CB, Hillyer CD. Nonfatal intravascular hemolysis in a pediatric patient after transfusion of a platelet unit with high-titer anti-A. Transfusion. 2007;47:1412–7. doi: 10.1111/j.1537-2995.2007.01283.x. [DOI] [PubMed] [Google Scholar]
- 4.Brandt JT, Julius CJ, Osborne JM, Anderson CL. The mechanism of platelet aggregation induced by HLA-related antibodies. Thromb Haemost. 1996;76:774–9. [PubMed] [Google Scholar]
- 5.Peerschke EI, Ghebrehiwet B. Platelet receptors for the complement component C1q: implications for hemostasis and thrombosis. Immunobiology. 1998;199:239–49. doi: 10.1016/S0171-2985(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg N, Heal JM, Hicks GL, Jr., Risher WH. Association of ABO-mismatched platelet transfusions with morbidity and mortality in cardiac surgery. Transfusion. 2001;41:790–3. doi: 10.1046/j.1537-2995.2001.41060790.x. [DOI] [PubMed] [Google Scholar]
- 7.Heckman KD, Weiner GJ, Davis CS, Strauss RG, Jones MP, Burns CP. Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/microL versus 20,000/microL. J Clin Oncol. 1997;15:1143–9. doi: 10.1200/JCO.1997.15.3.1143. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC Blood Disord. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanwell A, Andersson TM, Rostgaard K, Edgren G, Hjalgrim H, Norda R, Melbye M, Nyren O, Reilly M. Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox Sang. 2009;96:316–23. doi: 10.1111/j.1423-0410.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 10.Fialkow LB, Zucchiatti A, Cheng J, et al. ABO non-identical transfusions and red blood cell usage in blunt trauma patients. Transfusion. 2007;47:192A–193A. [Google Scholar]
- 11.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100:1717–23. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 2008;5:e177. doi: 10.1371/journal.pmed.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberth G, Kettisen J, Lisander B. Blood loss in prosthetic hip replacement is not influenced by the AB0 blood group. Eur J Surg. 2001;167:652–5. doi: 10.1080/11024150152619264. [DOI] [PubMed] [Google Scholar]
- 14.Welsby IJ, Jones R, Pylman J, Mark JB, Brudney CS, Phillips-Bute B, Mathew JP, Campbell ML, Stafford-Smith M. ABO blood group and bleeding after coronary artery bypass graft surgery. Blood Coagul Fibrinolysis. 2007;18:781–5. doi: 10.1097/MBC.0b013e3282f1029c. [DOI] [PubMed] [Google Scholar]
- 15.Minano A, Ordonez A, Espana F, Gonzalez-Porras JR, Lecumberri R, Fontcuberta J, Llamas P, Marin F, Estelles A, Alberca I, Vicente V, Corral J. AB0 blood group and risk of venous or arterial thrombosis in carriers of factor V Leiden or prothrombin G20210A polymorphisms. Haematologica. 2008;93:729–34. doi: 10.3324/haematol.12271. [DOI] [PubMed] [Google Scholar]
- 16.Blumberg N, Heal JM. Transfusion immunomodulation. In: Hillyer CD, Silberstein LE, Ness PM, Anderson KC, Roback JD, Eds Blood, editors. Banking and Transfusion Medicine. Churchill Livingstone Elsevier; Philadelphia, PA: 2007. [Google Scholar]
- 17.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 18.Heal JM, Liesveld JL, Phillips GL, Blumberg N. What would Karl Landsteiner do? The ABO blood group and stem cell transplantation. Bone Marrow Transplant. 2005;36:747–55. doi: 10.1038/sj.bmt.1705101. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin RJ, Antin JH. ABO-incompatible bone marrow transplantation: the transfusion of incompatible plasma may exacerbate regimen-related toxicity. Transfusion. 1999;39:1273–4. doi: 10.1046/j.1537-2995.1999.39111273.x. [DOI] [PubMed] [Google Scholar]
- 20.Lapierre V, Mahe C, Auperin A, Stambouli F, Oubouzar N, Tramalloni D, Benhamou E, Tiberghien P, Hartmann O. Platelet transfusion containing ABO-incompatible plasma and hepatic veno-occlusive disease after hematopoietic transplantation in young children. Transplantation. 2005;80:314–9. doi: 10.1097/01.tp.0000167758.63247.f4. [DOI] [PubMed] [Google Scholar]
- 21.Inaba K, Branco BC, Rhee P, Holcomb JB, Blackbourne LH, Shulman I, Nelson J, Demetriades D. Impact of ABO-identical vs ABO-compatible nonidentical plasma transfusion in trauma patients. Arch Surg. 2010;145:899–906. doi: 10.1001/archsurg.2010.175. [DOI] [PubMed] [Google Scholar]