Abstract
Hematopoietic humanized mice generated via transplantation of human hematopoietic stem cells (hHSCs) into immunodeficient mice are a valuable tool for studying development and function of the human immune system. This study was performed to generate a protocol that improves development and quality of humanized mice in the BALB/c-Rag2nullIl2rγnull strain, testing route of injection, in vitro culture and freezing of hHSCs, types of cytokines in the culture, and co-injection of lineage-depleted CD34− cells. Specific hHSC culturing conditions and the addition of support cells were found to increase the frequency, and human hematopoietic chimerism, of humanized mice. The optimized protocol resulted in BALB/c-Rag2nullIl2rγnull humanized mice displaying more consistent human hematopoietic and lymphoid engraftment. Thus, hematopoietic humanized mice generated on a BALB/c immunodeficient background represent a useful model to study the human immune system.
Keywords: hematopoietic stem cell, human cord blood, hematopoietic chimerism, immunodeficient mouse, cytokine, humanized mouse
1. Introduction
Hematopoietic humanized mice generated via transplantation of human hematopoietic stem cells (hHSCs) into immunodeficient mice, are becoming an important tool for studying the development and function of the human immune system (HIS). Major advances in the engraftment of a HIS in mice have been recently achieved through the use of novel recipient mice that bear mutations in the IL-2 receptor common gamma chain gene (Il2rγ) in combination with either the deletion of one of the recombination activating genes (Rag1 or Rag2) or the naturally occurring Prkdc-scid mutation [1]. Together these mutations lead to the development of mice with a largely dysfunctional immune system lacking B, T and NK cells, and allowing better xeno-engraftment [1; 2; 3]. The genetic background of the immunodeficient mouse strains is also known to contribute to HIS engraftment. The BALB/c [4; 5; 6] and the NOD [2; 3; 7; 8] represent successful recipients for HIS, while the C57BL/6 genetic background retains immunorejection capabilities [1]. Recent studies, however, have indicated that the NOD immunodeficient strain may be a better recipient for the development of a HIS than the BALB/c strain [9].
Although the increased human hematopoietic engraftment achieved with these novel immunodeficient recipient strains is currently a major advance, the model is still not optimized for many applications. A major obstacle is the large variability of human engraftment among recipients generated with HSCs isolated from different or even the same donors [4; 5; 7; 8]. This variability also extends to the type of human hematopoietic cells and their products. Furthermore, the stability of the human grafts is also variable, with many recipients losing chimerism over time while others maintain long-lived grafts that can be transferred to secondary recipients [7; 10]. This variability likely reflects differences in the potency and homing abilities of HSCs, as well as in their longevity. Understanding of human HSC biology has improved through research aimed at increasing the efficiency of bone marrow, umbilical cord blood and mobilized peripheral blood transplantation in the clinic. For instance, although the CD34 antigen is commonly accepted as a primitive hematopoietic stem cell marker, subsets within the CD34+ cells exist and display varying degrees of engraftment potential [11; 12; 13; 14; 15; 16]. In addition, effort has focused on expanding HSCs in culture, testing the effect of cytokines and media in this context [17; 18]. Thus, a more refined definition of hematopoietic stem cell phenotype and better conditions for HSC manipulations may improve experimental consistency in the generation of humanized mice.
The method to generate humanized mice varies greatly between investigators. Human HSCs are injected into either adult or newborn recipients, with the idea that young animals have a better tolerance to xenografts and/or more receptive tissue [9; 19]. Additionally, HSCs are transplanted intra-venous (i.v.), intra-hepatic (i.h.), intra-peritoneal, intra-splenic, intra-cardiac (i.c.), or directly into the bone marrow [4; 7; 9; 10; 20; 21; 22], with the notion that certain tissue microenvironments may increase the engraftment of these cells. Moreover, human HSCs are most commonly isolated from umbilical cord blood, but can also be obtained from fetal liver, adult bone marrow or G-CSF-mobilized peripheral blood [3; 6; 23; 24]. Finally, donor cells are injected either fresh or after freezing, and are often expanded in vitro for days or even weeks with different cytokine cocktails [11; 25]. It is often unclear how the methods for the generation of these mice affect their ultimate phenotype. Here, we performed a study aimed at optimizing a protocol for the generation of hematopoietic humanized mice displaying increased development and consistency of human hematopoietic chimerism and of human lymphocytes. Our study is based on the use of BALB/c-Rag2nullIl2rγnull (hereafter referred to as BALB/c-DKO) neonate mice as recipients of umbilical cord blood-derived HSCs [4; 5]. Parameters analyzed in this study were the injection route and culture conditions of CD34+ HSCs, and whether these cells could be frozen for future use. In addition, we tested whether co-injection of CD34− human cells supported the engraftment and differentiation of CD34+ HSCs by potentially providing factors that are important for the development and survival of human hematopoietic cells.
In accordance with previous reports, our data show the existence of a large variability in the rate and degree of human hematopoietic engraftment in humanized mice. However, we show that certain conditions significantly increase the numbers of engrafted mice as well as the degree of human leukocyte engraftment and the generation of human B and T cells, indicating that humanized BALB/c-DKO mice represent a useful animal model for the study of a human immune system.
2. Materials and Methods
2.1. CD34+ and CD34− cell preparation from human umbilical cord blood
Umbilical cord blood units were obtained from the University of Colorado Cord Blood Bank at ClinImmune Labs (Aurora, CO) as samples rejected due to low volume. Investigators in this study were blinded from donor identities, and the studies were performed in compliance with the National Jewish Health (NJH) Institutional Review Board. Blood mononuclear cells were isolated over Ficoll-density gradients. CD34+ cells were enriched using human CD34-specific magnetic beads and an AutoMACS (Miltenyi Biotech). The CD34+ cells were used immediately or cultured at 1×105 cells/ml in IMDM supplemented with 10% FBS, 50 μM β-mercaptoethanol, 2 mM Glutamax, 10 ng/ml interleukin-6 (IL-6) and 20 ng/ml stem cell factor (SCF) for up to 28 days. Flt3-Ligand (FL, 10 ng/ml), interleukin-3 (IL-3, 20 ng/ml), or thrombopoietin (TPO, 25 ng/ml) were added to some cultures, as indicated in the results section. All cytokines (R&D Systems) were prepared and stored according to manufacturer’s instructions and were added to cultures every 3–4 days. Cultured CD34+ cells were either used immediately or frozen in FBS and 10% DMSO at −80°C for future use. In some experiments, the CD34− cell fraction was further depleted of either T cells, or T and B cells, to generate “support” cells. T cell depletion was accomplished by negative selection using CD2 and CD3-specific magnetic beads on an AutoMACS (Miltenyi Biotech). CD20-specific magnetic beads were added for additional B cell depletion. T cell- and T,B cell-depleted CD34− “support” cells (also called ΔT and ΔT,B cells, respectively) were either mixed with CD34+ cells for immediate injection or frozen for later use. Frozen cell samples were quickly thawed at 60°C, resuspended in fresh media, and counted by trypan blue exclusion. Thawed cell samples were typically 60–85% viable.
2.2. Mice and hematopoietic stem cell transplantation
BALB/c-Rag2nullIl2rγnull (BALB/c-DKO) mice were bred and maintained on gentamycin-containing water under specific pathogen-free and BSL2 conditions at the Biological Resource Center at NJH. Animal care and experiments were approved by the NJH Institutional Animal Care and Use Committee. For cell transplantation, CD34+ enriched cells (5 ×104–1×106 cells/mouse) with or without CD34− “support” cells (4×105–5×106 cells/mouse) were resuspended in sterile PBS. Newborn BALB/c-DKO mice (1–3 days) were irradiated with 350 rad using a Cs-137 gamma irradiator. Irradiated pups were injected 2-to-6 hours after irradiation with 50 μl of cell suspension using a 30G needle into either the facial vein (i.v.) or the liver (i.h.).
2.3. Antibodies and flow cytometry
Antibodies used in flow cytometry were against: hCD3 (UCHT1; eBioscience), hCD5 (UCHT2, eBioscience), hCD20 (L27, BD Biosciences), (581, BD Biosciences), mCD45 (30-F11, eBioscience), and hCD45 (H130, eBioscience). Biotinylated samples were revealed with Pacific Blue conjugated streptavidin (Invitrogen). Cells were isolated from peripheral blood, bone marrow, thymus, spleen, and lymph nodes for flow cytometric analyses. Lymph nodes were found, on average, in 39% of the humanized mice that displayed hCD45+ cells in peripheral blood. The lymph nodes, which were in small numbers and appeared under-developed, were mostly mesenteric, although cervical, axillary, and inguinal lymph nodes were also rarely found. For mice in which lymph nodes were not visible by naked eye, small tissue samples at lymph node sites were nevertheless processed for flow cytometric analyses to assess presence of human hematopoietic cells. All cell samples were run on a Cyan analyzer (Beckman Coulter) and analyzed with FlowJo software (Tree Star, Inc.). Analyses were performed on live single cells (based on forward and side-scatter and doublet discrimination).
2.4. ELISAs
ELISA plates (Nunc) were coated overnight at 4°C with polyclonal goat anti-human IgM or IgG antibodies (Southern Biotech) at 5 μg/ml in PBS, then washed 4X with PBS, 0.5% Tween (wash buffer) and incubated for 1–4 hours with blocking buffer (PBS+1% BSA+0.05% NaN3) at room temperature. Serum samples were serially diluted with blocking buffer across the plate and incubated overnight at 4°C. Human IgM or IgG standards (Sigma) were added to each plate. Plates were washed 5X with wash buffer followed by the addition of alkaline phosphatase-conjugated goat anti-human IgM or IgG antibodies (Southern Biotech). Plates were incubated for 4–6 hours at 37°C, washed 5X, and incubated with the alkaline phosphatase substrate p-Nitrophenyl phosphate (Sigma-Aldrich). Optical density readings at 405 nm were measured using a VERSAmax microplate reader (Molecular Devices). Immunoglobulin concentrations in sera were calculated using SoftmaxPro software (Molecular Devices).
2.5. Statistical analysis
Statistical significance was assessed using Prism software (GraphPad Software, Inc). Data differing in only one variable were compared using a two-tailed Student’s t-test with equal variance, and Welch’s correction was employed when appropriate. The one-way analysis of variance (ANOVA) was used to assess statistical differences across groups. In all cases, differences were considered significant when the p-value was less than 0.05.
3. Results
3.1. Experimental design
In this study we used the BALB/c-DKO newborn model as a recipient of hHSCs [4]. Human HSCs were isolated from umbilical cord blood, although some experiments using HSCs recovered from untreated adult peripheral blood were also performed (see supplemental data). The number of HSCs injected ranged from 5×104 to 2.1×106 cells per mouse, and varied based on number of pups available for injection at any given time and whether the cells were cultured. The lower range is consistent with the literature reporting a minimum of 2–5×104 cells per mouse for the successful development of a HIS [2]. The highest cell numbers were generally injected when cells were expanded in culture, because we considered that the number of pluripotent HSCs would decrease during culture (e.g., some cells lost the CD34 cell marker). However, the number of injected cells in each experiment was recorded (Table 1), and the effect of cell number on human hematopoietic chimerism was later assessed.
Table 1.
Parameters for the generation of hematopoietic humanized BALB/c-DKO mice
| Injection group # | # Cord blood samples | CD34+ cell injection (cell #a) | # Days CD34+ culture | Cytokines (+ IL-6, SCF) | “Support” cell injectionb | HSC freezing | # Mice injected I.H. | # Mice injected I.V. | % chimeric micec |
|---|---|---|---|---|---|---|---|---|---|
| 1 | None | No | N/A | N/A | No | N/A | N/A | N/A | N/A |
| 2 | 4 | Yes (L,M) | 1–8 | IL-3, FL | No | No | 5 | 9 | 7 (0) |
| 3 | 2 | Yes (L,H) | 1–8 | IL-3, FL | ΔT | No | 0 | 6 | 67 (0) |
| 4 | 4 | Yes, (L,M) | 0 | N/A | No | No | 6 | 9 | 73 (47) |
| 5A | 2 | Yes (L,M) | 1–8 | FL | No | No | 5 | 0 | 80 (80) |
| 5B | 2 | Yes (M) | 1–8 | None | No | No | 3 | 2 | 80 (40) |
| 6 | 3 | Yes (L,M,H) | 9–28 | FL | No | No | 17 | 16 | 15 (3) |
| 7 | 5 | Yes (L,M,H) | 0 | N/A | ΔT | No | 11 | 4 | 93 (53) |
| 8A | 4 | Yes (L,M,H) | 1–8 | FL | ΔT | No | 9 | 12 | 76 (76) |
| 8B | 2 | Yes (M) | 1–8 | None | ΔT | No | 0 | 13 | 85 (54) |
| 9 | 2 | Yes (M) | 0 | N/A | ΔT,B | No | 0 | 11 | 54 (54) |
| 10 | 3 | Yes (L,H) | 1–8 | None | ΔT,B | No | 0 | 15 | 53 (40) |
| 11 | 3 | Yes (M,H) | 1–8 | TPO | ΔT | No | 7 | 2 | 78 (67) |
| 12 | 9 | Yes (L,M,H) | 1–8 | TPO | ΔT,B | No | 18 | 21 | 85 (51) |
| 13A | 1 | Yes (H) | 1–8 | FL | No | Yes | 1 | 8 | 78 (44) |
| 13B | 2 | Yes (M,H) | 1–8 | None | No | Yes | 0 | 14 | 36 (21) |
| 14 | 4 | Yes (L,H) | 1–8 | None | ΔT | Yes | 0 | 10 | 70 (60) |
| 15 | 1 | Yes (H) | 1–8 | None | ΔT,B | Yes | 6 | 0 | 100 (50) |
| 16 | 1 | Yes (H) | 1–8 | TPO | No | Yes | 0 | 9 | 89 (89) |
| 17 | 5 | Yes (M,H) | 1–8 | TPO | ΔT | Yes | 10 | 7 | 100 (94) |
| 18 | 1 | Yes (M) | 1–8 | TPO | ΔT,B | Yes | 2 | 0 | 0 (0) |
| 19 | 3 | No | N/A | N/A | ΔT | N/A | 2 | 5 | 0 (0) |
| 20 | 2 | No | N/A | N/A | ΔT,B | N/A | 3 | 2 | 0 (0) |
|
| |||||||||
| Total # | 105 | 175 | |||||||
Number of CD34+ cells injected per mouse. L=50,000–150,000; M=150,000–500,000; H=500,000–2,000,000.
ΔT=T cell depleted; ΔT,B=T cell and B cell depleted
Frequency of mice (injected i.h. and i.v.) displaying a PBL and/or thymus with >0.5% hCD45+ cells; the number in parenthesis is the % of mice displaying >2% hCD45+ in total (mouse + human) CD45+ cells in PBL, bone marrow and/or spleen.
N/A=not available
In our experimental design, we analyzed the following variables that may affect the generation of humanized mice (Table 1 and Fig. 1A): injection route of HSCs, CD34+ stem cell culture prior to injection, addition of cytokines to HSC cultures, co-injection of CD34− “support” cells, and injection of fresh versus frozen HSCs. Cord blood preparations were treated in one manner and injected into a cohort of mice such that each experimental group typically consisted of recipients generated with 1–5 individual cord blood samples (Table 1). Additionally, individual cord blood samples were used to test single parameters to ensure that differences observed were not due solely to variations in the source of HSCs. Human hematopoietic chimerism was measured in the peripheral blood, bone marrow, thymus, spleen, and lymph nodes of all experimental humanized mice. The lymph nodes were not found in all mice and when found were under-developed, but small tissue samples at lymph node sites were nevertheless processed for flow cytometric analyses to assess presence of human hematopoietic cells. T-tests were performed between groups of experimental mice varying in one parameter. To increase the power of our analysis in detecting the presence of small effects and to detect the effect of confounding factors, an additional ANOVA was performed across all groups. Chimeric mice (mice displaying human hematopoietic cells) were defined as those exhibiting a peripheral blood and/or a thymus composed of >0.5% human CD45+ cells (Table 1). The thymus was the tissue providing the most sensitive readout, and the 0.5% cutoff was based on background levels in non-transplanted BALB/c-DKO mice. The degree of human chimerism in mice was calculated as the percentage of hCD45+ cells in the total (human and mouse) CD45+ cell population (%hCD45/(%hCD45+%mCD45)) in defined tissues. The number of mice displaying a degree of chimerism above 2% in peripheral blood, bone marrow and/or spleen (a minimal frequency required for flow cytometric analyses) is reported in Table 1, but the effect of each parameter on the degree of human chimerism was assessed for all chimeric mice above the 0.5% cutoff. Overall, 280 mice were analyzed for these studies.
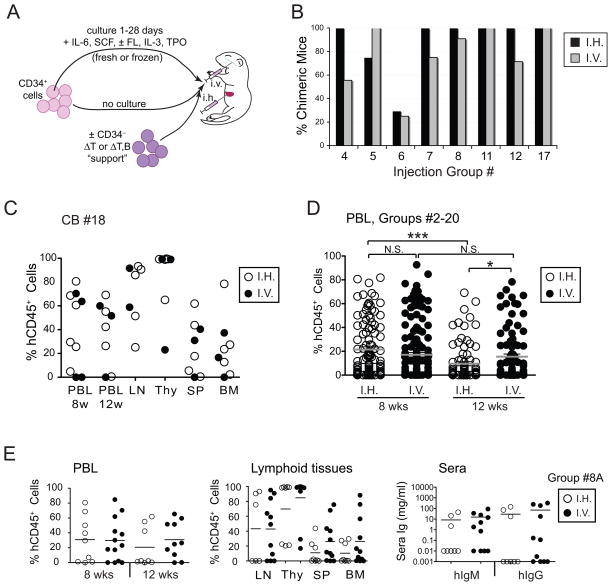
Figure 1. Intra-venous and intra-hepatic injection of cord blood HSCs into newborn BALB/c-DKO mice result in similar rates of human leukocyte engraftment.
(A) Schematic representation of experiments performed to test variables potentially affecting the generation of humanized BALB/c-DKO mice. (B) Rate of human chimeric mice achieved by either i.h. or i.v. injection of HSCs. Human chimeric mice were defined as recipient mice that harbored >0.5% human leukocytes either in peripheral blood and/or in the thymus 8 or more weeks after HSC injection. Data is shown for different experimental groups (Table 1) as indicated in figure. (C) Percentage of human leukocytes (%hCD45+ cells in total leukocytes) in peripheral blood (PBL) at 8 and 12 weeks post engraftment, and in lymph nodes (LN), thymus (Thy), spleen (SP) and bone marrow (BM) at 16 weeks post engraftment, of mice injected with HSCs from a single cord blood donor (CB#18) either i.h. (open circles) or i.v. (filled circles). Each symbol represents an individual mouse. No statistically significant difference was found between groups of i.h. (6 individuals) and i.v. (4 individuals) injected mice. (D) Frequency of human CD45+ leukocytes in PBL at 8 and 12 weeks post-engraftment among all study mice (groups #2–20 in Table 1) that were injected either i.h. (open circles) or i.v. (filled circles). Each circle represents an individual mouse. Horizontal bars represent arithmetic means. P values of <0.05 (*) and <0.001 (***) are indicated. N.S. = not significant. (E) Frequency of hCD45+ leukocytes in PBL at 8 and 12 weeks-post engraftment (left panel), and in lymphoid tissues at 16 weeks-post engraftment (middle panel), and of human IgM and IgG concentrations in the sera at 16 weeks-post engraftment (right panel) in mice of experimental group #8A (Table 1) that were injected either i.h. (open circles) or i.v. (filled circles) with 4 different cord blood samples. Each circle represents an individual mouse. Horizontal bars represent the mean frequencies of hCD45+ cells. No statistically significant differences were found between the i.h. and i.v. groups.
3.2. Injection of human HSCs via i.h. and i.v. produces similar rates and degree of human chimerism
A previous study reported better and more consistent human hematopoietic engraftment in adult NOD/SCID mice that received hHSCs i.v. instead than i.h. [10], but no direct comparison in the BALB/c-DKO newborn model has been reported so far to our knowledge.
Thus, groups of humanized BALB/c-DKO mice generated injecting hHSCs either i.v. or i.h. (Table 1) were analyzed to determine whether the injection route affected the rate and degree of human chimerism. Our analysis showed that i.v. and i.h. injection routes generated similar rate of human chimerism in these groups (Fig. 1B). Moreover, no significant difference was observed in the percentage of human cells in peripheral blood and lymphoid tissues among mice injected either i.v. or i.h. with HSCs from a single cord blood sample (Fig. 1C). Consistently, a one-way ANOVA comparing cell engraftment levels in the blood among all experimental mice in Table 1 indicated no difference between injection routes at 8 weeks post-injection. However, a small increase in the level of chimerism in mice injected i.v. over those injected i.h. was observed at 12 weeks post-cell transfer (Fig. 1D). This difference was probably related to the fact that the degree of chimerism slightly but significantly (p<0.0001) decreased in the blood of i.h.-injected mice from 8 to 12 weeks, while that of i.v.-injected mice did not (Fig. 1D).
We also compared human chimerism among mice within individual injection groups in which cord blood HSCs were similarly treated and injected either i.h. or i.v. (Table 1, groups 4, 5B, 6, 7, 8A, 8B, 11, 12 or 17). As exemplified with the analysis of group #8A, the degree of human chimerism varied largely (from undetectable to 80–100%) from mouse to mouse and donor to donor, but it was not significantly affected by the route of HSC injection (Fig. 1E), as also shown by the lack of differences in hIgM and hIgG in sera (Fig. 1E, right panel). Although no statistical difference was found, the degree of chimerism tended to be higher in thymus, bone marrow and spleen tissues for mice injected i.v., supporting the finding of the ANOVA (Fig. 1D).
Based on these analyses, we concluded that i.h. and i.v. injection of human HSCs into newborn BALB/c-DKO mice promote similar rates and degree of human chimerism, although i.v. injection may offer a small advantage by sustaining the chimerism over time. Because of the relatively small difference observed between mice injected i.v. or i.h., data from mice injected via both routes were combined for subsequent analyses of other parameters.
3.3. Short-term culture of hHSCs improves rate and degree of human chimerism
A previous report describing multi-lineage engraftment in the BALB/c-DKO newborn model used a protocol in which pups were injected with freshly purified CD34+ HSCs [4]. However, in other humanized mouse models and in human stem cell transplantation, HSCs are often cultured prior to their transfer into recipient organisms [11; 25; 26; 27]. This culture affords greater flexibility in protocol logistics by separating the time of HSC purification from that of the availability of recipients. This culture step, in addition, promotes expansion of HSCs, potentially resulting in larger cell numbers available for transplantation. Therefore, we performed experiments to test the effect of HSC culture on human hematopoietic engraftment in BALB/c-DKO mice.
HSCs were cultured for a variable period of time ranging from 1 to 28 days, and depending on the availability of recipient mice. In initial experiments we noticed that culturing HSCs for longer than 8 days had a drastically different effect than culturing the cells for less than 8 days, while minimal differences were observed within each time group (data not shown). To most effectively document the effect of culture, therefore, we arbitrarily categorized our experiments into three experimental groups: 1) fresh, 2) short-term cultured (1–8 days), and 3) long-term cultured (9–28 days) CD34+ HSCs.
Mice of experimental groups 4, 5A and 6 (Table 1) were analyzed to assess the effect of culture time on human chimerism. In these groups, cells were cultured in the presence of IL-6, stem cell factor (SCF) and Flt3-Ligand (FL) and, although the rate of cell expansion varied among individual cord blood preparations (data not shown), all cultures displayed high (>98%) cell viability. Analyses of human leukocytes and hIgs at 8–16 weeks post engraftment showed that the percentage of humanized mice was drastically decreased when HSCs were cultured for more than 8 days (Fig. 2A). In contrast, fresh and short-term cultured HSCs resulted in similar rates of chimerism, but this was true only when assessing for the presence of human leukocytes (Fig. 2A). In relation to hIgM or hIgG, the rate of chimeric mice was higher for groups injected with short-term cultured cells than for those transplanted with fresh HSCs (Fig. 2A), suggesting that short-term culture enhanced human HSC engraftment and/or their differentiation into the B cell lineage.
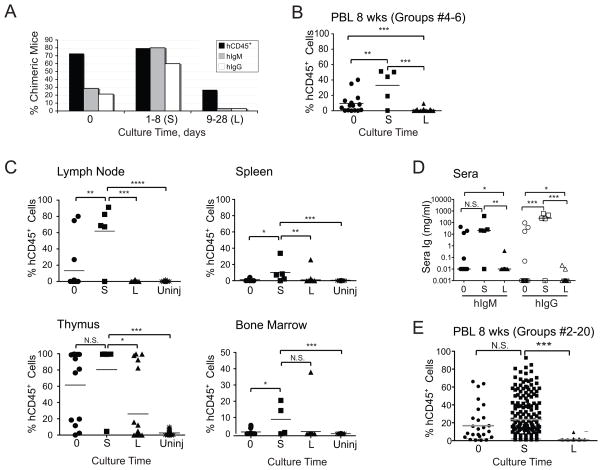
Figure 2. Short-term culture of cord blood HSCs increases rate and degree of human chimerism in BALB/c-DKO mice.
Cord blood CD34+ HSCs were injected on the day of purification (0) or after culture as described in Materials and Methods for shorter (1–8 days, S) or longer (9–26 days, L) times. (A) Rate of chimeric mice as established by the presence of >0.5% human CD45+ leukocytes in PBL and/or thymus (black bars), or by detection of human IgM (gray bars) or IgG (white bars) in sera of mice in experimental groups #2–10 (Table 1). (B–C) Frequency of hCD45+ leukocytes in PBL at 8 weeks (B) and lymphoid tissues at 16 weeks (C) in mice (groups #4–6, Table 1) that were injected with fresh or cultured HSCs. Data from uninjected (uninj) control mice is shown for comparison. D) Concentration of hIgM and hIgG in sera of mice at 16 weeks. (E) ANOVA of human engraftment levels in PBL among all study mice (groups #2–20, Table 1) at 8 weeks. Each symbol in panels B–D represents an individual mouse. Horizontal bars represent mean frequencies. P values of <0.05 (*), <0.01 (**), and <0.001 (***) are indicated. N.S. = not significant.
We found that the degree of human chimerism in mice injected with long-term cultured HSCs was greatly and significantly reduced when compared to mice injected with either fresh or short-term cultured HSCs (Fig. 2B, 2C). In fact, humanized mice generated with long-term cultured HSCs displayed hematopoietic human cell frequencies at levels that were only marginally higher than the background detected in non-injected recipients (Fig. 2C). In contrast, short-term culture of HSCs appeared to be beneficial for the establishment of human chimerism. Indeed, the level of human chimerism in peripheral blood (Fig. 2B), lymph node, spleen and bone marrow (Fig. 2C) was significantly higher in mice generated with short-term cultured HSCs than those generated with fresh HSCs. Analyses of absolute cell numbers showed similar trends in the lymph node, spleen and thymus, although the differences were not statistically significant because of higher variability (data not shown). The sera collected at 16 weeks contained hIg levels ranging from 0.05 to 100 μg/ml of IgM and from 0.05 to 500 μg/ml of IgG (Fig. 2D). The Ig concentration generally correlated with the amount of chimerism in the mouse (data not shown), and hIgM and hIgG levels were significantly reduced in mice established with long-term cultured HSCs, and were the highest in mice injected with short-term cultured cells (Fig. 2D). The conclusions obtained from the analysis of selected groups were in part supported from an ANOVA performed across all groups (Fig. 2E). Here, the degree of human chimerism was significantly lower in mice generated with HSCs from long-term cultures. A reduced degree of human chimerism was also observed in mice injected with fresh HSCs relative to those transplanted with short-cultured HSCs (Fig. 2E), but this difference was not statistically significant, potentially indicating the presence of a confounding factor. Finally, we found no effect on the stability of chimerism with either fresh or short and long cultured HSCs (data not shown).
In conclusion, short-term culture (1–8 days) of CD34+ HSCs promotes better rate and degree of human hematopoietic chimerism and levels of sera Igs in BALB/c-DKO mice.
3.3. Culturing hHSCs with Flt-3-Ligand, thrombopoietin, and/or IL-3
Investigators have reported growing hHSCs in the presence of different types of cytokines [17; 18; 25; 26; 28; 29]. Since the majority of studies report beneficial use of IL-6 and SCF [28; 30], we added these cytokines to all HSC cultures and tested the effect of FL, TPO, and IL-3 on the generation of BALB/c-DKO humanized mice. In these experiments, culture times were limited to short-term, between 1 and 8 days.
FL is important in the expansion and development of multiple hematopoietic lineages [31], while TPO promotes the production and differentiation of megakaryocyte and platelets and the survival and expansion of HSCs [32]. Both cytokines are sometimes added to the culture of CD34+ cells used for the generation of humanized mice [14; 26; 33]. We found that the rates of chimeric mice were generally similar regardless of the presence of FL (groups 5A,B, 8A,B, 13A,B, Table 1, and Fig. 3A) or TPO (groups 8B, 10–12, Table 1, and Fig. 3C) during culture. Analysis of lymphatic tissues, however, revealed that mice injected with FL-treated stem cells had a significantly higher percentage of hCD45+ cells in the lymph nodes, thymi and spleens, relative to mice that received HSCs cultured without FL (Fig. 3B). The addition of TPO to HSC cultures, in contrast, resulted in mice with comparable degree of human chimerism (Fig. 3D). Overall, the addition of either FL or TPO had no effect on hIgM or hIgG levels in the sera of chimeric mice (data not shown). The percentage of human leukocytes in the peripheral blood largely mirrored the chimerism in the lymphatic tissues, with higher chimerism resulting from the use of FL (Fig. 3E). However, a decrease in chimerism was observed from 8 to 12 weeks post transplantation among mice generated with TPO-treated HSCs (Fig. 3E). This difference was significant only among the i.h.-injected humanized mice, suggesting that TPO treatment and i.h. injection of HSCs synergize in reducing the survival of an HIS engraftment in mice.
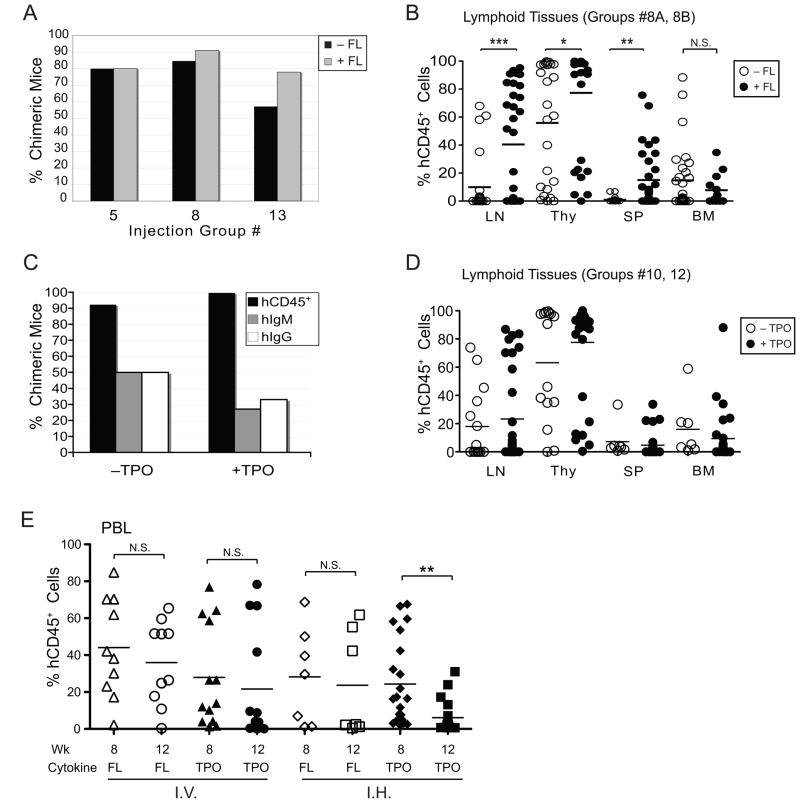
Figure 3. Addition of Flt-3 ligand to HSC cultures increases human hematopoietic engraftment while thrombopoietin supplementation has minimal effects.
Cord blood CD34+ HSCs were cultured with SCF, IL-6 and either FL (A and B) or TPO (C and D) for 1–8 days. (A) Rate of chimeric mice as established by the presence of >0.5% hCD45+ leukocytes in PBL and/or thymus of mice injected with HSCs cultured in the presence (gray bars) or absence (black bars) of FL. Data from mice of groups #5, 6, and 13 (Table 1) is shown. (B) Percentages of hCD45+ leukocytes in the indicated lymphoid tissues of recipient mice 10–18 weeks after receiving HSCs cultured in the presence (filled circles) or absence (open circles) of FL. Data from mice of groups #8A and #8B is shown. (C) Rate of chimeric mice as established by the presence of >0.5% hCD45+ leukocytes in PBL and/or thymus (black bars), or by the detection of human IgM (gray bars) or IgG (white bars) in sera of mice in experimental groups #8B, #10, #11 and #12 (Table 1). (D) Percentage of hCD45+ leukocytes in the indicated lymphoid tissues of recipient mice 10–18 weeks after receiving HSCs cultured in the presence (filled circles) or absence (open circles) of TPO. Data from mice of groups #10 and #12 is shown. No statistically significant differences were found between the groups with or without TPO in each tissue. (E) Frequency of hCD45+ cells in PBL at 8 and 12 weeks in mice that were injected i.v. or i.h. with HSCs that were cultured in either FL or TPO. Each symbol in panels B, D and E represents an individual mouse. Horizontal bars represent mean frequencies. P values of <0.05 (*), <0.01 (**), and <0.001 (***) are indicated. N.S. = not significant.
The cytokine IL-3 is reported to aid the proliferation and differentiation of pluripotent HSCs and has been added to cultures of human hHSCs used for transplantation into patients and immunodeficient mice [28; 34; 35]. In our experiments, proliferation and survival of cells cultured in IL-3 (and in the presence of IL-6, SCF and FL) were comparable to those cultured without IL-3 (data not shown). However, IL-3 treatment greatly diminished the rate of chimeric mice (Fig. 4A). Since 4 different cord blood preparations were used to establish the group of 14 mice that received IL-3-treated HSCs (group #2, Table 1), it is unlikely that the low human chimerism was due to HSC donor variability, or variability in the HSC preparation. We found that none of the mice injected with IL-3-treated HSCs had measureable levels of hIgM or hIgG in sera at 16 weeks (Fig. 4B). Moreover, IL-3 treatment of HSCs strongly impaired the establishment of human hematopoietic cells in all tissues examined (Fig. 4C).
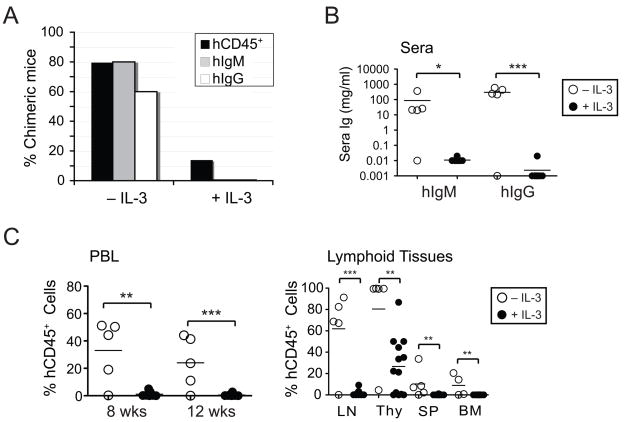
Figure 4. Addition of IL-3 to human HSC cultures severely weakens their engraftment potential in BALB/c-DKO mice.
Cord blood CD34+ HSCs were cultured as described in Materials and Methods with or without the addition of IL-3 for 1–8 days before transfer into BALB/c-DKO mice. (A) Human engraftment of mice injected with HSCs cultured in the presence or absence of IL-3 was determined by the presence of >0.5% hCD45+ leukocytes in PBL or thymus (black bars) and human IgM (gray bars) and IgG (white bars) in the sera. The frequency of mice displaying chimerism is shown for groups #2 (+IL-3) and #5A (−IL-3). (B) Concentrations of hIgM and hIgG in sera of mice transplanted with HSCs cultured with (filled circles) or without (open circles) IL-3. (C) Frequency of hCD45+ leukocytes in PBL at 8 and 12 weeks (left panel), and in lymphoid tissues at 16 weeks (right panel) of mice that were injected with HSCs cultured in the presence (filled circles) or absence (open circles) of IL-3. Each symbol in panels B and C represents an individual mouse. Horizontal bars represent mean frequencies. P values of <0.05 (*), <0.01 (**), and <0.001 (***) are indicated.
In summary, the addition of FL, but not that of TPO, to hHSC cultures improved the generation of BALB/c-DKO humanized mice. The addition of IL-3, in contrast, largely compromised the engraftment potential of hHSCs in mice.
3.5. Co-injection of T cell-depleted “support” cells aids hHSC engraftment
Engraftment of human HSCs and their differentiation in recipient mice relies grossly on the capacity of mouse and human cytokines and other factors to interact with and signal through heterologous receptors. Although many mouse cytokines bind human cytokine receptors, and vice versa [36], some of these interactions are known to be less functional [37; 38; 39]. Therefore, we investigated whether the co-injection of CD34− human cord blood cells with CD34+ HSCs could improve engraftment and differentiation rates, with the rationale that the CD34− “support” cells may secrete human cytokines and other factors that aid human HSC engraftment and maturation in vivo.
We isolated two potential populations of “support” cells from the CD34− cell fraction: 1) T cell-depleted (ΔT) cells, and 2) T cell and B cell-depleted (ΔT,B) cells. These cell depletion procedures were performed to prevent graft versus host disease by T cells and to avoid potential human chimerism by differentiated donor lymphocytes. Approximately 5×105–5×106 “support” cells were co-injected with HSCs into each recipient. In addition, two groups of mice were injected with “support” cells in the absence of HSCs to control for the contribution of “support” cells to human chimerism (Table 1, groups 19 and 20). Importantly, these control mice did not display any hCD45+ cells in the blood at 8 or 12 weeks (Fig. 5A, 5B, 5D), nor hIgM or hIgG in their sera (data not shown).
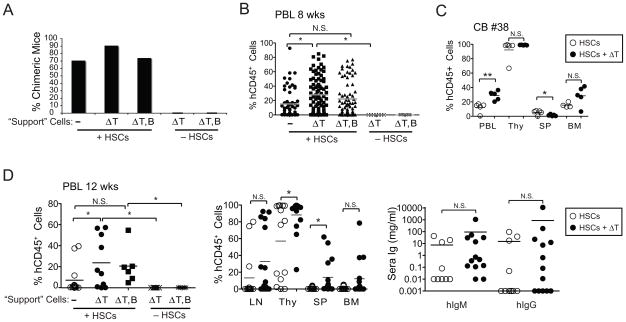
Figure 5. Co-injection of T cell-depleted CD34− cells supports engraftment and differentiation of human hematopoietic cells.
BALB/c-DKO mice were injected with cord blood HSCs in combination or not with CD34− cord blood “support” cells depleted of either T (ΔT) or T and B (ΔT,B) cells. (A) Rate of chimeric mice as established by the presence of >0.5% hCD45+ leukocytes in PBL and/or thymus of mice injected with HSCs with or without “support” cells. Frequency of chimeric mice injected with support cells in the absence of HSCs is shown as control. (B) Multiparameter ANOVA of the frequency of hCD45+ cells in PBL of 8 weeks old mice injected with HSCs in the presence or absence of “support” cells. Data from mice injected with support cells in the absence of HSCs is shown as control. Data from mice of all experimental groups besides groups #1–3, 6 (Table 1) is shown. (C) Percentage of hCD45+ leukocytes in PBL at 8 weeks and in indicated lymphoid tissues of mice injected with CD34+ HSCs with or without ΔT “support” cells from a single cord blood sample (CB#38). (D) Frequency of hCD45+ cells in PBL at 12 weeks (left panel) and in lymphoid tissues (middle panel), and concentration of hIgM and hIgG (right panel), in mice injected with HSCs in the presence or absence of “support” cells, or with “support” cells only. Data from experimental groups #4, #7, #9, #19, and #20 (Table 1) is shown. Each symbol in panels B–D represents an individual mouse. Horizontal bars represent mean frequencies. P values of <0.05 (*), <0.01 (**), and <0.001 (***) are indicated. N.S. = not significant.
We found that the addition of ΔT “support” cells marginally increased the rate of humanized mice, whereas that of ΔT,B “support” cells had no effect (Fig. 5A). Concordantly, an ANOVA performed across all experimental mice indicated a significant increase in human chimerism following the addition of ΔT, but not of ΔT,B CD34− cells (Fig. 5B). The analysis of mice that received cells from a single cord blood tissue generally confirmed the data from multiple mouse groups. In fact, a significant higher frequency of hCD45+ cells was observed in peripheral blood and bone marrow of mice that received “support” cells (Fig. 5C). However, the percentage of hCD45+ cells was slightly, but significantly, lower in the spleen of mice that received ΔT “support” cells in this cohort, perhaps indicating that differences between cord blood samples may mask effects mediated by other parameters.
In fact, when we compared the degree of human chimerism among groups of mice that were injected with HSCs from multiple cord blood sources (Fig. 5D and data not shown), we found a higher frequency of hCD45+ cells in all tissues examined from mice that received HSCs with ΔT “support” cells (Table 1, groups 7, 8) relative to those that were injected with HSCs only (Table 1, group 4) or with HSCs and ΔT,B cells (Table 1, groups 9, 10). These differences, although consistent in every tissue, did not always reach statistical significance. The addition of ΔT “support” cells appeared to improve human chimerism whether the mice were generated with fresh or cultured HSCs (data not shown), suggesting that this was the confounding factor that reduced the difference observed between groups of mice injected with either fresh or short-cultured HSCs in the ANOVA represented in Fig. 2E. The addition of ΔT cells also improved the production of hIgs, but the difference was not statistically significant (Fig. 5D, right panel).
Thus, overall our results indicate that the addition of CD34− human “support” cells, including B cells, aids the engraftment and development of human leukocytes in the BALB/c-DKO newborn model.
3.6. Increased consistency of B and T cell production in humanized mice generated with the optimized protocol
Based on the analyses of each of the above parameters, we designed an optimized protocol for the generation of humanized BALB/c-DKO mice consisting of co-injecting (i.v. or i.h.) newborn mice with human cord blood CD34+ HSCs cultured for 1–8 days in the presence of IL-6, SCF and FL, and T cell-depleted CD34− “support” cells. This optimized protocol generates humanized mice with significantly higher frequency of human leukocytes in peripheral blood (Fig. 6A) and lymphoid tissues (data not shown) relative to mice generated with a conventional protocol consisting of injecting only freshly purified CD34+ HSCs.
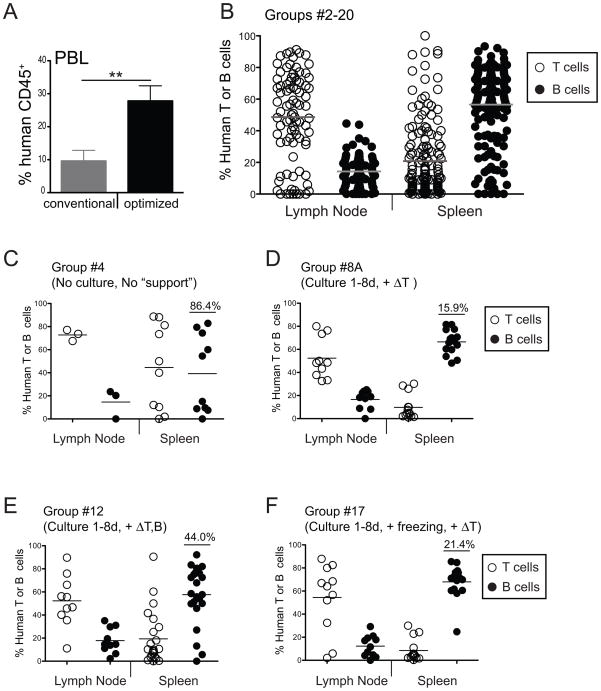
Figure 6. Protocol optimization increases the degree of consistency between humanized BALB/c-DKO mice.
(A) Average frequency of hCD45+ cells in PBL of humanized mice generated with either a conventional (freshly prepared non-cultured CD34+ hHSCs) or the optimized (CD34+ hHSCs cultured in the presence of IL-6/SCF/FL + ΔT CD34− support cells) protocol. P value of <0.01 (**) is indicated. (B–F) Lymph node and spleen cells isolated from BALB/c-DKO mice that were injected with human cord blood HSCs were analyzed by flow cytometry for the presence of CD3+CD5+ T cells (open circles) and CD20+ B cells (filled circles). The percentage of T and B cells in the hCD45+ leukocyte population of lymph nodes and spleens are shown for mice in all groups (groups #2–18, panel B), and for those of groups generated with either suboptimal protocols (groups #4 and #12 in panels C and E), or with optimized protocols (groups #8A and #17 in panels D and F). Each symbol represents an individual mouse. Horizontal bars represent mean frequencies. Numbers in graphs are coefficient of variation in the B cell population of each group of mice.
One common finding in hematopoietic humanized mice is the great variability in the reconstitution of T and B lymphocytes [4; 5; 7; 8]. In accordance with our initial goal to achieve consistent generation of lymphocytes in humanized mice, we analyzed the lymphocyte composition in lymphoid tissues. We found that the presence of T and B cells in the spleen and lymph nodes varied extensively among mice across all groups (Fig. 6B), indicating that protocol variations affect the generation of human lymphocytes in this model. Further analyses among experimental groups showed similar variability in the splenic T and B cell populations in mice generated with freshly purified CD34+ hHSCs (Fig. 6C, coefficient of variation for splenic B cells = 86.4%), a protocol previously used [4]. In contrast, we found greater consistency in mice generated with the protocol optimized in this study. Humanized mice generated with this protocol displayed greater consistency in the percentage of T and B cells in lymph nodes and spleen (Fig. 6D, coefficient of variation for splenic B cells = 15.9%). The co-injection of ΔT “support” cells, rather than ΔT,B “support” cells, was important for this increased consistency. In fact, mice that received HSCs with ΔT,B “support” cells displayed higher variability in the frequency of T and B cell in peripheral lymphoid organs (Fig. 6E, coefficient of variation for splenic B cells = 44.0%).
A difficult aspect of using newborns for the generation of humanized mice is the logistics associated with predicting the availability of recipient mice. For this reason we tested if freezing HSCs after short-term culture preserved HSC engraftment and differentiation abilities in recipient mice. We found that the frequency of chimeric mice and the degree of human chimerism was similar for mice generated with either fresh or frozen hHSCs (Fig. S1), indicating that HSC freezing does not reduce the capacity of these cells to engraft and differentiate in mice. Consistently, the degree of lymphocyte chimerism achieved when HSCs were injected with ΔT “support” cells after freezing (Fig. 6F, coefficient of variation for splenic B cells = 21.4%) was similar to that obtained with fresh cells.
The number of human CD34+ cells injected into each mouse could potentially affect the efficiency of human hematopoietic chimerism. To investigate this issue, we compared the level of human hematopoietic chimerism in the spleen of mice that were generated with a conventional protocol using freshly prepared, non-cultured, CD34+ cells to that of mice generated with the optimized protocol and comparable amounts of cultured CD34+ cells (groups #4 and 8A Table 1, Fig. S2). Humanized mice generated with low numbers (50,000–100,000) of cultured CD34+ cells in addition to ΔT support cells, displayed a significantly higher frequency of hCD45+ cells than mice generated with equivalent numbers of freshly prepared CD34+ cells (Fig. S2). Mice that received medium numbers (150,000–200,000) of cultured CD34+ cells had a slightly higher level of chimerism than mice injected with low cell numbers, on average, but these differences were not statistically significant. Injection of medium numbers of fresh CD34+ cells did not significantly increase the level of human chimerism relative to mice injected with low cell numbers. Interestingly, mice that were generated with the optimized protocol and received high numbers (1,000,000) of CD34+ cells did not display higher levels of chimerism relative to mice that received low or medium cell numbers (Fig. S2). Thus, these data suggest that the increased efficiency in the generation of humanized mice achieved with the optimized protocol is not significantly affected by the number of cells injected per mouse within the range used in this study (50,000 to 2,000,000 cells/mouse).
Generation of humanized mice with cord blood samples prohibits knowing the characteristics of the immune system transplanted into the mice prior to analysis. To study specific immune systems in humanized mice would require hHSCs isolated from pediatric or adult individuals with defined immunological characteristics. Using our optimized protocol, we tested whether it was possible to generate humanized mice with CD34+ cells isolated from peripheral blood of untreated adults. Transfer of peripheral blood CD34+ cells promoted the generation of a small frequency of chimeric mice (Fig. S3A). All of the mice, however, displayed an extremely low percentage of human chimerism in peripheral blood and spleen, too low to allow further analyses. The only tissue exhibiting a significant level of human chimerism in some of the mice was the thymus (Fig. S3B), but the absolute number of hCD45+ cells was extremely low (data not shown). These data indicate that CD34+ cells isolated from peripheral blood of untreated human individuals are not capable of engrafting and differentiating in immunodeficient mice.
In conclusion, the protocol for the generation of humanized BALB/c-DKO mice optimized in this study promotes higher rates, degree, and sustainability of cord blood-derived human hematopoietic cell engraftment, as well as more consistent generation of human B and T cells.
4. Discussion
Our study was performed to test parameters that potentially affected the survival, engraftment and/or differentiation capacity of umbilical cord blood-derived hHSCs in immunodeficient mice, and to generate a protocol optimized for the establishment of humanized mice in the BALB/c-DKO newborn model. We show that culturing hHSCs for 1 to 8 days in the presence of IL-6, SCF, and FL, and co-injecting these cells with T cell-depleted CD34− cells into sublethally irradiated newborn BALB/c-DKO mice, increases the frequency of humanized mice and the generation of human leukocytes in all lymphoid tissues. Moreover, we found that this protocol improves the consistency of B cell and T cell production between mice generated with the same or different cord blood preparations.
Our study was performed by analyzing the effect of various parameters on the generation of approximately 280 humanized mice. Analysis of variance (ANOVA) was used to determine the presence of small effects and to control for the effect of confounding factors. However, most differences or lack thereof observed by the ANOVA analyses were confirmed by comparing groups of mice that differed by only one parameter.
In our study, culture of hHSCs proved to be an important factor in engraftment success. In particular, short-term culture (less than 9 days) of CD34+ hHSCs improved engraftment and leukocyte generation, while longer culture drastically reduced the engraftment and differentiation capacity of HSCs. We observed minimal (non-significant) differences in human hematopoietic chimerism in mice injected with hHSCs that were cultured for different time within the window of 1–8 days (data not shown). In contrast, hHSCs drastically lost their ability to reconstitute a human hematopoietic compartment in humanized-mice when cultured for longer than 8 days, and the reasons for this sudden loss of function were not defined. The positive effect of short-term culture over no culture was statistically significant only when the comparison was performed among groups of mice that only differed for this factor, while it was not significant in ANOVA. We propose that the reason for this discrepancy is due to the effect of an other important factor, the co-injection of support cells, which appears to positively affect even mice injected with non-cultured cells (see below). Mice that received support cells were not included in the groups analyzed by t-test, but were included in the ANOVA. Freshly isolated CD34+ cells, which consist of multiple cell subsets including self-renewing stem cells, are prevalently in G0 phase, but enter the cell cycle during cell culture with SCF and/or other cytokines [14; 40]. In addition, the frequency of cells in G1 cycling phase progressively increases with time in culture [40]. Human HSCs in G0 phase have been proved to be superior at engrafting NOD/SCID mice compared to G1 cycling HSCs [41], while HSCs in the G1 phase can aid the engraftment capacity of G0 cells [14]. Thus, the different engraftment levels achieved with short-term and long-term cultured cells in our study may be due to a difference in the ratio of non-cycling and cycling cells at different times of culture. Specifically, short-term cultures may contain an optimal number of both G0 and G1 HSCs, which promote engraftment, while long-term cultures may have too many G1 and too few G0 HSCs for proper engraftment. We favor this interpretation of our data over a potential effect caused by the dose of CD34+ cells (more often higher for cultured cells) because: 1) the dose effect on chimerism was not significantly different, and 2) the highest CD34+ cell dose did not improve human hematopoietic engraftment of cells cultured for over 8 days. Successful engraftment into immunodeficient mice with CD34+ hHSCs cultured for longer than 8 days has been previously reported [25; 26; 27]. The difference between our and those previous studies may be related to the culture conditions, and specifically the types of medium, FBS, and cytokines, whereby some may be capable of maintaining the survival of long-living self-renewing HSCs.
While our HSC cultures always contained IL-6 and SCF, we found that the presence of additional cytokines had variable effects on the generation of humanized mice. The addition of FL improved human hematopoietic chimerism in the peripheral lymphoid organs of humanized mice. Specifically, the frequency of human hematopoietic cells in the bone marrow of humanized mice was not affected by the use of FL, while the frequency was significantly higher in the spleen and lymph nodes of mice that received FL-treated HSCs. Differences in the thymus were small and, in large part, non significant. How FL mediates its positive effect on peripheral lymphoid tissues is presently unclear, but different scenarios can be suggested. The fact that the frequency of hCD45+ cells was the same in the bone marrow (these were mostly CD19+ B cells, data not shown) suggests that FL-treated or non-treated hHSCs had similar seeding capacity and hematopoietic production in this tissue. One possibility is that FL-treated hHSCs have enhanced capacity to seed the spleen and, thus, promote hematopoiesis in the spleen in addition to the bone marrow. FL has been shown to increase migration of hHSCs to SDF-1 [42], which may promote increased homing to and retention in the spleen. Another possibility is that FL alters permanently the capacity of hHSCs to differentiate into lymphoid and non-lymphoid hematopoietic cells by promoting epigenetic changes. A third possibility, is that FL-treated hHSCs produce factors that alter the murine microenvironment allowing better differentiation and survival of human hematopoietic cells.
The use of IL-3 in cultures of HSCs is more controversial [43; 44]. Our results support those studies indicating a negative effect of IL-3, as we found that IL-3-treatment of HSCs strongly inhibited their capacity to generate a HIS in BALB/c-DKO mice. IL-3 has been shown to promote strong proliferation of hHSCs [40], which could potentially limit cell engraftment by reducing the numbers of HSCs in G0 phase [14]. In contrast to IL-3, the addition of TPO to HSC cultures had only minimal effect on the establishment of a HIS in BALB/c-DKO mice. While we could not discern a significant positive effect of TPO, we noticed that it slightly reduced the longevity of the human hematopoietic graft, thus not justifying its use. Culturing of HSCs affected expression of markers such as CD34, CD38, CXCR4, and CD45 (data not shown). However, these changes were similar in the different culture conditions, thus not correlating with the engraftment abilities of HSCs in mice.
Despite the advancement achieved with the use of Rag and Il2rγ double-deficient mice, these mice are still unable to provide an optimal environment for the generation of a normal HIS. The problem is most likely related to the inability of some mouse and human cytokines and chemokines to efficiently bind and promote signaling via heterologous receptors [37; 38; 39]. Cytokines and chemokines play a pivotal role in the survival, expansion, localization and differentiation of hematopoietic stem cells and hematopoietic cell lineages. We found that co-injecting T cell-depleted CD34− cells alongside CD34+ HSCs increases the frequency, degree and consistency of human chimerism in mice. The presence of B cells in the “support” cell preparation appeared important but not essential for the supporting function. Our findings are in line with unpublished observations of another group that reported higher human engraftment in mice injected with T cell-depleted cord blood cell preparations relative to mice that received only CD34+ enriched cells [9]. Of note, the co-injection of T cell-depleted CD34− cells improved human chimerism of mice that received either cultured or non-cultured hHSCs (data not shown), suggesting that the supportive blood cell is different from that arising during culture (i.e., G1 CD34+ cells). Thus, our data support the possibility that blood cells express factors that help HSCs to survive, although the natures of the exact cell type and factor(s) are presently unknown.
We found that cultured hHSCs could be frozen for later use without impairing their engraftment abilities. Injection of hHSCs into neonate immunodeficient mice results in higher human hematopoietic engraftment [9]. Thus, the possibility of freezing hHSCs increases the flexibility of using BALB/c-DKO newborns for the generation of hematopoietic humanized mice, since it is not always easy to predict the availability of recipients.
A recent study indicated no differences in the level of human hematopoietic engraftment in neonate NOD-scid IL2rγnull mice that received hHSCs i.h. or i.c., where the i.c. route is considered equivalent to the i.v. route [9]. We found that both i.h. and i.v. injections of HSCs were suitable for human engraftment into BALB/c-DKO newborns. However, human leukocytes were maintained for longer time in mice injected i.v. than i.h in our study. The difference was small, and significant only in the ANOVA of all mice. Our results suggest that long-term HSCs have increased capacity of survival and/or homing to the bone marrow if they are injected i.v. instead than i.h., maybe because the liver stops being a site of hematopoiesis before birth. Although technically challenging, i.v. injection into the facial vein of 1–3 days old mice was reliable. Thus, we favor injection of hHSCs into mice via i.v. over i.h. because of both its reliability and its promotion of a longer human hematopoietic engraftment.
Very limited success was obtained when mice were injected with CD34+ cells isolated from peripheral blood of adult individuals. Although up to 30% of the mice had detectable numbers of human leukocytes, these numbers were only slightly above the background observed in intact recipients. Of interest, human chimerism was more easily detected in the thymus and was the highest in this organ than in any other lymphoid tissue in all our humanized mice. This fact was particularly important for detecting chimerism in humanized mice with extremely low level of engraftment, such as those generated with PBL-derived HSCs. The limited human hematopoietic chimerism obtained with PBL cells reinforces the notion that CD34+ cells that are normally present in the peripheral blood of adults are not totipotent HSCs, but they are likely cells that have already differentiated into a certain hematopoietic lineage. The use of humanized mice for the study of an adult HIS, therefore, requires a different source of HSCs, such as bone marrow or G-CSF mobilized peripheral blood.
Transplantation of hHSCs into immunodeficient mice had, in general, variable success, where the level of chimerism varied from 0 to >90% from mouse to mouse and, especially, between different cord blood sample donors. The protocol optimized in this study for the generation of humanized mice in BALB/c-DKO newborns resulted in less variability. This protocol consistently generated chimeric mice in >90% of recipients, with mice displaying, on average, 20%–40% hCD45+ cells in blood and lymphoid tissues, depending on the tissue. Most importantly, the frequency of B and T cells in the human leukocyte population was also less variable between mice generated with the optimized protocol, allowing studies aimed at understanding human B cell development and selection with this system. In addition, the higher level of chimerism achieved with the optimized protocol correlated with the development of lymph nodes (data not shown, manuscript in preparation). These lymph nodes, although underdeveloped, bore a sufficient number of cells for analysis. A recent study has shown that NOD-Rag1nullIL2rγnull mice are better recipients for hHSCs than BALB/c-Rag1nullIL2rγnull mice [9]. Although we did not perform a direct comparison between NOD and BALB/c strains ourselves, we observed that our optimized protocol generated humanized BALB/c-DKO mice with levels of human hematopoietic cell engraftment similar to those published for NOD-Rag1nullIL2rγnull mice generated with a standard protocol. It has been suggested that the BALB/c-DKO (BALB/c-Rag2nullIL2rγnull) strain we used in our study, and the BALB/c-Rag1nullIL2rγnull strain that was compared to NOD may have different engraftment characteristics [9]. This difference may be the reason why our BALB/c-DKO humanized mice appear qualitatively similar to the NOD humanized mice previously reported. However, our data clearly indicate that our protocol optimization improves the level of human chimerism in BALB/c immunodeficient mice, and we suggest that it may also improve chimerism in other immunodeficient strains. The availability of different mouse genetic backgrounds for the generation of humanized mice is advantageous, as the genetic background of the mouse has the potential to affect the development of the HIS. For instance, the NOD expresses the atypical I-Ag7 MHC allele that promotes the development of autoimmunity and has high similarities with the HLA-DQ8 human allele that is associated with the development of human autoimmunity [45]. Given that in humanized mice human T cells may be at least partially selected on mouse MHC [46], the human T cell repertoire generated in NOD and BALB/c humanized mice may be different and promote diverse immune functions.
5. Conclusions
Our study used a multi-parameter approach to setup an optimized protocol for the generation of humanized mice in the BALB/c-Rag2nullIL2rγnull strain. This protocol results in higher levels and lower variation of human hematopoietic engraftment in mice, allowing easier comparison of human immune systems between mice generated with different hHSC samples.
Supplementary Material
Cord blood CD34+ HSCs were cultured for 1–8 days and injected either fresh or after freezing into BALB/c-DKO mice. (A) Rate of chimeric mice that were generated by injection of either fresh or frozen HSCs, and with or without “support” cells. Human chimerism was established by the presence of >0.5% human CD45+ leukocytes in PBL and/or thymus. (B) Frequency of hCD45+ leukocytes in PBL of mice at 8 weeks after injection of either fresh or frozen HSCs. Data from all mice of experimental groups #4,5,7–18 (Table 1) is shown. (C) Percentage of hCD45+ cells in indicated tissues of 8 weeks mice that were injected with either fresh (open circles) or frozen (filled circles) HSCs isolated from a single cord blood sample (CB#38). (D) Frequency of human leukocytes in lymphoid tissues of mice injected with either fresh (open circles, groups #5, #11, Table 1) or frozen (filled circles, groups #13, #17, Table 1) HSCs. Each symbol in panels B-D represents an individual mouse. Horizontal bars represent mean frequencies. N.S. = not significant.
Groups of humanized mice were generated either with a conventional protocol consisting of fresh (non-cultured) CD34+ hHSCs only (group #4, Table 1) or with the optimized protocol consisting of a combination of cultured hHSCs and ΔT support cells (group 8A, Table 1). The graph shows the frequency of hCD45+ cells in the total (mouse + human) leukocyte population of the spleen of 16 week old mice that were injected with low (50,000–100,000), medium (150,000–200,000) or high (1,000,000) CD34+ cells.
(A) CD34+ cells were purified from adult peripheral blood of healthy and untreated individuals with the same methodology utilized for the enrichment of cord blood CD34+ cells described in Material and Methods. Blood samples were collected in the Clinical Division of National Jewish Health. Investigators in this study were blinded from donor identities, and the studies were performed in compliance with the National Jewish Health (NJH) Institutional Review Board. PBL-derived CD34+ cells were injected on the day of purification (0), or after culture for shorter (1–8 days, S) or longer (9–40 days, L) time period prior to injection without (black bars) or with (gray bars) T cell-depleted (ΔT) CD34− “support” cells. Cell cultures were performed as described for cord blood HSCs. Injections of “support” cells without CD34+ cells were included as controls (white bars). The percentage of injected mice displaying human chimerism (>0.5% hCD45+ cells in PBL and/or thymus) is shown. (B) Percentage of hCD45+ cells in the PBL at 8 weeks and in the spleen and thymus at 10–16 weeks of mice that received PBL-derived CD34+ cells with or without “support” cells. The dotted horizontal line in the right panel represents the minimum level of detection of human chimerism, defined as two standard deviations above the mean of uninjected controls. Each symbol represents an individual mouse. Horizontal bars represent mean frequencies. The only statistically significant difference is indicated as a P value of <0.05 (*).
Acknowledgments
This study was supported by the National Institute of Health with the R21-AI073629 grant, and by Arthritis Foundation with an Innovative Research Grant. The BALB/c-Rag2nullIl2rγnull (BALB/c-DKO) mice were a generous gift from Dr. Irving Weissman (Stanford University, CA). We are deeply thankful to the ClinImmune Labs for providing us the umbilical cord blood samples for our studies. We thank Drs. James Murphy and Doug Everett (NJH) for assisting with statistical analyses, and Dr. Sarah Rowland for proof reading.
Abbreviations
- hHSC
human hematopoietic stem cell
- HIS
human immune system
- SCF
stem cell factor
- FL
Flt3-Ligand
- TPO
thrombopoietin
- IL
interleukin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 2.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 3.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 4.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 5.Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, Wijnands E, Blom B, Spits H. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004;104:3886–93. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 6.Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/− (RAG-hu) mouse model. Retrovirology. 2006;3:76. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, Cuthbert A, Burzenski L, Gott B, Lyons B, Foreman O, Rossini AA, Greiner DL. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol. 2008;154:270–84. doi: 10.1111/j.1365-2249.2008.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, Burzenski L, Gott B, Foreman O, Kavirayani A, Herlihy M, Rossini AA, Shultz LD, Greiner DL. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa F, Livingston AG, Wingard JR, Nishikawa S, Ogawa M. An assay for long-term engrafting human hematopoietic cells based on newborn NOD/SCID/beta2-microglobulin(null) mice. Exp Hematol. 2002;30:488–94. doi: 10.1016/s0301-472x(02)00784-1. [DOI] [PubMed] [Google Scholar]
- 11.Danet GH, Lee HW, Luongo JL, Simon MC, Bonnet DA. Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34(+) cells after ex vivo expansion. Exp Hematol. 2001;29:1465–73. doi: 10.1016/s0301-472x(01)00750-0. [DOI] [PubMed] [Google Scholar]
- 12.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, Guetta E, Barkai G, Nagler A, Lapidot T. Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–91. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa F, Livingston AG, Minamiguchi H, Wingard JR, Ogawa M. Human cord blood long-term engrafting cells are CD34+ CD38. Leukemia. 2003;17:960–4. doi: 10.1038/sj.leu.2402878. [DOI] [PubMed] [Google Scholar]
- 14.Byk T, Kahn J, Kollet O, Petit I, Samira S, Shivtiel S, Ben-Hur H, Peled A, Piacibello W, Lapidot T. Cycling G1 CD34+/CD38+ cells potentiate the motility and engraftment of quiescent G0 CD34+/CD38−/low severe combined immunodeficiency repopulating cells. Stem Cells. 2005;23:561–74. doi: 10.1634/stemcells.2004-0060. [DOI] [PubMed] [Google Scholar]
- 15.Knaan-Shanzer S, van der Velde-van Dijke I, van de Watering MJ, de Leeuw PJ, Valerio D, van Bekkum DW, de Vries AA. Phenotypic and functional reversal within the early human hematopoietic compartment. Stem Cells. 2008;26:3210–7. doi: 10.1634/stemcells.2007-0117. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima M, Ueda T, Migita M, Oue Y, Shima Y, Shimada T, Fukunaga Y. Hematopoietic capacity of preterm cord blood hematopoietic stem/progenitor cells. Biochem Biophys Res Commun. 2009;389:290–4. doi: 10.1016/j.bbrc.2009.08.139. [DOI] [PubMed] [Google Scholar]
- 17.Kelly SS, Sola CB, de Lima M, Shpall E. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44:673–81. doi: 10.1038/bmt.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koestenbauer S, Zisch A, Dohr G, Zech NH. Protocols for hematopoietic stem cell expansion from umbilical cord blood. Cell Transplant. 2009;18:1059–68. doi: 10.3727/096368909X471288. [DOI] [PubMed] [Google Scholar]
- 19.Macchiarini F, Manz MG, Palucka AK, Shultz LD. Humanized mice: are we there yet? J Exp Med. 2005;202:1307–11. doi: 10.1084/jem.20051547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yahata T, Ando K, Sato T, Miyatake H, Nakamura Y, Muguruma Y, Kato S, Hotta T. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood. 2003;101:2905–13. doi: 10.1182/blood-2002-07-1995. [DOI] [PubMed] [Google Scholar]
- 21.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, Spits H. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205:2033–42. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson T, Greiner DL, Shultz LD. Current Protocols in Immunology. John Wiley & Sons, Inc; 2008. Creation of “humanized” mice to study human immunity; pp. 15.21–15.21.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumura T, Kametani Y, Ando K, Hirano Y, Katano I, Ito R, Shiina M, Tsukamoto H, Saito Y, Tokuda Y, Kato S, Ito M, Motoyoshi K, Habu S. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gammac(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp Hematol. 2003;31:789–97. doi: 10.1016/s0301-472x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 24.Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac−/−, Balb/c-Rag1−/−gammac−/−, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giassi LJ, Pearson T, Shultz LD, Laning J, Biber K, Kraus M, Woda BA, Schmidt MR, Woodland RT, Rossini AA, Greiner DL. Expanded CD34+ human umbilical cord blood cells generate multiple lymphohematopoietic lineages in NOD-scid IL2rgamma(null) mice. Exp Biol Med (Maywood) 2008;233:997–1012. doi: 10.3181/0802-RM-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger M, Fagioli F, Piacibello W, Sanavio F, Mareschi K, Biasin E, Bruno S, Gammaitoni L, Gunetti M, Nesi F, Madon E, Aglietta M. Role of different medium and growth factors on placental blood stem cell expansion: an in vitro and in vivo study. Bone Marrow Transplant. 2002;29:443–8. doi: 10.1038/sj.bmt.1703390. [DOI] [PubMed] [Google Scholar]
- 27.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–9. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brugger W, Mocklin W, Heimfeld S, Berenson RJ, Mertelsmann R, Kanz L. Ex vivo expansion of enriched peripheral blood CD34+ progenitor cells by stem cell factor, interleukin-1 beta (IL-1 beta), IL-6, IL-3, interferon-gamma, and erythropoietin. Blood. 1993;81:2579–84. [PubMed] [Google Scholar]
- 29.Mattia G, Milazzo L, Vulcano F, Pascuccio M, Macioce G, Hassan HJ, Giampaolo A. Long-term platelet production assessed in NOD/SCID mice injected with cord blood CD34+ cells, thrombopoietin-amplified in clinical grade serum-free culture. Exp Hematol. 2008;36:244–52. doi: 10.1016/j.exphem.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Kollet O, Aviram R, Chebath J, ben-Hur H, Nagler A, Shultz L, Revel M, Lapidot T. The soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro maintenance and proliferation of human CD34(+)CD38(−/low) cells capable of repopulating severe combined immunodeficiency mice. Blood. 1999;94:923–31. [PubMed] [Google Scholar]
- 31.Small D, Levenstein M, Kim E, Carow C, Amin S, Rockwell P, Witte L, Burrow C, Ratajczak MZ, Gewirtz AM, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A. 1994;91:459–63. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaushansky K. Thrombopoietin and hematopoietic stem cell development. Ann N Y Acad Sci. 1999;872:314–9. doi: 10.1111/j.1749-6632.1999.tb08475.x. [DOI] [PubMed] [Google Scholar]
- 33.Luens KM, Travis MA, Chen BP, Hill BL, Scollay R, Murray LJ. Thrombopoietin, kit ligand, and flk2/flt3 ligand together induce increased numbers of primitive hematopoietic progenitors from human CD34+Thy-1+Lin- cells with preserved ability to engraft SCID-hu bone. Blood. 1998;91:1206–15. [PubMed] [Google Scholar]
- 34.Joo SY, Choi BK, Kang MJ, Jung DY, Park KS, Park JB, Choi GS, Joh J, Kwon CH, Jung GO, Lee SK, Kim SJ. Development of functional human immune system with the transplantations of human fetal liver/thymus tissues and expanded hematopoietic stem cells in RAG2−/−gamma(c) −/− MICE. Transplant Proc. 2009;41:1885–90. doi: 10.1016/j.transproceed.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 35.Bordeaux-Rego P, Luzo A, Costa FF, Olalla Saad ST, Crosara-Alberto DP. Both interleukin-3 and interleukin-6 are necessary for better ex vivo expansion of CD133+ cells from umbilical cord blood. Stem Cells Dev. 2010;19:413–22. doi: 10.1089/scd.2009.0098. [DOI] [PubMed] [Google Scholar]
- 36.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–71. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One. 2008;3:e3192. doi: 10.1371/journal.pone.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Lent AU, Dontje W, Nagasawa M, Siamari R, Bakker AQ, Pouw SM, Maijoor KA, Weijer K, Cornelissen JJ, Blom B, Di Santo JP, Spits H, Legrand N. IL-7 enhances thymic human T cell development in “human immune system” Rag2−/−IL-2Rgammac−/− mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–55. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell RM, Balazs AB, Rao DS, Kivork C, Yang L, Baltimore D. Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ladd AC, Pyatt R, Gothot A, Rice S, McMahel J, Traycoff CM, Srour EF. Orderly process of sequential cytokine stimulation is required for activation and maximal proliferation of primitive human bone marrow CD34+ hematopoietic progenitor cells residing in G0. Blood. 1997;90:658–68. [PubMed] [Google Scholar]
- 41.Gothot A, van der Loo JC, Clapp DW, Srour EF. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34(+) cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 1998;92:2641–9. [PubMed] [Google Scholar]
- 42.Kassmer SH, Niggemann B, Punzel M, Mieck C, Zanker KS, Dittmar T. Cytokine combinations differentially influence the SDF-1alpha-dependent migratory activity of cultivated murine hematopoietic stem and progenitor cells. Biol Chem. 2008;389:863–72. doi: 10.1515/BC.2008.099. [DOI] [PubMed] [Google Scholar]
- 43.Yonemura Y, Ku H, Hirayama F, Souza LM, Ogawa M. Interleukin 3 or interleukin 1 abrogates the reconstituting ability of hematopoietic stem cells. Proc Natl Acad Sci U S A. 1996;93:4040–4. doi: 10.1073/pnas.93.9.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossmanith T, Schroder B, Bug G, Muller P, Klenner T, Knaus R, Hoelzer D, Ottmann OG. Interleukin 3 improves the ex vivo expansion of primitive human cord blood progenitor cells and maintains the engraftment potential of scid repopulating cells. Stem Cells. 2001;19:313–20. doi: 10.1634/stemcells.19-4-313. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida K, Corper AL, Herro R, Jabri B, Wilson IA, Teyton L. The diabetogenic mouse MHC class II molecule I-Ag7 is endowed with a switch that modulates TCR affinity. J Clin Invest. 2010;120:1578–90. doi: 10.1172/JCI41502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, Ito R, Ito M, Minegishi M, Minegishi N, Tsuchiya S, Sugamura K. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–58. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cord blood CD34+ HSCs were cultured for 1–8 days and injected either fresh or after freezing into BALB/c-DKO mice. (A) Rate of chimeric mice that were generated by injection of either fresh or frozen HSCs, and with or without “support” cells. Human chimerism was established by the presence of >0.5% human CD45+ leukocytes in PBL and/or thymus. (B) Frequency of hCD45+ leukocytes in PBL of mice at 8 weeks after injection of either fresh or frozen HSCs. Data from all mice of experimental groups #4,5,7–18 (Table 1) is shown. (C) Percentage of hCD45+ cells in indicated tissues of 8 weeks mice that were injected with either fresh (open circles) or frozen (filled circles) HSCs isolated from a single cord blood sample (CB#38). (D) Frequency of human leukocytes in lymphoid tissues of mice injected with either fresh (open circles, groups #5, #11, Table 1) or frozen (filled circles, groups #13, #17, Table 1) HSCs. Each symbol in panels B-D represents an individual mouse. Horizontal bars represent mean frequencies. N.S. = not significant.
Groups of humanized mice were generated either with a conventional protocol consisting of fresh (non-cultured) CD34+ hHSCs only (group #4, Table 1) or with the optimized protocol consisting of a combination of cultured hHSCs and ΔT support cells (group 8A, Table 1). The graph shows the frequency of hCD45+ cells in the total (mouse + human) leukocyte population of the spleen of 16 week old mice that were injected with low (50,000–100,000), medium (150,000–200,000) or high (1,000,000) CD34+ cells.
(A) CD34+ cells were purified from adult peripheral blood of healthy and untreated individuals with the same methodology utilized for the enrichment of cord blood CD34+ cells described in Material and Methods. Blood samples were collected in the Clinical Division of National Jewish Health. Investigators in this study were blinded from donor identities, and the studies were performed in compliance with the National Jewish Health (NJH) Institutional Review Board. PBL-derived CD34+ cells were injected on the day of purification (0), or after culture for shorter (1–8 days, S) or longer (9–40 days, L) time period prior to injection without (black bars) or with (gray bars) T cell-depleted (ΔT) CD34− “support” cells. Cell cultures were performed as described for cord blood HSCs. Injections of “support” cells without CD34+ cells were included as controls (white bars). The percentage of injected mice displaying human chimerism (>0.5% hCD45+ cells in PBL and/or thymus) is shown. (B) Percentage of hCD45+ cells in the PBL at 8 weeks and in the spleen and thymus at 10–16 weeks of mice that received PBL-derived CD34+ cells with or without “support” cells. The dotted horizontal line in the right panel represents the minimum level of detection of human chimerism, defined as two standard deviations above the mean of uninjected controls. Each symbol represents an individual mouse. Horizontal bars represent mean frequencies. The only statistically significant difference is indicated as a P value of <0.05 (*).