Abstract
Objective
To assess the effects of different bariatric surgical procedures on the treatment of obesity and insulin resistance in high fat diet-induced obese (DIO) mice.
Background
Bariatric surgery is currently considered the most effective treatment for morbid obesity and its comorbidities; however, a systematic study of their mechanisms is still lacking.
Methods
We developed bariatric surgery models, including gastric banding, sleeve gastrectomy, Roux-en-Y gastric bypass (RYGB), modified RYGB (mRYGB) and biliopancreatic diversion (BPD), in DIO mice. Body weight, body fat and lean mass, liver steatosis, glucose tolerance and pancreatic beta cell function were examined.
Results
All bariatric surgeries resulted in significant weight loss, reduced body fat and improved glucose tolerance in the short term (4 weeks), compared to mice with sham surgery. Of the bariatric surgery models, sleeve gastrectomy and mRYGB had higher success rates and lower mortalities and represent reliable restrictive and gastrointestinal (GI) bypass mouse bariatric surgery models, respectively. In the long term, the GI bypass procedure produced more profound weight loss, significant improvement of glucose tolerance and liver steatosis than the restrictive procedure. DIO mice had increased insulin promoter activity, suggesting over-activation of pancreatic beta cells, which was regulated by the mRYGB procedure. Compared to the restrictive procedure, the GI bypass procedure showed more severe symptoms of malnutrition following bariatric surgery.
Discussions
Both restrictive and GI bypass procedures provide positive effects on weight loss, fat composition, liver steatosis and glucose tolerance; however, in the long term, the GI bypass shows better results than restrictive procedures.
Introduction
Obesity is associated with an increased risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular disease. It has been described as the greatest current threat to human health and represents a major public health crisis (1-3). Bariatric surgery is currently considered the most effective treatment for obesity and its comorbidities (2, 4). Beneficial effects of bariatric surgery include weight loss, reduced insulin resistance, and decreased risk factors for cardiovascular disease (2). Based on the nutrient pass patterns, bariatric surgery is divided into two classes: restrictive procedures (e.g. gastric banding and sleeve gastrectomy) and gastrointestinal (GI) bypass procedures (e.g. Roux-en-Y gastric bypass, RYGB, and biliopancreatic diversion, BPD). Gastric banding is a purely restrictive procedure, and other surgeries, including RYGB and BPD, however, produce restrictive diet intake as well as malabsorption. Both restrictive and GI bypass procedures produce significantly greater weight loss and more profound improvements in glucose tolerance than medical treatments(5, 6). However, the degree of improvement varies with the type of procedure used and the precise techniques utilized.
The mechanisms of the metabolic and cardiovascular improvements associated with bariatric surgery are not clearly defined. While weight loss is associated with commensurate decrements in insulin resistance, recent clinical results suggest that the GI bypass, in contrast to the restrictive procedure, normalizes insulin sensitivity even before achievement of ideal body weight (7, 8). A weight-independent response has been thought to initiate this amelioration in insulin resistance. Research has been carried out on a variety of large animal models of bariatric surgery, including the pig and dog (9-11). These experiments support the notion that weight loss is related to reduced stomach volume and changes in the regulation of intestinal hormones. However, the mechanisms of bariatric surgery on glucose metabolism, and on hormone secretion and action remain to be elucidated. A bariatric surgery model has been developed in rats and recent findings from these studies show changes in meal patterns, satiety, food choice, glucose metabolism and energy expenditure (12-14). These studies concluded that the improvement in glucose tolerance is due to increased insulin sensitivity. However, systematic studies that compare different effects of restrictive and GI bypass surgeries on weight loss and glucose metabolism remain to be performed. We have developed a repertoire of mouse bariatric surgical models to provide tools to study the physiology of bariatric surgery that can be applied to the vast number of genetic mouse models of metabolic disease. The application of bariatric surgical techniques to genetic mouse models provides a unique opportunity to test the mechanisms associated with the effects of bariatric surgery on glucose metabolism and hormone action, and elucidates the neural and immunological effects of bariatric surgery.
In the current study, we developed bariatric surgery models in high fat diet-induced obese (DIO) mice. The models include (i) gastric banding, (ii) sleeve gastrectomy, (iii) RYGB, (iv) modified RYGB (mRYGB) and (v) BPD. We evaluated the feasibility and reliability of different bariatric surgery models. We investigated the short-term and long-term impacts of bariatric surgery on weight loss, glucose tolerance, pancreatic beta-cell viability and liver steatosis.
Methods
Mice
C57BL/6 (H-2b) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice expressing luciferase under the control of a NF-κB promoter (NF-κB-luc) on a C57BL/6 background or a mouse insulin promoter (MIP-luc) on a FVB background were kindly provided by Dr. Timothy Blackwell and Dr. Alvin Powers, Department of Medicine at Vanderbilt University Medical Center (15, 16). Mice were housed at 23°C on a 07:00-19:00 light cycle. At 6 weeks of age the mice were placed on a 60% Kcal fat diet (Research Diets, Inc.) for 12 weeks to establish diet-induced obesity (DIO). The mice were maintained on the same high fat diet after bariatric surgery. All experiments and surgical preparations were performed according to the protocol approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee (IACUC). The mice remained under the care of the Division of Animal Care (DAC) at Vanderbilt University in compliance with NIH guidelines and the Principles of Laboratory Animal Care, and the Guide for the Care and Use of Laboratory Animals.
Bariatric Surgical preparations
Animals were fasted for 4 to 6 hours prior to the surgical preparations. Anesthesia was induced and maintained throughout the procedure with isoflurane (2-3% with O2). Following aseptic preparation a midline laparotomy was performed to gain exposure to the GI tract. At the conclusion of the bariatric procedure, the midline incision was closed and the mice were recovered on a water-circulated heating pad. Body weight and body composition were evaluated after surgeries. In the selected mice, glucose tolerance, liver steatosis and complete blood count (CBC) were also examined compared to naïve lean (C57BL/6) mice and DIO mice without surgeries.
Gastric Banding
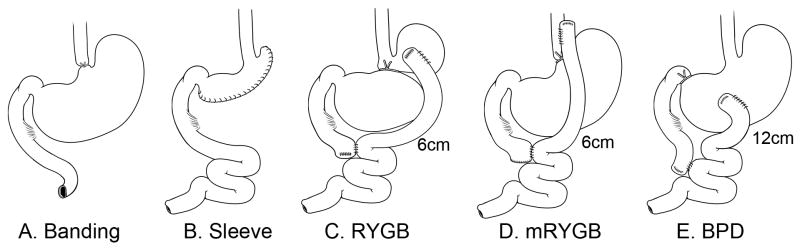
The gastroesophageal junction was isolated and an elastic silicon rubber string (0.23mm) was placed around the gastroesophageal junction (Figure 1A). The ends of the string were tied together to form an elastic circular band with the tension of the band adjusted such that the expansion capacity of the junction is restricted as food passes into the stomach. This procedure is purely a gastric restrictive procedure. Sham procedures involved the mobilization of the esophagus and stomach, and the silicone string was placed around the gastroesophageal junction, tied and then removed.
Figure 1. Mouse bariatric surgery models.
Bariatric surgeries include: A. gastric banding (Banding); sleeve gastrectomy (Sleeve); C. Roux-en Y gastric bypass (RYGB); D. modified RYGB (mRYGB); and E. biliopancreatic diversion (BPD). Surgical procedures were described in the Methods.
Sleeve Gastrectomy
The procedure removed the greater curvature and the entire fundus (70–80% of total stomach, which includes 90% of the forestomach and 70% of the glandular stomach). A gastric tube was fashioned along the lesser curvature with the incision line starting at the body, 1 cm distal to the gastroesophageal junction and extending to around 1 cm proximal to the pylorus along the lower great curvature. The stomach tube (1 cm in diameter) was closed using 9-0 Ethilon sutures (Figure 1B). In this fashion, gastric continuity is maintained and the greater curvature region of the stomach was eliminated. In the sham procedure, the stomach, duodenum and jejunum were mobilized and the stomach was clamped without incision.
RYGB
The upper gastrointestinal anatomy of the mouse does not permit the replication of the procedure that is utilized in humans. We developed a procedure that closely resembles RYGB by ligating the stomach between the glandular portion and the gastric fundus (forestomach). A portion of the jejunum, 4 cm from the Ligament of Treitz and 6 cm from the site of gastroenterostomy, was transected. The distal segment was anastomosed to the forestomach using 9-0 Ethilon in a side-to-side fashion. GI continuity is established by performing a side-to-side jejuno-jejunostomy (Figure 1C). The sham procedure involved mobilization of the forestomach and proximal and distal jejunum and ileum without any intersection.
Modified RYGB (mRYGB)
mRYGB was developed because the forestomach of the mouse lacks sufficient muscle to push nutrients through the anastomosis in an RYGB procedure, resulting in a high mortality within a few days of surgery. mRYGB was performed in a similar fashion to the RYGB, but the upper side-to-side anastomosis of the jejunum was performed with the lower portion of the esophagus. The stomach ligation was accomplished by placing a suture to close the gastroesophageal junction distal to the anastomosis. The distances of jejuno-jejunostomy to the Ligament of Treitz and the site of gastroenterostomy are 4 cm and 6 cm, respectively, as described in Figure 1D. The sham procedure included isolation of the esophagus, stomach and proximal and distal jejunum without any intersection and anastomosis.
Biliopancreatic diversion (BPD)
A portion of the jejunum, 4 cm from the Ligament of Treitz and 12 cm from the site of gastroenterostomy, was transected. The distal segment was anastomosed to the greater curvature of the stomach using 10-0 Ethilon in a side-to-side fashion. Continuity of the GI tract was established by performing a side-to-side anastomosis. This procedure results in an isolation of the duodenum and uppermost segment of the jejunum from the GI tract. Unlike the human procedure, the proximal duodenum is ligated at the pyloric-duodenal junction using 6-0 silk, but not transected as shown in Figure 1E. The sham procedure involved isolation and mobilization of the stomach, proximal and distal jejunum and ileum without any intersection and anastomosis.
Gastrointestinal imaging following bariatric surgery
We evaluated GI continuity by imaging with contrast 14 days after the bariatric procedures. Mice were fasted 6hr prior to imaging (MicroCat-II, Siemens) and anesthetized with isoflurane. Contrast (Optiray 320) was administered by gavage with a volume of 0.8 ml and continuous imaging was performed.
Whole body composition
Body mass was measured using mq10 NMR analyzer (Bruker Optics Inc, Billerica, MA) following 2 hr of fasting. The NMR analyzer allows for the measurement of whole-body composition parameters, including total body fat, muscle and body fluids, in conscious rodents (17). Fat, muscle and body fluids were calculated as grams of total mass.
Intraperitoneal glucose tolerance tests (IPGTT)
Mice were fasted for 4 hr prior to the IPGTT. Blood was sampled from the tail vein before and at 30, 60, 90 and 120 min after an intraperitoneal injection of 2 mg/g body weight of dextrose (20%). Blood glucose levels (mg/dL) were measured using a blood glucose meter (SureStep, Lifescan, Inc.). The area under the cure (AUC) was calculated using the trapezoidal rule (18).
Bioluminescence imaging (BLI)
Bioluminescence imaging was performed under anesthesia with isoflurane. Luciferin (Roche Diagnostics, Indianapolis, IN) was injected intravenously at a dose of 50mg/kg. Mice were housed inside a light-tight box and imaged with an ICCD camera (Hamamatsu C2400-32, Hopkinton, MA). Light emission through the ventral body was detected as photon counts over a standardized area of the organs of interest using the ARGUS-50 software for image processing (19-21). Islet viability was evaluated by BLI analysis using FVB mice expressing luciferase under the control of a mouse insulin promoter (MIP-luc). MIP-luc mice were fed a high fat diet for more than 12 weeks and subjected to BLI examinations. NF-κB activation in the abdomen was measured by BLI using NF-κB-luc mice.
Pathological examination
Selected mice were sacrificed and the livers were collected for pathological examinations (H&E) following bariatric surgery. Liver histopathological features were evaluated by Brunt's grading. Significant lesions included steatosis, ballooning, and intra-acinar and portal inflammation; and the lesions were graded as mild (1+, up to 33%), moderate (2+, 33-66%) and severe (3+, >66%) (22).
Statistics
Statistical significance was analyzed using ANOVA test (Statview 4.5, Abacus Concepts, Berkeley, CA). P-value of < 0.05 was considered significant. For in vitro data analysis, results are presented as mean ± SEM, and comparisons between the values were performed using the 2-tailed Student's t test.
Results
Development of mouse bariatric surgery models
We have developed bariatric surgery models in mice that correspond to the surgical approaches used in humans. Each surgical procedure uniquely reconfigures the GI tract such that the physiological roles of specific segments (e.g. stomach, duodenum and jejunum) can be isolated and characterized (Figure 1). The banding procedure is usually considered a relatively simple restrictive procedure; however, it is difficult to define the banding tension (diameter) of the lower esophagus in mice. Of banding-treated mice (n = 12), no mouse died of surgical procedure; however, 4 mice (33.3%) died of banding restriction within one month post-surgery when the ligation was too tight. Another 4 mice (33.3%) with the banding procedures failed to produce weight loss due to ineffective banding (Table 1). Surgical success rate of sleeve gastrectomy was 100% (n = 14). In the RYGB procedure, the forestomach of the mouse lacks the elasticity and motility to develop pressures necessary to force the meal past the anastomosis, resulting in high mortality from gastric obstruction. Surgical success rate of RYGB was 87.5% (7/8); however, 2 mice died and 5 mice required euthanization due to the obstruction and severe malnutrition two weeks post-surgery. We modified the procedure by the anastomosis of the jejunum to the lower portion of the esophagus and animals with the mRYGB could eat and drink freely. Surgical success rate was 75% in 12 mRYGB-treated mice and 3 of 9 mice died of severe malnutrition (33.3%). Although success rate of BPD was 90% (9/10), most of the mice (8/9 or 88.9%) died of severe malnutrition (Table 1). Thus sleeve gastrectomy and mRYGB represent reliable mouse gastric restrictive and GI bypass models, respectively.
Table 1. Mouse bariatric surgery model.
| Model | Number | Success %* | Mortality%** |
|---|---|---|---|
| Banding | 12 | 100 (12/12) | 33.3 (4/12, restriction) |
| Sleeve | 14 | 100 (14/14) | 0 |
| RYGB | 8 | 87.5 (7/8) | 100 (7/7, obstruction) |
| mRYGB | 12 | 75 (9/12) | 33.3 (3/9, malnutrition) |
| BPD | 10 | 90 (9/10) | 88.9 (8/9, malnutrition) |
| Sham*** | 23 | 100 (23/23) | 0 |
Success, surgical success rate.
Mortality, mice with complications, such as the restriction of banding, obstruction in the site of anastomosis (RYGB) and severe malnutrition (RYGB, mRYGB and BPD), died or were euthanized two months, post-surgery.
Sham surgeries include all sham surgeries related to individual bariatric surgery.
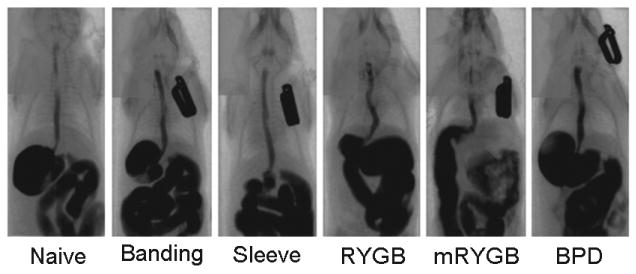
Normal contrast-enhanced CT examinations in bariatric surgical procedures confirmed the specific GI reconfiguration along with GI continuity resulting from the specific bariatric surgical procedure (Figure 2). Slight reduction of stomach volume was observed in the gastric banding procedure (Banding). The stomach volume was significantly reduced in sleeve gastrectomy (Sleeve) and RYGB procedures. The contrast passed directly into the jejunum in mRYGB procedure. No bowel obstruction or anastomotic leaks were observed.
Figure 2. Imaging of bariatric surgery.
After 7 to 10 days of bariatric surgery, imaging was performed using MicroCat-II. The contrast (Optiray 320, 0.8ml) was administered into the mouse by gavage. Continuous photos were taken every 4 seconds, for a total of 16 photos per mouse. Each figure represents one of 16 photos.
Effects of bariatric surgery on total body weight loss and changes in fat mass
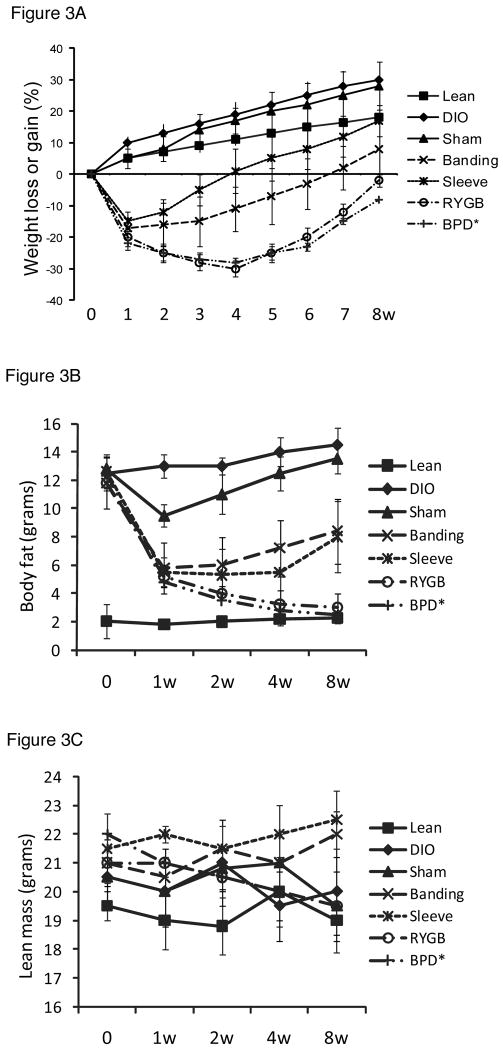
The bariatric surgical procedures resulted in weight loss in all mice. The sham procedures did not result in significant weight loss. There were no significant differences in body weight or glucose tolerance tests among the different sham procedures; thus, data collected from all sham surgeries was pooled and are referred to as “sham.” Both restrictive and GI bypass procedures produced weight loss (p < 0.05 in all groups, compared to untreated and sham-treated DIO mice at all time points, post-surgery). The most significant weight loss was observed in mice with GI bypass procedures (mRYGB and BPD) as compared to restrictive procedures (banding and sleeve, Figure 3A).
Figure 3. Body weight and composition.
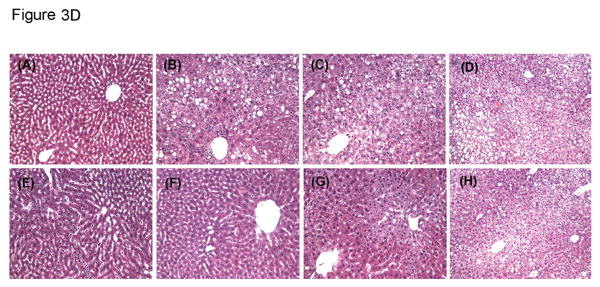
A. Weight loss and gain. DIO mice were weighed after bariatric and sham surgeries (n = 5 in each group except BPD* that only one mouse was alive at 8 weeks). All mice lost weight in a few days after surgeries; however, mice with sham surgeries gained weight after one week of surgery, and mice with bariatric surgeries maintained their weight loss for more than one month. B. Body composition was measured by a NMR analyzer, as described in the Methods. Fat composition was expressed as grams per body weight (n = 4 in each group except BPD* that only one mouse was alive at 8 weeks). C. Lean mass was also measured by a NMR analyzer (N = 4 in each group except BPD* that only one mouse was alive at 8 weeks). D. Bariatric surgery improves hepatic steatosis (H&E, × 100, the figure represents one of three pathological examinations in each group). (A), naïve lean mouse; (B), naïve DIO mouse; (C), DIO mouse with sham surgery at 4 weeks; (D), DIO mouse with sham surgery at 8 weeks; (E), DIO mouse with mRYGB at 4 weeks; (F), DIO mouse with mRYGB at 8 weeks; (G), DIO mouse with sleeve gastrectomy at 4 weeks; and (H), DIO mouse with sleeve gastrectomy at 8 weeks.
Our results show that fat mass in DIO mice was significantly higher than that in lean mice and there was no significant difference of lean mass (Figures 3B and C). Within one week, bariatric procedures resulted in significant reductions in fat mass with the most prominent results achieved with mRYGB and BPD, followed by sleeve gastrectomy and banding. There were no significant differences in lean mass among the groups (Figure 3C). Although the sham surgery also impacted body mass, it was comparable to untreated DIO mice after two weeks of surgery.
Liver steatosis is a morphological pattern of DIO liver and may progress into steatohepatitis (23). To test whether bariatric surgery can improve liver steatosis, we harvested livers at 4 and 8 weeks, post-surgery, for pathological examination (H&E). According to the standards of liver steatosis grading (22), the severity of all DIO livers were graded 2 to 3 (i.e. moderate to severe lesions, ≥ 66 percent of hepatocytes are affected). Liver steatosis was significantly improved by sleeve gastrectomy and mRYGB procedures at 4 weeks (0 - 1), compared to untreated and sham-treated DIO liver (Figure 3D). However, mRYGB, but not sleeve gastrectomy, persistently improved liver steatosis at 8 weeks, post-bariatric surgery.
Bariatric surgery improves glucose tolerance and regulates pancreatic islet viability
Untreated DIO or sham surgery DIO mice showed glucose intolerance, compared to chow-fed mice (naïve B6) mice, as evidenced by abnormal IPGTT. Sleeve gastrectomy improved glucose tolerance in the early period (less than 4 weeks); however, there was no significant difference between untreated DIO mice and sleeve-treated DIO mice at 8 weeks, post-surgery. mRYGB and BPD procedures persistently improved glucose tolerance (Tables 2 - 5).
Table 2. IPGTT in mice with bariatric surgeries at 1 week post-surgery (mg/dL).
| Model* | 0min | 30min | 60min | 90min | 120min |
|---|---|---|---|---|---|
| Lean (n=4) | 148±13 | 314±49 | 181±9 | 156±4 | 145±1 |
| DIO (n=6) | 182±14 | 464±36 | 401±38 | 354±22 | 301±13 |
| Sham (n=3) | 141±30 | 376±67 | 278±21 | 211±39 | 174±42 |
| Banding (n=3) | 126±12 | 298±102 | 210±82 | 190±127 | 183±76 |
| Sleeve (n=3) | 126±30 | 412±62 | 203±57 | 160±35 | 139±30 |
| mRYGB (n=3) | 105±18 | 373±88 | 203±66 | 163±55 | 119±25 |
| BPD (n=3) | 117±2 | 368±113 | 205±78 | 142±31 | 110±16 |
Model: Lean, chow-fed lean C57BL/6 mice; DIO, high fat diet-induced obesity; Sham, DIO mice with sham surgery; Banding, DIO mice with gastric banding; Sleeve, DIO mice with sleeve gastrectomy; mRYGB, DIO mice with mRYGB; and BPD, DIO mice with BPD.
Table 5. IPGTT in mice with bariatric surgeries at 8 weeks post-surgery (mg/dL).
| Model* | 0min | 30min | 60min | 90min | 120min |
|---|---|---|---|---|---|
| Lean (n=4) | 152±6 | 301±33 | 195±11 | 170±9 | 151±3 |
| DIO (n=4) | 176±10 | 479±24 | 366±17 | 274±22 | 238±29 |
| Sham (n=3) | 155±17 | 442±33 | 338±58 | 327±82 | 274±88 |
| Banding (n=3) | 157±5 | 540±20 | 486±8 | 422±26 | 352±38 |
| Sleeve (n=3) | 201±54 | 381±60 | 444±50 | 405±53 | 332±62 |
| mRYGB (n=3) | 113±7 | 312±24 | 184±19 | 135±2 | 125±2 |
| BPD** | 151 | 407 | 177 | 168 | 152 |
Model: Lean, chow-fed lean C57BL/6 mice; DIO, high fat diet-induced obesity; Sham, DIO mice with sham surgery; Banding, DIO mice with gastric banding; Sleeve, DIO mice with sleeve gastrectomy; mRYGB, DIO mice with mRYGB; and BPD, DIO mice with BPD (n = 3 - 4 mice in each group).
Only one mouse was alive at 8th week post-BPD surgery.
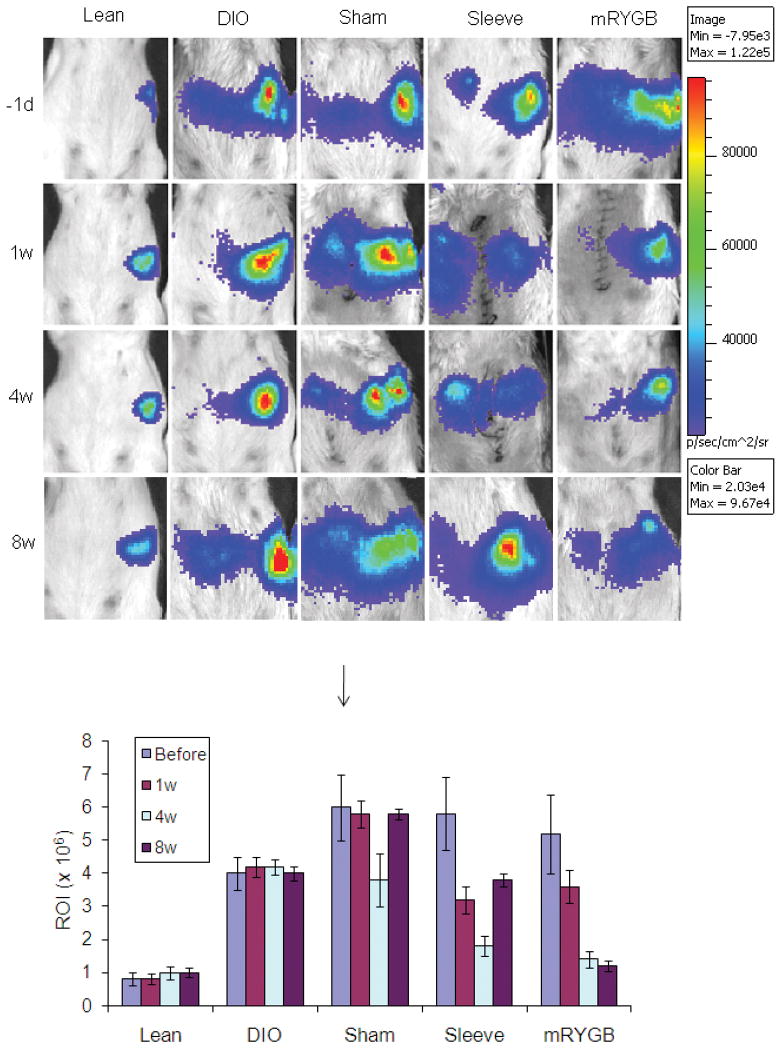
The effect of high fat diet and bariatric surgery on the viability of pancreatic islets was examined. Enhanced luciferase activity was observed in DIO mice, compared to regular chow-fed mice of the same age (lean, Figure 4), suggesting that DIO induces over-activation of pancreatic beta-cells. The luciferase activity was reduced in sleeve gastrectomy and mRYGB groups (versus untreated DIO mice, p < 0.05 and p < 0.01, respectively) at 4 weeks, post-surgery. However, mRYGB procedure, but not sleeve gastrectomy, persistently improved beta cell function 8 weeks after surgery (Figure 4).
Figure 4. Bioluminescence (BLI) for islet viability.
DIO was induced in mice expressing luciferase under the control of an insulin promoter (MIP-luc), and BLI was performed after bariatric surgeries, compared to untreated DIO mice (DIO) and lean mice (Lean). Luciferase was expressed as the regions of interest (ROI). The figures represent one of four BLI examinations. Data in the bar chart represent means ± SEM.
DIO induces elevated NF-κB activation
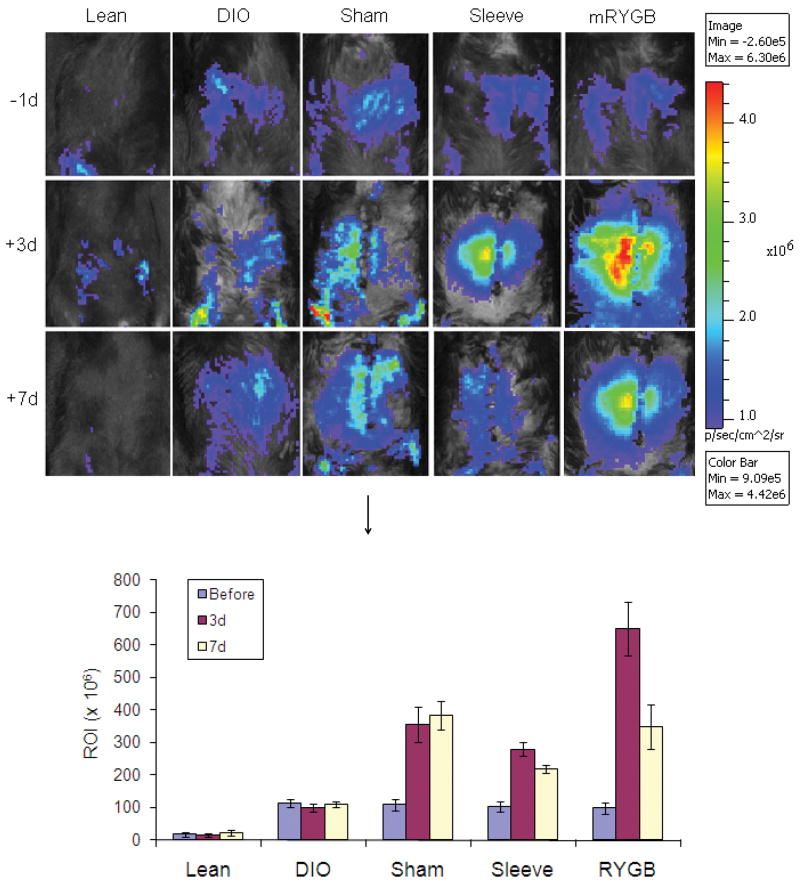
BLI was performed using NF-κB-driven luciferase transgenic mice. NF-κB activation was increased in the abdomen of DIO mice (ROI, 10.5 × 106), compared to regular chow-fed mice (ROI, 1.14 ×106, p < 0,001). All bariatric surgical procedures resulted in significant activation of NF-κB, with the mRYGB causing the highest activation; the activation was ameliorated one week after all bariatric procedures with the highest effect noted following mRYGB (Figure 5).
Figure 5. Bioluminescence (BLI) for NF-κB activation.
DIO was induced in mice expressing luciferase under the control of an NF-κB promoter (NF-κB-luc), and BLI was performed after bariatric surgeries, compared to untreated DIO mice (DIO) and lean mice (Lean). Luciferase was expressed as the regions of interest (ROI). The figures represent one of three BLI examinations. Data in the bar chart represent means ± SEM.
Complications of bariatric surgeries
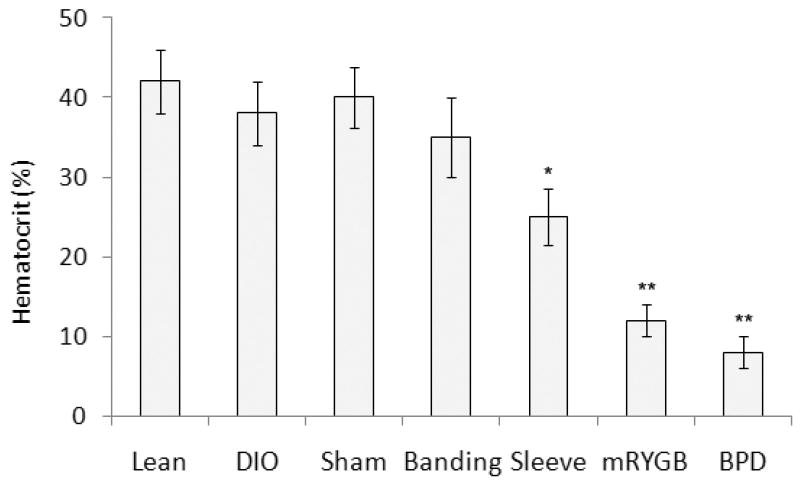
The bariatric procedures were not associated with short-term complications (e.g. bleeding, leaking, infection and intestinal obstruction) (Table 1). The CT imaging examinations showed the patterns of bariatric surgery without leaking and obstruction of the digestive system (Figure 2). The bariatric surgical procedures were associated with significant long-term complications, primarily malnutrition. Consistent with human data (24-26), GI bypass procedures, and most prominently BPD and mRYGB, resulted in more severe anemia than noted with the restrictive procedures (gastric banding and sleeve gastrectomy). Compared to naïve lean mice (50.5% ± 3.2), hematocrit levels at one month were 22.0% ± 5.5 following RYGB (p < 0.001), 19.5% ± 3.5 following BPD (p < 0.01), 38.4% ± 3.5 following sleeve gastrectomy (p = 0.414), and 33.8% ± 6.8 following gastric banding mice (p > 0.05, Figure 6). Mice with GI bypass procedures (mRYGB and BPD) experienced reduced percentages of red blood cells (RBCs, 6.4 and 3.7, n = 2), hemoglobin (HB, 7.9 and 4.5, n = 2) and mean cell volume (MCV, 35.5 and 29.7, n = 2). Additional symptoms of malnutrition included hair loss and significant weight loss 2 to 8 weeks post-surgery. 40% (mRYGB) and 80% (BPD) of the mice presented symptoms of severe malnutrition (Table 1).
Figure 6. Hematocrit (HCT) levels in bariatric surgery-treated mice.
HCT was performed in DIO mice with different bariatric surgeries one month after bariatric surgery, compared to sham surgery (Sham), untreated-DIO (DIO) and untreated-lean mice (Lean, n = 3 - 4 in each group). Data in Figure 6 represent means ± SEM. *: p < 0.05; ** p < 0.01.
Discussion
Bariatric surgery is the most effective treatment for morbid obesity(2, 4). Gastric banding, RYGB and sleeve gastrectomy are widely used in the U.S. for the treatment of obesity and its comorbidities, with some centers performing BPD. A rat bariatric surgery model has been developed and used to support the notion that improvements in weight loss and diabetes are related to a variety of factors, including reduced stomach volume, changes in the regulation of intestinal hormones and increases in insulin sensitivity (9, 14, 27-31). We have developed five different bariatric surgery models in DIO mice. Our results are similar in many respects to those obtained in human subjects following the commonly used bariatric procedures, namely gastric banding, sleeve gastrectomy, RYGB, mRYGB and BPD. However, in our mouse bariatric surgeries, sleeve gastrectomy and mRYGB represent reliable restrictive and GI bypass models, respectively, due to higher surgical success rates.
It is clear from our studies that both restrictive and GI bypass procedures produce positive effects on weight loss, fat composition and glucose tolerance in the short term (4 weeks); however, in the long term (8 weeks), restrictive procedures were least effective in decreasing body weight and body fat, while GI bypass procedures were quite effective in treating obesity and insulin resistance. It is important to note that our model of mRYGB is a modified one which requires the anastomosis of jejunum directly to the esophagus and differs from the human RYGB model where the jejunum is anastomosed to a small gastric pouch. Although RYGB and BPD resulted in significant losses of body weight and fat mass, mRYGB procedure produces the similar positive effects in the treatment of obesity and insulin resistance without the high mortality. It is also important to note that while the mouse BPD model was the most effective in treating obesity, it was associated with a much higher incidence of malnutrition and mortality. Human data suggest that BPD is extremely effective in ameliorating diabetes as 90% of patients have normoglycemia and increased insulin sensitivity up to 20 years, post-surgery (32).
Several studies have shown that bariatric procedures, and most significantly RYGB and BPD, ameliorate insulin resistance associated with obesity and reverse type 2 diabetes mellitus(33) through poorly understood mechanisms. Several of the studies have shown that the improvements associated with bariatric surgery are not solely related to weight loss. Our results offer some insight into potential mechanisms for which the models developed here can be used to gain further understanding. Abdominal adiposity significantly affects both lipid (FFAs) and glucose metabolism and, thus, is closely associated with insulin resistance (34, 35). Recent findings suggest that high intrahepatic fat, but not visceral fat, is the primary marker of metabolic complications of obesity (36, 37). Additionally, fatty liver or nonalcoholic fatty liver disease (NAFLD) is increasingly recognized as a condition associated with obesity that may progress to end-stage liver disease. Whether or not bariatric surgery has beneficial effects on patients with NAFLD is impossible to assess at this time due to the lack of clinical studies. The current study suggests that severe liver steatosis is observed in DIO mice and that bariatric procedures, especial mRYGB, significantly improve liver steatosis. The improvement of fatty liver in DIO mice is associated with improved glucose tolerance as evidenced by a diminished glycemic excursion in response to an IPGTT in bariatric surgery-treated DIO mice. Although both restrictive and GI bypass procedures exerted positive effects on glucose tolerance, in the long term, improved glucose tolerance and liver steatosis were observed in mRYGB-treated DIO mice, suggesting that the GI bypass procedure seems to be more effective than the restrictive procedure in treating insulin resistance.
Obese subjects maintain normoglycemia for a sustained interval before the onset of frank T2DM. This is presumably due to hyper-secretion of insulin that is sufficient to overcome insulin resistance. The availability of genetically modified mice whose islets express luciferase protein under the control of mouse insulin promoter (MIP-luc) provided us with the opportunity to visualize the viability of pancreatic islets. Utilizing BLI technology, we observed over-activation of pancreatic islets in DIO MIP-luc mice, compared to lean MIP-luc mice. Both restrictive and GI bypass significantly improved the over-activation of islet cells in DIO mice in 4 weeks, suggesting that the compensatory oversecretion of insulin associated with insulin resistance is rapidly reversed. However, beta cell over-activation was observed in sleeve-treated DIO mice at 8 weeks, whereas luciferase activity in mRYGB-treated DIO mice was still comparable to chow-fed lean mice. The results suggest that restrictive procedure ameliorates beta cell over-activation in the short term, and GI bypass improves beta cell function persistently, which is consistent with the improvement of glucose tolerance. Thus, GI bypass provides an approach for the reversal of insulin resistance and the regulation of beta cell activation.
It is increasingly recognized that obesity is characterized by the activation of an inflammatory process in metabolically active sites such as the liver and adipose (38-40). Adipocyte hypertrophy induces the release of monocyte chemokines. The infiltrated macrophages, in turn, release inflammatory proteins causing further recruitment of macrophages to adipose tissue. NF-κB is a potent proinflammatory signal transduction molecule in innate and adaptive immune reactions (41-43). NF-κB is activated in obesity and stimulation of IκB produces beneficial effects in the treatment of obesity and its comorbidities (44, 45). While NF-κB activation has been implicated in T2DM, the visualization of NF-κB activation in DIO mice is lacking. Our results show that NF-κB activation is upregulated in the abdomen of DIO mice, compared to lean mice. Surgical procedures further stimulate elevated NF-κB activation, which is decreased one week after bariatric surgery. This observation could be important in formulating strategies for the evaluation of NF-κB activation in obesity-related T2DM and bariatric surgery.
Malabsorption causes nutritional deficiencies following bariatric surgery. The deficiencies includes macronutrients, such as protein deficiency, and micronutrients, such as vitamins and trace elements (46). Both mRYGB and BPD produce more severe malnutrition than gastric banding and sleeve procedures, as evidenced by anemia, hair loss and severe weight loss one month after surgery. The deficiency of iron, vitamin B12 and other micronutrients, such as copper, vitamins A and E and zinc, may contribute to bariatric surgery-induced malnutrition and anemia (47).
We have chosen the mouse for these studies based on the following reasons: (i) to date the only animal species in which obesity and the obesity related pathologies can be induced and consistently reproduced are rodents, particularly mice; (ii) gene transfected and knockout mice are currently available that provide us with unique opportunities to test the diverse mechanisms of bariatric surgery on metabolism, neural regulation, endocrine function and immunological effects. However, we know that obesity and diabetes result from a combination of genetic mutants and environmental factors. Neither beta-cell depletion nor frank diabetes was observed in DIO mice; therefore, genetic mutants should be included, which is under investigation.
In conclusion, we have developed mouse bariatric surgery models that can be utilized to address the effects of bariatric surgery on the treatment of obesity and T2DM. Sleeve gastrectomy and mRYGB represent reliable restrictive and GI bypass procedures, respectively, in mice. Both procedures produce positive effects on obesity and insulin resistance in the short term; but mRYGB improves metabolism in the long term. These procedures can provide significant information about the mechanisms associated with the effects of surgical intervention on obesity and its comorbidities.
Table 3. IPGTT in mice with bariatric surgeries at 2 weeks post-surgery (mg/dL).
| Model* | 0min | 30min | 60min | 90min | 120min |
|---|---|---|---|---|---|
| Lean (n=4) | 120±11 | 308±36 | 176±11 | 155±11 | 147±10 |
| DIO (n=5) | 157±13 | 490±36 | 419±40 | 341±11 | 319±24 |
| Sham (n=3) | 142±27 | 402±81 | 368±73 | 271±68 | 225±66 |
| Banding (n=3) | 72±6 | 381±68 | 384±108 | 396±102 | 358±90 |
| Sleeve (n=3) | 130±25 | 257±76 | 182±9 | 153±27 | 132±32 |
| mRYGB (n=3) | 106±25 | 297±76 | 214±32 | 134±3 | 110±11 |
| BPD (n=3) | 91±7 | 178±32 | 139±18 | 111±18 | 98±14 |
Model: Lean, chow-fed lean C57BL/6 mice; DIO, high fat diet-induced obesity; Sham, DIO mice with sham surgery; Banding, DIO mice with gastric banding; Sleeve, DIO mice with sleeve gastrectomy; mRYGB, DIO mice with mRYGB; and BPD, DIO mice with BPD (n = 3 - 4 mice in each group).
Table 4. IPGTT in mice with bariatric surgeries at 4 weeks post-surgery (mg/dL).
| Model* | 0min | 30min | 60min | 90min | 120min |
|---|---|---|---|---|---|
| Lean (n=3) | 155±7 | 286±43 | 187±12 | 162±17 | 149±4 |
| DIO (n=5) | 158±13 | 498±40 | 431±39 | 376±22 | 319±9 |
| Sham (n=3) | 175±12 | 426±39 | 317±68 | 268±64 | 196±38 |
| Banding (n=3) | 142±19 | 431±105 | 391±124 | 353±118 | 172±88 |
| Sleeve (n=3) | 174±9 | 445±60 | 267±49 | 197±31 | 167±25 |
| mRYGB (n=3) | 103±16 | 314±27 | 141±26 | 132±27 | 108±17 |
| BPD (n=3) | 128±11 | 438±39 | 219±63 | 138±16 | 121±16 |
Model: Lean, chow-fed lean C57BL/6 mice; DIO, high fat diet-induced obesity; Sham, DIO mice with sham surgery; Banding, DIO mice with gastric banding; Sleeve, DIO mice with sleeve gastrectomy; mRYGB, DIO mice with mRYGB; and BPD, DIO mice with BPD (N = 3 - 4 mice in each group).
Acknowledgments
We thank Dr. Kelli Boyd, associate professor of Pathology, Vanderbilt University Medical Center, for assistance on the analysis of animal malnutrition, Dr. Todd Peterson, assistant professor, and Dr. Mohammed Tantawy, research fellow, Department of Imaging Science at Vanderbilt University Medical Center, for assistance on imaging analyses, and Mimi Eckhard, director, Division of Media Services, Section of Surgical Science at Vanderbilt University Medical Center, for helpful proofreading of the manuscript.
Footnotes
This study was supported by NIH grant R01 DK050277 and DK059637 to D.W., JDRF grant 1-2008-159 to D.Y. and NIH grant R01 DK070860 to N.N.A.
References
- 1.Frachetti KJ, Goldfine AB. Bariatric surgery for diabetes management. Curr Opin Endocrinol Diabetes Obes. 2009;16(2):119–24. doi: 10.1097/MED.0b013e32832912e7. [DOI] [PubMed] [Google Scholar]
- 2.Goldfine AB, Shoelson SE, Aguirre V. Expansion and contraction: treating diabetes with bariatric surgery. Nat Med. 2009;15(6):616–7. doi: 10.1038/nm0609-616. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Saber AA, Elgamal MH, McLeod MK. Bariatric surgery: the past, present, and future. Obes Surg. 2008;18(1):121–8. doi: 10.1007/s11695-007-9308-7. [DOI] [PubMed] [Google Scholar]
- 5.Cunneen SA. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2008;4(3 Suppl):S47–55. doi: 10.1016/j.soard.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Phillips E, Ponce J, Cunneen SA, Bhoyrul S, Gomez E, Ikramuddin S, et al. Safety and effectiveness of Realize adjustable gastric band: 3-year prospective study in the United States. Surg Obes Relat Dis. 2009;5(5):588–97. doi: 10.1016/j.soard.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Camastra S, Gastaldelli A, Maria Sironi A, Natali A, Muscelli E, et al. beta-cell function in obesity: effects of weight loss. Diabetes. 2004;53 3:S26–33. doi: 10.2337/diabetes.53.suppl_3.s26. [DOI] [PubMed] [Google Scholar]
- 8.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55(7):2025–31. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 9.Gentileschi P, Gagner M, Milone L, Kini S, Fukuyama S. Histologic studies of the bypassed stomach after Roux-en-Y gastric bypass in a porcine model. Obes Surg. 2006;16(7):886–90. doi: 10.1381/096089206777822322. [DOI] [PubMed] [Google Scholar]
- 10.Davis KG, Wertin TM, Schriver JP. The use of simvastatin for the prevention of gallstones in the lithogenic prairie dog model. Obes Surg. 2003;13(6):865–8. doi: 10.1381/096089203322618678. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Margallo FM, Loscertales B, Diaz-Guemes I, Uson J. Technical feasibility of laparoscopic Finney pyloroplasty examined in a canine model. Surg Endosc. 2007;21(1):136–9. doi: 10.1007/s00464-005-0798-x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1273–82. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17(10):1839–47. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackwell TS, Yull FE, Chen CL, Venkatakrishnan A, Blackwell TR, Hicks DJ, et al. Multiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammation. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1095–101. doi: 10.1164/ajrccm.162.3.9906129. [DOI] [PubMed] [Google Scholar]
- 16.Fowler M, Virostko J, Chen Z, Poffenberger G, Radhika A, Brissova M, et al. Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation. 2005;79(7):768–76. doi: 10.1097/01.tp.0000152798.03204.5c. [DOI] [PubMed] [Google Scholar]
- 17.Shearer J, Duggan G, Weljie A, Hittel DS, Wasserman DH, Vogel HJ. Metabolomic profiling of dietary-induced insulin resistance in the high fat-fed C57BL/6J mouse. Diabetes Obes Metab. 2008;10(10):950–8. doi: 10.1111/j.1463-1326.2007.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–32. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 19.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, et al. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol. 2006;176(8):4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 20.Sadikot RT, Blackwell TS. Bioluminescence imaging. Proc Am Thorac Soc. 2005;2(6):537–40. 11–2. doi: 10.1513/pats.200507-067DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M, Swijnenburg RJ, Gunawan F, Cao YA, Yang Y, Caffarelli AD, et al. In vivo visualization of cardiac allograft rejection and trafficking passenger leukocytes using bioluminescence imaging. Circulation. 2005;112(9 Suppl):I105–10. doi: 10.1161/CIRCULATIONAHA.104.524777. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 23.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(3):809–20. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009;56(5):1105–21. doi: 10.1016/j.pcl.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9(4):489–96. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 26.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25(11-12):1150–6. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre V, Stylopoulos N, Grinbaum R, Kaplan LM. An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity (Silver Spring) 2008;16(12):2585–92. doi: 10.1038/oby.2008.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez PP, Nicholson SE, Burkhardt GE, Johnson RA, Johnson FK. Development of a Sleeve Gastrectomy Weight Loss Model in Obese Zucker Rats. J Surg Res. 2008 doi: 10.1016/j.jss.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mistry SB, Omana JJ, Kini S. Rat models for bariatric surgery and surgery for type 2 diabetes mellitus. Obes Surg. 2009;19(5):655–60. doi: 10.1007/s11695-009-9811-0. [DOI] [PubMed] [Google Scholar]
- 30.Kampe J, Brown WA, Stefanidis A, Dixon JB, Oldfield BJ. A rodent model of adjustable gastric band surgery-implications for the understanding of underlying mechanisms. Obes Surg. 2009;19(5):625–31. doi: 10.1007/s11695-008-9751-0. [DOI] [PubMed] [Google Scholar]
- 31.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 32.Scopinaro N, Marinari GM, Camerini GB, Papadia FS, Adami GF. Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study. Diabetes Care. 2005;28(10):2406–11. doi: 10.2337/diacare.28.10.2406. [DOI] [PubMed] [Google Scholar]
- 33.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52(10):2461–74. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 34.Rijkelijkhuizen JM, Doesburg T, Girman CJ, Mari A, Rhodes T, Gastaldelli A, et al. Hepatic fat is not associated with beta-cell function or postprandial free fatty acid response. Metabolism. 2009;58(2):196–203. doi: 10.1016/j.metabol.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 36.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbrini E, Tamboli RA, Magkos F, Marks PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O'Brien KD, et al. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59(2):386–96. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15(8):846–7. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 40.Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138(5):961–75. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazeki K, Masuda N, Funami K, Sukenobu N, Matsumoto M, Akira S, et al. Toll-like receptor-mediated tyrosine phosphorylation of paxillin via MyD88-dependent and -independent pathways. Eur J Immunol. 2003;33(3):740–7. doi: 10.1002/eji.200323375. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Aljada A, Garg R, Ghanim H, Mohanty P, Hamouda W, Assian E, et al. Nuclear factor-kappaB suppressive and inhibitor-kappaB stimulatory effects of troglitazone in obese patients with type 2 diabetes: evidence of an antiinflammatory action? J Clin Endocrinol Metab. 2001;86(7):3250–6. doi: 10.1210/jcem.86.7.7564. [DOI] [PubMed] [Google Scholar]
- 45.Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, et al. Suppression of nuclear factor-kappaB and stimulation of inhibitor kappaB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab. 2001;86(3):1306–12. doi: 10.1210/jcem.86.3.7309. [DOI] [PubMed] [Google Scholar]
- 46.Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 39(1):109–24. doi: 10.1016/j.gtc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 47.von Drygalski A, Andris DA. Anemia after bariatric surgery: more than just iron deficiency. Nutr Clin Pract. 2009;24(2):217–26. doi: 10.1177/0884533609332174. [DOI] [PubMed] [Google Scholar]