Abstract
Alcohol and nicotine use disorders are often treated as separate diseases, despite evidence that approximately 80–90% of alcohol dependent individuals are also heavy smokers. Both nicotine and ethanol have been shown to interact with neuronal nicotinic acetylcholine receptors (nAChRs), suggesting these receptors are a common biological target for the effects of nicotine and ethanol in the brain. There are few studies that have examined the effects of co-administered nicotine and ethanol on the activity of nAChRs in rodents. In the present study, we show that Sprague-Dawley rats, a strain often used for nicotine studies but not as often for voluntary ethanol intake studies, will consume 20% ethanol using both the intermittent-access two-bottle-choice and operant self-administration models without the need for sucrose fading. We show that nicotine (0.2mg/kg and 0.8mg/kg, s.c.) significantly increases operant 20% ethanol self-administration and varenicline (2mg/kg, s.c), a partial agonist at nAChRs, significantly decreases operant ethanol self-administration and nicotine-induced increases in ethanol self-administration. This suggests that nAChRs play an important role in increasing ethanol self-administration and that varenicline may be an efficacious treatment for alcohol and nicotine co-dependencies.
Keywords: ethanol, nicotine, nicotinic receptors, Sprague-Dawley, varenicline
Introduction
Alcohol dependence impacts millions of individuals and constitutes one of the most serious public health problems worldwide. Epidemiological studies have shown a high correlation between alcohol consumption and tobacco use (Falk et al., 2006) and the prevalence of smoking in alcoholics is as high as 90% (Batel et al., 1995; DiFranza and Guerrera, 1990; Falk et al., 2006; Istvan and Matarazzo, 1984) compared to roughly 20% (Centers for Disease Control and Prevention, CDC) for the general population. Preclinical studies have demonstrated that nicotine administration increases voluntary ethanol consumption (Blomqvist et al., 1996; Olausson et al., 2001; Potthoff et al., 1983; Smith et al., 1999) and operant ethanol self-administration (Lê et al., 2003). Additionally, nicotine has been shown to induce reinstatement of ethanol–seeking in animals (Lê et al., 2010; Lê et al., 2003) and in humans (Barrett et al., 2006; Marks et al., 1997). It has been proposed that nicotine increases the reinforcing properties of ethanol as they both increase dopamine release in the nucleus accumbens (Tizabi et al., 2007). Furthermore, nicotine reduces the aversive effects of ethanol, including ethanol ataxia, ethanol-induced sedation and cognitive-impairment (Gould et al., 2001; Melia et al., 1996). Ethanol’s action in the brain has been shown to be at least partly mediated by the activation of neuronal nicotinic acetylcholine receptors (nAChRs) while most of the behavioral effects of nicotine are known to be regulated by the nAChRs (Blomqvist et al., 1992; Chatterjee and Bartlett, 2010; Chatterjee et al., 2010; Picciotto et al., 1998; Tapper et al., 2004). Central nAChRs are pentameric ligand-gated ion channels consisting of α2–α10, and β2–β4 subunits that assemble into multiple combinations (Champtiaux et al., 2003; Gotti et al., 1997).
Varenicline, an FDA approved treatment for smoking cessation which targets the nAChRs (Gonzales et al., 2006; Rollema et al., 2007), has recently been shown to reduce ethanol self-administration and heavy drinking in rats (Steensland et al., 2007), mice (Hendrickson et al., 2010; Kamens et al., 2010), and in humans (McKee et al., 2009). Although varenicline has been shown to modulate dopamine levels in the nucleus accumbens following acute injections of ethanol and nicotine (Ericson et al., 2009), varenicline’s effect on nicotine-induced increases in ethanol self-administration is unknown.
Sprague-Dawley (SD) rats have been used extensively to study the effects of nicotine (Caggiula et al., 2002; Donny et al., 1995; Levin and Simon, 1998; Malin et al., 1992; Rollema et al., 2007; Shoaib et al., 1997; Stolerman et al., 1973), but have rarely been used to study the effects of ethanol. Unlike Long-Evans and Wistar rats which display high voluntary, oral ethanol consumption in the two-bottle choice and operant self-administration settings, SD rats achieve low to moderate intake levels (Khanna et al., 1990; Linseman, 1987; Melchior and Myers, 1976). Several investigators have examined the effects of involuntary ethanol exposure on the strain with the use of oral intubation (Cagetti et al., 2003; Kokka t al., 1993), i.p. injections (Ellis, 1966), or vapor inhalation (Karanian et al., 1986; Rogers et al., 1979). However, the full potential of SD rats in studies examining voluntary ethanol consumption has yet to be realized.
We and others have previously shown standard laboratory rats exposed to 20% ethanol using an intermittent-access two-bottle choice paradigm are transformed into high ethanol-consuming rats (Simms et al., 2008; Wise, 1973). We have also shown that Long-Evans rats self-administer 20% ethanol without sucrose fading using an operant self-administration protocol (Simms et al., 2010). Both methods produce very robust and reproducible levels of high voluntary ethanol consumption that are maintained over a long period of time. In the present study, we applied the 20% ethanol intermittent-access two-bottle choice and 20% ethanol operant self-administration models to train standard SD rats to consume ethanol. We then adapted the model described by Lê (2003), to study the effects of systemic nicotine administration (0.2mg/kg and 0.8mg/kg) on 20% ethanol operant self-administration and examined the effect of varenicline on nicotine-induced increases in ethanol self-administration.
Material and Methods
Animals and Housing
Adult, male, ethanol-naïve, SD rats weighing 150–175g upon arrival (Charles River, Wilmington, MA USA), were individually housed in ventilated Plexiglas cages (Thoren Caging Systems Inc., Hazelton, PA, USA) in a climate-controlled room on a 12-h reverse light/dark cycle (lights off at 10 a.m.). Rats were given at least 1 week to acclimate to individual housing conditions and handling procedures. Food and water were available ad libitum in the home cage throughout the entire experiment. Operant-sessions occurred between 12 and 4 PM, with the exception of initial self-administration training as outlined below. All procedures were pre-approved by the Ernest Gallo Clinic and Research Center Institutional Animal Care and Use Committee and were in accordance with NIH Guide for the Care and Use of Laboratory Animals.
Drugs and Treatment Schedules
Ethanol and sucrose solutions were prepared in tap water using 95% (v/v) ethanol (Gold Shield Chemical Co., Hayward, CA, USA). Blood ethanol concentrations were analyzed using a nicotinamide adenine dinucleotide (NAD)-alcohol dehydrogenase (ADH) spectrophotometric assay (Sigma-Aldrich, St. Louis, MO, USA). Nicotine solutions were prepared using (−) nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO, USA) dissolved in saline (vehicle) and administered subcutaneously (s.c.) .Varenicline (6,7,8,9-tetrahydro-6,10-methano-6H pyrazino[2,3-h][3]benzazepine tartrate) (Coe et al., 2005) was generously provided by Pfizer Global Research and Development (Groton, CT) and dissolved in saline (vehicle) and administered subcutaneously (s.c.). All drug solutions were prepared immediately before each injection and administered in a volume of 1 ml/kg.
Voluntary ethanol consumption using two-bottle choice paradigms
Ethanol intake procedures
All fluids were presented in 100-ml graduated glass cylinders with stainless-steel drinking spouts inserted through two grommets in front of the cage 15 min after the lights went off in the reversed light/dark cycle room. Bottles were weighed 30 min and 24 hours after the fluids were presented, and measurements were taken to the nearest gram. The weight of each rat was measured daily Monday through Friday to monitor health and calculate the grams of ethanol intake per kilogram of body weight.
Intermittent-access 20% ethanol
To determine whether the 20% ethanol intermittent-access protocol would produce high voluntary ethanol intake in SD rats, two groups (n=31 total) were trained to consume 20% ethanol using the intermittent-access drinking paradigm. The rats were given access to one bottle of 20% ethanol and one bottle of water for three 24-hour-sessions per week (Mondays, Wednesdays and Fridays) as previously described (Simms et al., 2008; Wise, 1973). The rats had unlimited access to two bottles of water between the ethanol-access periods. The placement of the ethanol bottle was alternated each ethanol drinking session to control for side preferences. We maintained the rats on 20% ethanol intermittent access two-bottle choice paradigm for 8 weeks (24 ethanol-access sessions).
Continuous-access 10% ethanol with sucrose fade
A modified sucrose-fading procedure was adapted from Samson (1986) where the animals were given access to both a water bottle and a 10% ethanol solution continuously (n=10), 7 days a week. On the first day of exposure, a 10S/10E mixture of 10% sucrose (w/v) and 10% ethanol (v/v) was presented for 5 consecutive days. This procedure was repeated for a 5S/10E and 2S/10E solution until a 10% ethanol solution alone was presented in the home cage. Measurements for this group were collected 5 days a week starting Monday morning and at every 24-hour interval following data collection. The placement of the ethanol bottle was alternated daily to control for side preferences.
Blood Ethanol Concentration (BEC) assay
Blood samples were collected from the lateral tail vein 30 minutes after the presentation of the bottles in animals that had established stable baseline ethanol consumption. Whole blood was centrifuged at 4°C for 13 minutes at 8000 rpm and sera was separated and stored at −80°C until analysis. Sera was then analyzed using the nicotinamide adenine dinucleotide (NAD)-alcohol dehydrogenase (ADH) spectrophotometric assay (Zapata et al., 2006). The BECs were determined using a standard calibration curve and then correlated with ethanol consumed (g/kg/30min).
Operant self-administration paradigm
Operant apparatus
Self-administration testing was conducted in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA). Details regarding the apparatus have been extensively described elsewhere (Richards et al., 2008; Steensland et al., 2007).
Operant self-Administration procedure for 20% or 10% ethanol
Following eight weeks of two-bottle choice exposure, animals were switched to an operant setting to investigate whether prior long-term ethanol exposure would lead to the initiation of operant responding for either 20% or 10% ethanol, respectively, without the use of sucrose fading. Sessions occurred five days per week (Monday–Friday) regardless of their previous two-bottle-choice regimen. Food and water were available ad libitum at all times in the home cage throughout the training. For the first two days of training animals were placed in the operant conditioning chambers for a 14-h overnight session on an FR1 schedule of reinforcement (0.1 ml after a single active lever press) with 20% or 10% ethanol solution as the reinforcer. Once the active lever is pressed, the stimulus light above the lever was illuminated for 3 seconds and was accompanied by a 3 second tone to indicate availability of the reinforcer in the dipper receptacle. The dipper port was illuminated for 10 seconds whilst the dipper cup was available. If the cup was not actively licked during the 10 seconds, the cup fell and this event was recorded as a null response. Lever pressing during the 10 seconds were also recorded but did not result in any programmed activity. During the overnight sessions, only the active lever was available for the rat to press, to facilitate learning. Following the completion of these sessions, rats were then exposed to 45-minute FR1 sessions for 1 week (5 sessions). Subsequently, training sessions were reduced to 30 minutes and the work ratio was increased to FR3 schedule of reinforcement (three active lever presses required for a 0.1 ml reward). The second (inactive) lever was also introduced at this time. Upon pressing the inactive lever, no reinforcer, cue light, or auditory stimuli were presented and the event was merely recorded as a measure of nonspecific behavior. Rats continued on the FR3 protocol with 20% ethanol as the reinforcer for a minimum of 24 sessions. Any animals that failed to receive any reinforcers (<1) during the last 5 operant sessions were excluded from further study. When the animals had maintained a stable baseline (20 sessions) blood samples were collected from the lateral tail vein immediately following a 30-minute FR3 session, samples were then prepared and analyzed as described above.
Nicotine and varenicline administration effects on 20% ethanol operant self-administration
We examined the effect of daily administration of nicotine (0.2 mg/kg and 0.8 mg/kg, s.c.) in SD rats self-administering 20% ethanol using the method adapted from Lê (2003). Operant sessions were extended from 30 to 60 minutes using the FR3 schedule, and following 5 operant sessions, animals were separated into two balanced groups (according to ethanol intake levels in the 60 minute sessions) and assigned to either the nicotine or vehicle group. Nicotine was dissolved in saline (0.8 mg/kg, free base) and all injections were delivered via subcutaneous injection 15 minutes prior to the beginning of the daily session. Injections were given 5 days a week for 3 weeks (15 sessions) to observe the effects of chronic, high-dose nicotine pretreatment. To evaluate the effect of varenicline pretreatment on nicotine-induced increases in ethanol self-administration, varenicline was administered 30 minutes prior to the 16th and 17th nicotine pretreatment (45 minutes prior to start of the operant session). All rats received two doses (vehicle or 2 mg/kg, s.c.) over the course of 2 days in a counterbalanced design, and thus each rat served as its own control. The varenicline doses were chosen because they have previously been shown to effectively decrease nicotine self-administration (Rollema et al., 2007) and ethanol consumption (Steensland et al., 2007). Following varenicline testing, nicotine administration was terminated for five consecutive sessions to observe ethanol intake levels in the 20% ethanol operant self-administration model. We then evaluated the effect of a lower dose of nicotine, 0.2mg/kg, in the 20% ethanol operant self-administration model. The animals were separated back into their original nicotine administration group (nicotine or vehicle) but their treatment was switched to counterbalance from previous testing. As a result, animals that previously received vehicle injections would now be receiving nicotine (0.2mg/kg) injections and vice versa.
Statistics
Statistical analysis was performed using SigmaStat version 3.5 (Systat Software, San Jose, CA). The behavioral data were analyzed using t-test and two-way ANOVA where appropriate, followed by Newman-Keuls post hoc analysis when a significant overall main effect was found (p < 0.05). The BEC data was plotted against the ethanol intake (g/kg/30min) measured prior to blood collection and analyzed by linear regression.
Results
Sprague-Dawley rats voluntarily consume 20% ethanol using the intermittent-access two-bottle choice drinking paradigm
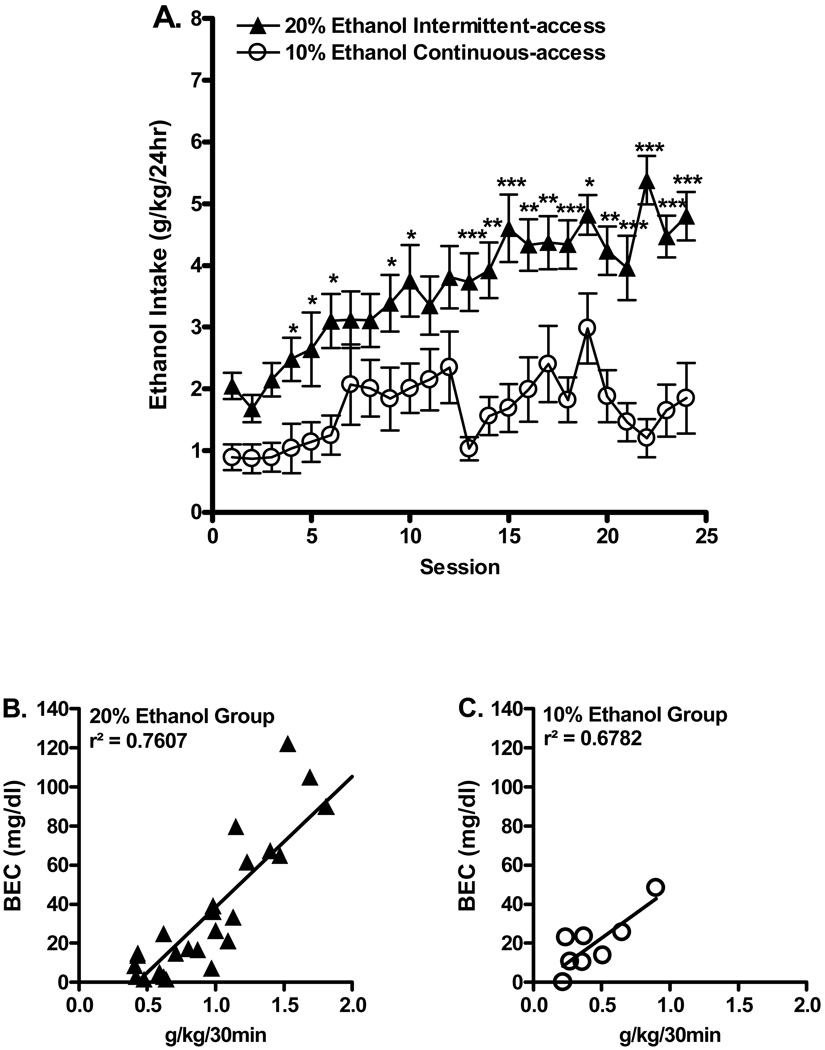
A steady escalation in ethanol consumption was found in the 20% ethanol group over 24 ethanol exposure sessions (Figure 1A). Ethanol consumption measured over the last three sessions (22 to 24) were significantly higher in the 20% ethanol intermittent (4.8 ± 0.4 g/kg/24hr, n=31) compared to the 10% ethanol continuous (1.5 ± 0.7 g/kg/24hr, n=10) group (T test; p<0.001). Two-way ANOVA comparing ethanol consumption (g/kg/24hr) in the 20% ethanol intermittent access group and the 10% ethanol continuous access group for 24 sessions revealed an overall main effect of group [F(1,823)=11.0, p<0.01], an overall main effect of session [F(23, 823)=8.4, p<0.001], and an overall significant interaction (group × session) [F(23, 823)=2.5, p<0.05]. Post hoc analysis revealed significant differences in ethanol consumption between the groups (Figure 1A).
Figure 1.
High ethanol consumption in Sprague Dawley Rats using the intermittent access 20% ethanol two-bottle choice paradigm. (A.) Ethanol consumption (g/kg) was significantly greater for SD rats following intermittent-access 20% ethanol compared to continuous-access 10% ethanol with a sucrose fade. The values are expressed as the mean ethanol intake (g/kg/24hr) ± SEM (two-way ANOVA followed by Newman-Keuls post hoc test). The amount of ethanol consumed significantly correlated with the measured blood ethanol concentrations BECs: intermittent 20% ethanol (B.): r2=0.7607, p<0.001; continuous 10% ethanol (C.): r2=0.6782, p < 0.05. Blood Ethanol Concentrations (BECs, mg/dl) for each animal were measured following 30 minutes of voluntary oral ethanol access using either intermittent access or continuous access protocols. * p< 0.05, ** p<0.01, *** p<0.001, n=31 for the 20% ethanol group and n=10 for the 10% ethanol group.
The amount of ethanol consumed after 30 minutes significantly correlated with the blood ethanol concentrations (BECs) measured in both the 20% ethanol intermittent group, r2=0.7607, p<0.001 (Fig 1B) and in the 10% ethanol continuous group, r2=0.6782, p<0.05 (Figure 1C). The BECs were much higher in animals consuming 20% ethanol with a range between 6 to 122 mg/dl in comparison to the 10% continuous ethanol consuming animals (10 to 48 mg/dl).
Sprague-Dawley rats self-administer 20% ethanol using operant self-administration
Mean baseline ethanol consumption measured during three sessions (20–22) showed the 20% ethanol self-administration group (0.96 ± 0.10 g/kg/30min, n=23) consumed significantly higher levels of ethanol compared to the 10% ethanol self-administration group (0.39 ± 0.10 g/kg/30min, n=10) (T test; p<0.001). Two-way ANOVA comparing the daily consumption (g/kg/30 min) of the 20% ethanol group compared to the 10% ethanol group revealed an overall main effect of group [F(1,810)=14.1, p<0.001], and an overall main effect of session [F(27, 810)=1.9, p<0.01], but there was no significant interaction (group × session) [F(27, 810)=0.7, n.s.]. Post hoc analysis found significant differences for all 28 baseline days (Figure 2A). The amount of ethanol self-administered in each group significantly correlated with BECs (20% ethanol intermittent: r2=0.6667, p<0.001, Figure 2B; 10% ethanol continuous: r2=0.4782, p<0.05, Figure 2C). Two animals were excluded from BEC analysis because their BEC was well below what would be expected for the amount of ethanol they pressed for, indicating that the animals were not drinking the full 0.1 ml at each reward presentation. Together these results show that SD rats will self-administer and voluntarily consume high amounts of ethanol that are comparable to other out-bred rats.
Figure 2.
SD rats self-administer 20% ethanol using an operant self-administration paradigm. (A.) Animals that were previously exposed to the intermittent-access, 20% ethanol two-bottle choice model (n=23) exhibited significantly higher intake levels in comparison to animals that were previously exposed to 10% ethanol (n=9). The values are expressed as the mean ethanol intake (g/kg/24hr) ± SEM (two-way ANOVA followed by Newman-Keuls post hoc test). Blood samples were collected from the lateral tail vein immediately following the 30-minute FR3 session for BEC determination. The amount of ethanol consumed for the 20% ethanol- and 10% ethanol-responding animals significantly correlated with the measured BECs (linear regression): 20% ethanol (B.): r2=0.6667, p<0.001; 10% ethanol (C.): r2=0.4782, p < 0.05. * p< 0.05, ** p<0.01, *** p<0.001, n=23 for the 20% ethanol group and n=9 for the 10% ethanol group.
Nicotine administration increases 20% ethanol operant self-administration
Nicotine administration (0.2mg/kg and 0.8mg/kg) significantly increases ethanol consumption. Two-way ANOVA of ethanol consumption comparing the 0.8mg/kg nicotine and vehicle treated groups revealed an overall main effect of pretreatment [F(1,315)=114.2, p<0.001], an overall main effect of session [F(14,315)=2.1, p<0.05], but no overall significant interaction (pretreatment × session) [F(14,315)=1.4, n.s.]. Post hoc analysis found significant differences in ethanol consumption starting from session 3 (Figure 3). Two-way ANOVA of active lever presses revealed a significant effect of pretreatment [F(1,292=11.4, p<0.01], a significant interaction between pretreatment × session [F(14,292) =2.1, p<0.05] and a significant interaction of pretreatment × session [F(14,292)=2.1, p<0.05]. Post hoc analysis found significant differences in active pressing in 11 of the 15 sessions. The amount of ethanol self-administered in each group significantly correlated with BECs (T test; p<0.001, data not shown). A two-way ANOVA of ethanol consumption comparing the 0.2mg/kg nicotine and vehicle treated group revealed an overall main effect of pretreatment [F(1,84)=5.4, p< 0.05] but no overall main effect of session [F(7,84)=1.04, n.s.] nor significant interaction [F(7,84)=0.3, n.s.]. Post hoc analysis found significant differences in ethanol consumption between the nicotine and saline treated groups (Table 1).
Figure 3.
Nicotine administration increases 20% ethanol operant self-administration. (A.) Nicotine (0.8 mg/kg, s.c.), but not vehicle (saline), administered 15 minutes prior to the session significantly increases ethanol intake. The values are expressed as mean ethanol intake g/kg/60min ± SEM (two-way ANOVA followed by Newman-Keuls post hoc test). * p< 0.05, ** p<0.01, *** p<0.001, n=12 for the nicotine group and n=11 for the vehicle group.
Table 1.
Nicotine administration (0.2mg/kg, s.c.) increases 20% ethanol operant self-administration (60 minute, FR3 schedule).
| Treatment | ||
|---|---|---|
| Session | Nicotine 0.2mg/kg | Vehicle |
| Baseline (No injections) | 0.99 ± 0.18 | 0.92 ± 0.13 |
| 1 | 1.48 ± 0.33 | 1.03 ± 0.26 |
| 2 | 1.48 ± 0.14 | 1.02 ± 0.25 |
| 3 | 1.53 ± 0.24* | 0.89 ± 0.22 |
| 4 | 1.37 ± 0.26 | 1.09 ± 0.11 |
| 5 | 1.51 ± 0.30* | 0.92 ± 0.21 |
| 6 | 1.43 ± 0.18* | 0.86 ± 0.12 |
p<0.05, compared to the vehicle pretreated group
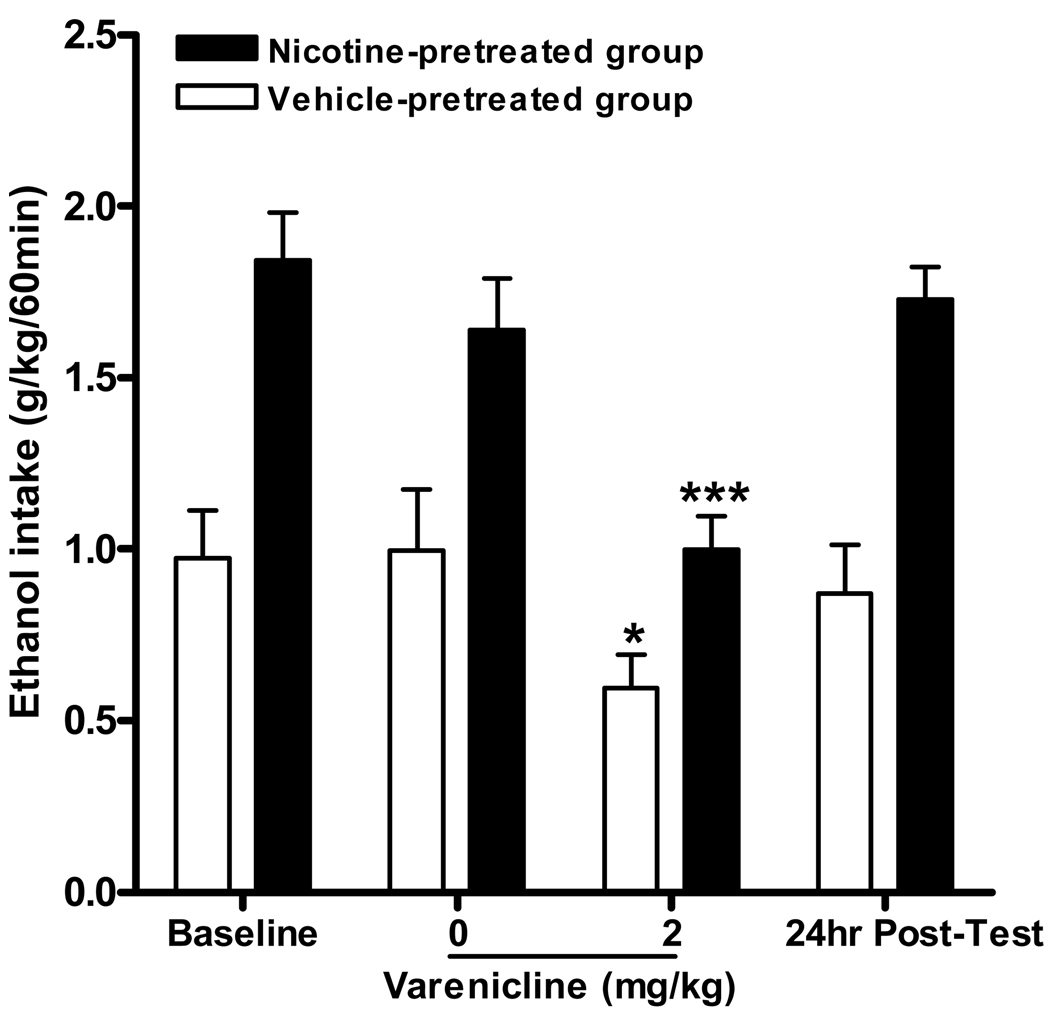
Varenicline significantly decreases both ethanol and nicotine-induced increases on operant ethanol self-administration
Varenicline significantly attenuated both 20% ethanol self-administration and the increases in 20% ethanol operant self-administration caused by chronic nicotine administration. Two-way ANOVA analysis of ethanol consumption revealed a significant effect of pretreatment (0.8mg/kg nicotine or vehicle) [F(1,20=19.9, p<0.001], an effect of varenicline treatment (2mg/kg or vehicle) [F(1,20=16.1, p<0.001] but no interaction between pretreatment × treatment [F(1,20) =1.5, n.s.]. Post hoc analysis revealed an effect of varenicline pretreatment for the saline and the nicotine administered group (Figure 4). Two-way ANOVA of active lever presses revealed a significant effect of nicotine administration (0.8mg/kg or vehicle) [F(1,20=6.0, p<0.05], an effect of varenicline pretreatment (2mg/kg or vehicle) [F(1,20=20.4, p<0.001] but no interaction between nicotine administration × varenicline pretreatment [F(1, 20) =0.44, n.s.]. Post hoc analysis further reveals varenicline significantly decreased active lever pressing for both nicotine and vehicle pretreated groups. There was no overall main effect on the number of inactive lever presses for either nicotine administration or varenicline pretreatment (data not shown).
Figure 4.
Varenicline significantly decreases nicotine induced increases in 20% ethanol operant self-administration in SD rats. Varenicline (2 mg/kg s.c.) was administered 30 minutes prior to nicotine (0.8 mg/kg s.c that was administered 15 mins prior to the operant session. The values are expressed as mean ethanol consumed (g/kg/60 min) ± SEM (two-way repeated measures ANOVA followed by Newman-Keuls post hoc test). * p<0.05, *** p<0.001 compared to varenicline vehicle within treatment group, n = 11 to 12 per group.
Discussion
We show that SD rats, a strain that is not traditionally utilized for voluntary, oral ethanol intake studies because of their low ethanol consumption, will self-administer high amounts of 20% ethanol without the use of sucrose fading or home cage fluid deprivation in both a two-bottle choice and operant drinking paradigm. The intake levels were significantly greater in comparison to animals trained to consume 10% ethanol using a continuous-access protocol with sucrose fading and were maintained for several weeks. We then show that both 0.2mg/kg and 0.8mg/kg nicotine increase 20% ethanol operant responding; an effect which is attenuated by pretreatment with varenicline, a partial agonist at nAChRs.
Our results indicate that SD rats achieve high levels of ethanol consumption using the intermittent-access two-bottle choice drinking paradigm. A recent study reported the effectiveness of the intermittent model in inducing high ethanol preference in SD rats in short-term (2 weeks) and limited-access (3 hrs per day) regimens (Moorman and Aston-Jones, 2009), but in this present study we focused on long-term exposure. Using the intermittent-access drinking paradigm SD rats achieve high levels of ethanol consumption that are maintained over several weeks (4.8 ± 0.4 g/kg/24hr, 8 weeks) and do not need sucrose fading to initiate ethanol consumption. They reach similar consumption levels to Long-Evans rats (5–6 g/kg/24hr) trained in the same model while both strains consume slightly less than Wistar rats (6–8 g/kg/24hr). We found SD rats achieved BECs (6 to 122 mg/dl) that were similar to Long-Evans rats (10 to 100 mg/dl), indicating similar ethanol clearance between these strains. As noted in our previous work, Wistar rats have to consume more ethanol in order to reach similar BECs to Long-Evans rats which may, at least partially, explain the differences in intake (Simms et al., 2008). In addition to high intake levels and BECs, our findings also revealed a higher preference for 20% ethanol vs. 10% ethanol, an effect demonstrated in previous work (Amit et al., 1970; Simms et al., 2008). The high ethanol intake levels and preference observed in SD rats in the present study reiterates the 20% ethanol intermittent-access as a robust model for inducing high ethanol intake in a traditionally low consuming strain.
Additionally, we have demonstrated that SD rats will consume significantly higher amounts of 20% ethanol (0.96 ± 0.10 g/kg/30min) compared to 10% ethanol (0.39 ± 0.10 g/kg/30min) with pharmacologically relevant BECs in an operant self-administration paradigm. These results coincide with previous evidence in the literature that suggests that out-bred rats may consume greater amounts of ethanol when higher concentrations of ethanol are presented (Amit et al., 1970; Samson et al., 1999; Samson et al., 1988; Simms et al., 2010; Wise, 1973, 1975). However, it is difficult to prove that the SD rats in the present study have an inherent preference for higher ethanol concentrations because of the long-term ethanol exposure these animals experienced in their home cage. In the future, it would be interesting to observe the performance of ethanol-naive, adult SD rats in the operant setting to evaluate the impact of prior chronic ethanol exposure on subsequent operant self-administration.
In the present study, we show that 0.8mg/kg nicotine administration in SD rats induced a steady escalation in ethanol intake that was statistically significant within the first week of nicotine pretreatment. Chronic nicotine (0.8mg/kg) caused a significant increase in ethanol intake by the third day of treatment that was maintained throughout the remainder of the treatment regimen with the average operant ethanol consumption of daily nicotine administration to be 2.09 ± 0.14 g/kg/60min. We also found that administration of a lower dose of nicotine (0.2mg/kg) induces an escalation in ethanol intake, however, this increase in ethanol intake was modest and far less robust in comparison to the higher dose. These findings support previous studies that have reported increased levels of ethanol consumption and self-administration following acute or chronic nicotine treatment (Blomqvist et al., 1996; Ericson et al., 2000; Le et al., 2000; Le et al., 2010; Le et al., 2003; Olausson et al., 2001; Potthoff et al., 1983; Smith et al., 1999). Acute administration of nicotine and ethanol has been shown to increase dopamine release in the nucleus accumbens, and when nicotine and ethanol are co-administered, there is an additive increase in dopamine release (Tizabi et al., 2007).
Following the termination of the nicotine treatment all rats exhibiting increased 20% ethanol operant responding returned immediately to pretreatment operant self-administration levels (data not shown), which is consistent with previous reports (Lê et al., 2003). In contrast to these findings, studies have shown that nicotine, administered 30 minutes prior to the self-administration session (Sharpe and Samson, 2002) or after daily ethanol self-administration sessions (Nadal and Samson, 1999), reduces ethanol consumption and lever responding in rats. Furthermore, following the termination of nicotine administration, the increase (Blomqvist et al., 1996) or the decrease (Sharpe and Samson, 2002) in ethanol intake has been reported to persist for at least a week. These discrepancies could be due to experimental factors such as difference in the strain of rats, dosages used, the history of ethanol self-administration prior to nicotine treatment and/or the amount of ethanol access time given during each self-administration session following nicotine treatment.
Varenicline is a high affinity partial agonist at α4β2* nAChRs, low affinity partial agonist at α3β4*, α3β2*, α6β2* nAChRs, and a low affinity agonist at α7 nAChRs (Coe et al., 2005). Acute varenicline administration decreases nicotine and ethanol induced increases in dopamine release in the nucleus accumbens (Ericson et al., 2009) and reduces ethanol intake in rodents (Kamens et al., 2010; Steensland et al., 2007) as well as alcohol self-administration in humans (McKee et al., 2009). Consistent with our previous findings (Steensland et al., 2007), we show that varenicline decreases baseline ethanol as well as reduces nicotine-induced increase in 20% self-administration. However, varenicline did not reduce nicotine-induced increases in ethanol intake below its effects on ethanol intake alone. This suggests that varenicline-insensitive nAChRs or non-nicotinic receptors as suggested by Ericson (2000) may also mediate the effects of nicotine to increase ethanol self-administration. Taken together, our data suggests that nAChRs play an essential role in ethanol self-administration and that varenicline may prove to be an effective treatment for the co-administration of ethanol and nicotine.
The societal costs of alcohol and nicotine use disorders are devastating and there remains a critical need for more effective treatment options. The present study establishes the utility of out-bred SD rats in investigating the behavioral effects of voluntary ethanol exposure and provides an opportunity to incorporate electrophysiological studies examining the mechanisms involved in ethanol consumption and seeking in this strain. Additionally, our data provides further evidence that nAChRs play an important role in mediating the effects of nicotine and ethanol and suggests that the smoking cessation aid varenicline may be a promising novel treatment for concomitant alcohol and nicotine dependency in humans.
Acknowledgements
We thank Rui Li and Yuanyuan Wang for excellent technical assistance and Allison Feduccia for manuscript preparation. We thank Pfizer Inc for generously providing us with varenicline. This work was supported by funding the NIH, 1RO1AA017924-01 to S.E.B and the State of California for Medical Research through UCSF to S.E.B., Department of Defense Grant W81XWH-07-1-0075 to S.E.B. The experiments contained herein comply with the current laws of the United States of America. All procedures were pre-approved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the Humane Care and Use of Laboratory Animals.
Footnotes
Disclosure/Conflict of Interest
The authors declare that, except for income received from my primary employer. S.E.B. has received financial support for research for a varenicline clinical study but has not received compensation from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holding that could be perceived as constituting a potential conflict of interest.
Authors Contribution
J.J.B, J.A.S, and S.E.B were responsible for the development and design of the study and manuscript. JJB and J.A.S were responsible for collecting behavioral data. J.H. performed BEC assay and data analysis. JJB drafted the manuscript. JAS, SC, and SEB provided critical supplemental content and revisions. JAS, SC, JH and SEB provided significant critiques to the manuscript. All authors reviewed contents of the study and have approved the final version for publication.
References
- Amit Z, Stern MH, Wise RA. Alcohol preference in the laboratory rat induced by hypothalamic stimulation. Psychopharmacologia. 1970;17:367–377. doi: 10.1007/BF00403808. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE. Partial agonists of the α3β4* neuronal nicotinic acetylcholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- Ericson M, Engel JA, Soderpalm B. Peripheral involvement in nicotine-induced enhancement of ethanol intake. Alcohol. 2000;21:37–47. doi: 10.1016/s0741-8329(99)00099-3. [DOI] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Collins AC, Wehner JM. Nicotine enhances latent inhibition and ameliorates ethanol-induced deficits in latent inhibition. Nicotine Tob Res. 2001;3:17–24. doi: 10.1080/14622200020032060. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanian J, Yergey J, Lister R, D'Souza N, Linnoila M, Salem N., Jr Characterization of an automated apparatus for precise control of inhalation chamber ethanol vapor and blood ethanol concentrations. Alcohol Clin Exp Res. 1986;10:443–447. doi: 10.1111/j.1530-0277.1986.tb05121.x. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Sharma H. Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol. 1990;7:429–434. doi: 10.1016/0741-8329(90)90027-a. [DOI] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Lê AD, Lo S, Harding S, Juzytsch W, Marinelli PW, Funk D. Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl) 2010;208:475–486. doi: 10.1007/s00213-009-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Linseman MA. Alcohol consumption in free-feeding rats: procedural, genetic and pharmacokinetic factors. Psychopharmacology (Berl) 1987;92:254–261. doi: 10.1007/BF00177925. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J Subst Abuse Treat. 1997;14:521–527. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior CL, Myers RD. Genetic differences in ethanol drinking of the rat following injection of 6-OHDA, 5,6-DHT or 5,7-DHT into the cerebral ventricles. Pharmacol Biochem Behav. 1976;5:63–72. doi: 10.1016/0091-3057(76)90289-6. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Samson HH. Operant ethanol self-administration after nicotine treatment and withdrawal. Alcohol. 1999;17:139–147. doi: 10.1016/s0741-8329(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Potthoff AD, Ellison G, Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav. 1983;18:489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C. Chronic ethanol self-administration in a continuous-access operant situation: the use of a sucrose/ethanol solution to increase daily ethanol intake. Alcohol. 1999;19:151–155. doi: 10.1016/s0741-8329(99)00032-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Pfeffer AO, Tolliver GA. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcohol Clin Exp Res. 1988;12:591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Repeated nicotine injections decrease operant ethanol self-administration. Alcohol. 2002;28:1–7. doi: 10.1016/s0741-8329(02)00238-0. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans Rats Acquire Operant Self-Administration of 20% Ethanol Without Sucrose Fading. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl) 1999;142:408–412. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30:329–342. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wise RA. Maximization of ethanol intake in the rat. Adv Exp Med Biol. 1975;59:279–294. doi: 10.1007/978-1-4757-0632-1_19. [DOI] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]