Abstract
Rationale
Infants and children with chronic lung disease of prematurity (CLDP) are at increased risk for respiratory morbidities. We sought to determine (1) whether socio-economic status, race/ethnicity, and/or sex are risk factors for respiratory morbidities and (2) whether disparities in care existed for major therapy decisions such as home supplemental oxygen and gastrostomy tubes as well as initial length of stay in the neonatal intensive care unit.
Methods
Between January 2008 and February 2010 sociodemographic and respiratory morbidity data were collected on premature (<32 weeks gestation) infants and children (<3 years old) with CLDP. Associations between risk factors and respiratory morbidities and treatment parameters were examined using adjusted regression models.
Results
Data were collected on 135 subjects (Gestational age: 26.2 ± 2.0 weeks). Self-reported Nonwhites were more likely to report rescue medication use in the past 7 days (Adjusted OR: 2.87, [1.286.45], p=0.011) and the use of systemic steroids for respiratory symptoms since the last clinic visit (Adjusted OR: 2.12, [1.02-4.43], p=0.045). Lower median household income was associated with increased activity limitations (Adjusted OR: 2.79, [1.16-6.70], p=0.022) and public insurance coverage was associated with a decreased risk for hospitalizations (Adjusted OR: 0.36, [0.13-0.98], p=0.045). Major therapy decisions were not associated with disparities of care.
Conclusions
A key finding was that Non-whites were more likely to report rescue medication and systemic steroid use than Whites, but there was no difference in the frequency of respiratory symptoms or preventative inhaled corticosteroid use. Etiologies for these findings remain unclear and require further research.
Keywords: Bronchopulmonary dysplasia, chronic lung disease, prematurity, race, ethnicity, beta-agonist, corticosteroids, disparity
Introduction
In 2008, 12.3% of all infants born in the United States were born prematurely (<37 weeks gestation) (www.marchofdimes.com). Ongoing advances in neonatology have led to dramatic improvements in survival, but survivors frequently are affected by a variety of medical problems, such as developmental delay, retinopathy of prematurity, and chronic lung disease of prematurity (CLDP). CLDP may manifest with varying severity ranging from minimal symptoms to hypoxia requiring home supplemental oxygen to chronic respiratory failure requiring home mechanical ventilation.
CLDP remains a significant cause of both morbidity and mortality for preterm infants. Infant mortality in the United States was 6.86 infant deaths per 1,000 live births in 2005.1 Very preterm infants (<32 weeks gestation) accounted for 55% of all of infant deaths, but only 2% of live births in 2005 in the United States. Beyond the initial 12 hours of life, the most common causes of mortality for extremely low birth weight infants are respiratory-related.2 Infants with CLDP frequently have prolonged initial hospitalizations in neonatal intensive care units (NICU) for months, and after discharge have frequent “sick” office visits, emergency department visits, and re-hospitalizations for respiratory symptoms and infections. Many of these infants also require outpatient subspecialty care from pulmonologists and neonatologists as well as oxygen, diuretics, and/or respiratory medications.
The complex treatment and follow-up regimens prescribed for these children require knowledgeable caregivers, ready access to healthcare providers, insurance coverage, and financial resources. In other chronic respiratory diseases of childhood such as asthma and cystic fibrosis, respiratory health outcomes can be affected by any of these social factors. Median household income and insurance status have been shown to affect lung function in cystic fibrosis,3-5 with similar findings for pediatric asthma.6 Besides socio-economic factors contributing to disparities in healthcare outcomes, race/ethnicity and sex may also play a role. Worse respiratory outcomes have been observed in females and minorities in cystic fibrosis,7, 8 as well as with minorities in asthma,6, 9, 10 but not by sex in children with asthma.10 Our first set of analyses encompass the hypothesis that socio-economic factors (income, caregiver education level, and insurance status), race/ethnicity, and/or sex affect outcomes in CLDP as measured by the primary outcomes of acute care use, such as hospitalizations, and secondary outcomes of the presence of respiratory symptoms.
It has also been established that differential prescription of therapies may occur between patients of different sexes, races/ethnicities, or socio-economic status. For example, an IOM report requested by Congress notes that minorities are less likely to receive desirable therapies such as guideline-based therapy for asthma and more likely to receive less desirable therapies such as amputations for diabetes.11 Our second set of analyses encompass the null hypothesis that socio-economic factors, race/ethnicity, and/or sex do not affect major clinician decisions, specifically the prescription of gastrostomy tubes and home supplemental oxygen as well as length of initial stay in the NICU. We examined our hypotheses using data obtained from a pediatric pulmonary registry with subjects recruited from a specialty clinic for infants and children with CLDP.
Methods
Study Population
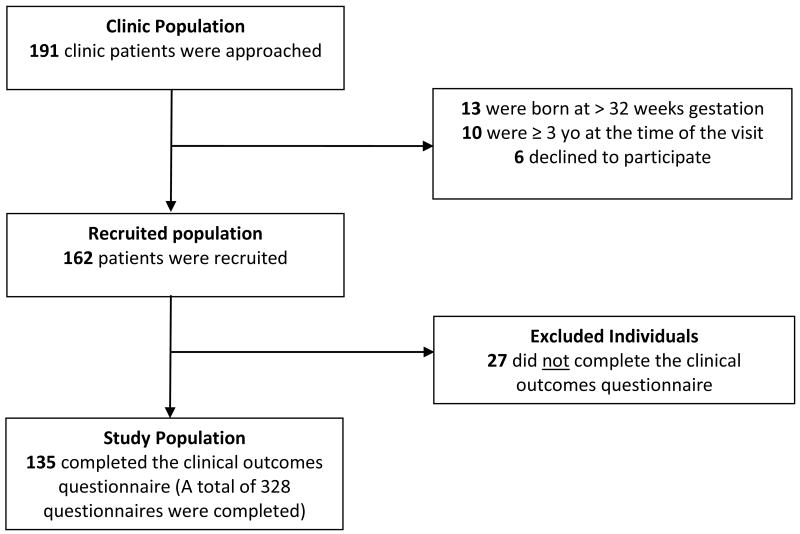
Subjects for this study were recruited from the Johns Hopkins Chronic Lung Disease of Prematurity (CLDP) Clinic between January 2008 and February 2010 under the auspices of the Johns Hopkins University IRB. Inclusion criteria included (1) a diagnosis of BPD at the time of NICU discharge, (2) a diagnosis of CLDP by the staffing pediatric pulmonologist as defined by the presence of respiratory symptoms (tachypnea or wheezing), the use of chronic respiratory medications (diuretics or inhaled corticosteroids), and/or the use of home supplemental oxygen in the at their first clinic visit, (3) born at ≤32 weeks of gestation, (4) less than 3 years of age at the time of the visit, and (5) a caregiver or guardian who consented to participate in a pediatric pulmonary registry. Of the 162 eligible subjects, 27(17%) were excluded on basis of not having a completed questionnaire documenting all four primary outcomes of emergency department visits, hospital admissions, systemic steroid use, and/or antibiotic use since the last clinic visit (Figure 1). Although excluded subjects (n=27) did not differ from the study population (n=135) on the basis of sex (p=0.52), race/ethnicity distribution (p=0.76), gestational age (p=0.67), age at first clinic visit (p=0.07), or median household income (p=0.31), excluded subjects were more likely to be covered by public insurance (p<0.001) and have a caregiver reporting a lower level of education (p=0.001) (Table 1). It is possible that caregivers with lower levels of education may have had more difficulties completing questionnaires during a short clinic visit, thus leading to exclusion due to a lack of completed forms. The study population does include 8 sets of twins.
Figure 1. Study Population Derivation.
Table 1. Recruited and Study Populations.
| Variable Mean ± SD [Range] | Recruited Population (n = 162) | Study Population (n = 135) | Excluded Individuals (n = 27) | Included vs. Excluded P value1 | ||
|---|---|---|---|---|---|---|
| Demographics | Sex (% Male) | 61.1 | 62.2 | 55.6 | 0.52 | |
| Race/Ethnicity (%Non-White) | 71.6 | 71.1 | 74.1 | 0.76 | ||
| Gestation (weeks) | 26.2 ± 2.0 [22.7 – 32.0] |
26.2 ± 2.0 [23.0 – 31.9] |
26.4 ± 2.0 [22.7 – 32.0] |
0.67 | ||
| Age at first clinic visit (months) | 6.9 ± 3.9 [2.0 – 29.0] |
6.6 ± 3.6 [2.0 – 29.0] |
8.1 ± 4.8 [3.2 – 25.6] |
0.07 | ||
| Socio-Economic Status | Median household income ($′000) | 42.8 ± 16.9 [11.1 – 101.4] (n = 150) |
43.4 ± 17.1 [11.1 – 101.4] (n = 124) |
39.8 ± 15.4 [11.5 – 65.7] (n = 26) |
0.31 | |
| Education: Primary caregiver (%) | <High School | 7.7 | 3.8 | 36.4 | ||
| H.S. Graduate | 25.3 | 23.8 | 36.4 | |||
| Some College | 37.4 | 38.8 | 27.3 | |||
| College Grad. | 15.4 | 17.5 | 0.0 | 0.001 | ||
| Adv. Degree | 14.3 (n = 91) |
16.3 (n = 80) |
0.0 (n = 11) |
|||
| Insurance status (%Public) | 58.0 | 51.9 | 88.9 | <0.001 | ||
| Exposures | Secondhand smoke (%Ever exposed)2 | - | 25.8 (n = 128) |
- | - | |
| Daycare (%Ever attended)2 | - | 20.2 (n = 124) |
- | - | ||
| Disease Severity | Supplemental oxygen use at first clinic visit (%Yes) | 35.8 | 39.3 | 18.5 | 0.04 | |
| Gastrostomy tube present at first clinic visit (%Yes) | 14.2 | 14.1 | 14.8 | 0.92 | ||
| Ventilator use at first clinic visit (%Yes) | 1.2 | 1.5 | 0.0 | 0.53 | ||
| Chronic inhaled corticosteroid use (%Ever) | 68.5 | 68.2 | 70.4 | 0.82 | ||
T-test or chi-square test P values for study population vs. those excluded.
Data is not available for excluded individuals.
Socio-Economic Variables
Three separate socio-economic factors were examined in this analysis, namely household income, primary caregiver education level, and insurance status. Median household income was derived from 2000 U.S. Census tract data; some subjects resided at addresses registered after this census and thus are not included (n=11). Primary caregiver education level was obtained via questionnaire and insurance coverage (private vs. public) from billing records; the 2 subjects who carried both types of coverage were classified as “private.” For analyses, income at less than 200% of the poverty level (2000 U.S. poverty level defined as $17,463 for a family of four including 2 children) was defined as low SES, primary caregiver education level of high school graduate or less was defined as low SES, and public insurance was defined as low SES. In addition to SES factors, sex and race/ethnicity were also analyzed with male sex and any self-reported Non-white race/ethnicity considered being potential risk factors.
Exposure Variables
Two potentially confounding exposures were included in this analysis, namely secondhand smoke exposure in the home and daycare attendance. Both were assessed through questionnaires at each clinic visit (Supplemental Table 1). Exposure to environmental tobacco smoke or daycare reflected an exposure during at least one clinical encounter (Table 1 and Supplemental Table 2), whereas for visit comparisons (Tables 2 and 3), adjustments for secondhand smoke or daycare exposure reflected an exposure reported on a specific clinic visit.
Table 2. Adjusted Odds Ratios of Primary Outcomes by Visit.
| Outcome | Risk Factor | n visits | Odds Ratio [95% C.I.]1 |
P value |
|---|---|---|---|---|
| Emergency Department Visit | Median Household Income | 251 | 0.86 [0.38 – 1.94] |
0.72 |
| Education | 166 | 0.55 [0.12 – 2.50] |
0.44 | |
| Insurance Status | 263 | 0.72 [0.31 – 1.69] |
0.45 | |
| Race/Ethnicity | 263 | 1.30 [0.54 – 3.10] |
0.56 | |
| Sex | 263 | 0.65 [0.31 – 1.39] |
0.27 | |
| Hospitalization | Median Household Income | 251 | 0.23 [0.05 – 1.14] |
0.07 |
| Education | 166 | No hospitalizations in low SES group | - | |
| Insurance Status | 263 | 0.36 [0.13 – 0.98] |
0.045 | |
| Race/Ethnicity | 263 | 0.92 [0.27 – 3.11] |
0.90 | |
| Sex | 263 | 0.44 [0.15 – 1.27] |
0.13 | |
| Systemic Steroid Usage | Median Household Income | 251 | 0.89 [0.44 – 1.83] |
0.76 |
| Education | 166 | 0.53 [0.13 – 2.21] |
0.38 | |
| Insurance Status | 263 | 1.06 [0.53 – 2.12] |
0.87 | |
| Race/Ethnicity | 263 | 2.12 [1.02 – 4.43] |
0.045 | |
| Sex | 263 | 0.71 [0.37 – 1.35] |
0.29 | |
| Antibiotic Usage | Median Household Income | 251 | 0.82 [0.44 – 1.53] |
0.53 |
| Education | 166 | 0.45 [0.12 – 1.73] |
0.24 | |
| Insurance Status | 263 | 0.53 [0.27 – 1.02] |
0.06 | |
| Race/Ethnicity | 263 | 1.48 [0.80 – 2.73] |
0.21 | |
| Sex | 263 | 1.20 [0.70 – 2.05] |
0.51 |
All logistic regressions clustered by subject and adjusted for age in months at the time of visit, sex, race/ethnicity, exposures of secondhand smoke exposure and daycare attendance, and disease severity as measured by the presence of oxygen use at the first clinic visit and gestational age.
Table 3. Adjusted Odds Ratios of Symptomatology by Visit.
| Outcome | Risk Factor | n visits | Odds Ratio [95% C.I.]1 |
P value |
|---|---|---|---|---|
| Days with trouble breathing | Median Household Income | 249 | 1.33 [0.66 – 2.67] |
0.42 |
| Education | 165 | 1.94 [0.67 – 5.58] |
0.22 | |
| Insurance Status | 261 | 0.95 [0.48 – 1.90] |
0.88 | |
| Race/Ethnicity | 261 | 1.05 [0.46 – 2.37] |
0.91 | |
| Sex | 261 | 1.96 [0.88 – 4.36] |
0.10 | |
| Rescue medication use | Median Household Income | 249 | 1.11 [0.53 – 2.32] |
0.78 |
| Education | 165 | 1.15 [0.29 – 4.48] |
0.84 | |
| Insurance Status | 261 | 1.95 [0.92 – 4.14] |
0.08 | |
| Race/Ethnicity | 261 | 2.87 [1.28 – 6.45] |
0.011 | |
| Sex | 261 | 1.56 [0.76 – 3.18] |
0.22 | |
| Activity limitations | Median Household Income | 248 | 2.79 [1.16 – 6.70] |
0.022 |
| Education | 163 | 3.45 [0.86 – 13.80] |
0.08 | |
| Insurance Status | 259 | 1.19 [0.49 – 2.91] |
0.70 | |
| Race/Ethnicity | 259 | 1.59 [0.69 – 3.67] |
0.28 | |
| Sex | 259 | 0.60 [0.27 – 1.34] |
0.21 | |
| Caregiver awake at night | Median Household Income | 251 | 1.21 [0.56 – 2.59] |
0.63 |
| Education | 166 | 0.74 [0.21 – 2.65] |
0.64 | |
| Insurance Status | 263 | 0.71 [0.37 – 1.39] |
0.32 | |
| Race/Ethnicity | 263 | 0.87 [0.42 – 1.79] |
0.68 | |
| Sex | 263 | 0.70 [0.33 – 1.46] |
0.34 |
All logistic regressions clustered by subject and adjusted for age in months at the time of visit, sex, race/ethnicity, exposures of secondhand smoke exposure and daycare attendance, and disease severity as measured by the presence of oxygen use at the first clinic visit and gestational age.
Outcome Variables
Primary outcome variables were based on acute care use reported since last clinic visit on questionnaires, and included emergency department (ED) visits, hospitalizations, systemic steroid use, and antibiotic use (Supplemental Table 1). Secondary outcomes of interest were divided into two categories, symptoms within the past week and use of medical therapies. The first category encompassed the presence of difficulty breathing, use of rescue medications, presence of activity limitation, and the presence of nighttime symptoms. The second category encompassed the use of home supplemental oxygen or gastrostomy tube as recorded at the first clinic visit, and time from birth to initial discharge from the hospital. Although outcomes related to airway hyperreactivity (i.e. systemic steroid or rescue medication use) may not encompass all subjects, abnormal spirometry is seen in 56% of 11 year olds who were born prematurely,12 and is likely higher in our younger study population given that the use of inhaled corticosteroids approaches 70%, which does not account for subjects with milder hyperreactivity not requiring daily preventative therapy. All outcomes were treated as dichotomous variables except age at the time of initial discharge, which was treated as a continuous variable.
Statistical Methods
Descriptive frequencies of sociodemographic characteristics and exposures were generated using means and proportions as appropriate. Statistical comparisons between populations deemed to be at risk and not at risk were made using t tests and chi-square tests. Multivariable logistic regression models were constructed to examine the association of low SES or other risk factors and the outcomes. Regression models in Tables 2 and 3 were adjusted for age at the time of form completion, race/ethnicity, sex, daycare and smoke exposures, and disease severity as measured by gestational age and oxygen use at the first clinic visit. Regression models in Table 4 were adjusted for age at the time of the first pulmonary clinic visit, race/ethnicity, sex, and disease severity as measured by gestational age. In addition, the models in Tables 2 and 3, which are based on multiple clinical visits per subject, were adjusted for clustering by subject using Generalized Estimating Equations (GEE) methodology to account for subjects who had more than one clinical encounter over the course of the study. We estimated that 246 questionnaires would provide 75% power to identify an OR of 2.0 or greater for a particular SES factor.13 Intercooled STATA 10 (StataCorp LP, College Station, TX) was used for all statistical analyses. P-values <0.05 were considered statistically significant.
Table 4. Adjusted Odds Ratios and Coefficients of Secondary Outcomes at First Clinic Visit.
| Outcome | Risk Factor | n subjects | Odds Ratio [95% C.I.]1 |
P value |
|---|---|---|---|---|
| Home Oxygen | Median Household Income | 124 | 1.12 [0.51 – 2.48] |
0.77 |
| Education | 80 | 1.70 [0.60 – 4.87] |
0.32 | |
| Insurance Status | 135 | 0.83 [0.40 – 1.73] |
0.62 | |
| Race/Ethnicity | 135 | 1.15 [0.49 – 2.68] |
0.75 | |
| Sex | 135 | 1.14 [0.51 – 2.53] |
0.74 | |
| Gastrostomy Tube | Median Household Income | 124 | 0.31 [0.07 – 1.40] |
0.13 |
| Education | 80 | 0.06 [0.00 – 3.27] |
0.17 | |
| Insurance Status | 135 | 1.33 [0.41 – 4.25] |
0.64 | |
| Race/Ethnicity | 135 | 1.04 [0.29 – 3.74] |
0.96 | |
| Sex | 135 | 0.49 [0.14 – 1.71] |
0.26 | |
|
Co-efficient [95%C.I.]2 |
||||
| Length of Initial Hospitalization | Median Household Income | 124 | -0.52 [-1.31 – 0.26] |
0.19 |
| Education | 80 | 0.30 [-0.63 – 1.22] |
0.53 | |
| Insurance Status | 135 | 0.05 [-0.68 – 0.77] |
0.90 | |
| Race/Ethnicity | 135 | 0.72 [-0.11 – 1.54] |
0.09 | |
| Sex | 135 | 0.15 [-0.62 – 0.93] |
0.69 |
All logistic regressions adjusted for age in months at the time of visit, sex, race/ethnicity, and disease severity as measured by gestational age.
All linear regressions adjusted for age in months at the time of visit, sex, race/ethnicity, and disease severity as measured by gestational age.
Results
Demographics
The study population (n=135) consisted of 62% males and was predominantly Non-white (71%); of those who were Non-white, 97% self-reported as Black (Table 1). The mean gestational age was 26.2 weeks (n=135), with a mean birth weight of 837 ± 293 grams (n =130). Subjects were first seen in pulmonary clinic at a mean age of 6.6 ± 3.6 months; 59% of subjects were assessed for the study at their first visit. The mean estimated median household income was $43,437 ± 17,161 (n=124), which was slightly higher than the U.S. median household income ($41,994 in 2000). Of the 80 primary caregivers in the study population who reported their highest level of education, only 3.8% reported less than the equivalent of a high school education. Approximately half of the subjects (52%) were covered by public insurance, which was predominantly Maryland Medicaid. The mean number of forms completed per subject was 2.4 ± 1.7 [Range: 1-9], and by linear regression, sex, race/ethnicity, insurance status, and estimated income were not associated with the number of forms completed. Primary caregiver education level was associated with the number of forms completed (p=0.01); those with a high school education or less completed a mean of 1.9 ± 1.2 forms, and those with more than a high school education completed a mean of 2.9 ± 1.9 forms.
Primary Outcomes
Self-reported Non-white status was associated with increased systemic steroid use and public insurance status was associated with fewer hospitalizations: In logistic regression analysis, Non-whites were twice (Adjusted OR: 2.12; p=0.045) as likely to report the use of systemic steroids for respiratory symptoms since the last clinic visit as Whites (Table 2). No other socio-economic factor studied was found to be associated with systemic steroid use, nor was race/ethnicity associated with any of the other primary outcomes studied. In logistic regression analysis, subjects covered by public insurance were less likely (Adjusted OR: 0.36; p=0.045) to report a hospitalization since their last clinic visit than subjects covered by private insurance (Table 2). No other socio-economic factor studied was found to be associated with hospitalizations, nor was insurance status associated with any of the other outcomes studied.
Secondary Outcomes (Recent Symptoms)
Self-reported Non-white status was associated with increased rescue medication use for respiratory symptoms and lower household income was associated with increased activity limitations: Non-whites were 2.5 times more likely to report using rescue medications at least once in the past week than Whites (p=0.019) (Table 3). Despite the difference in usage of rescue medications in the past week, Non-whites did not report the presence of daytime (p=0.70) or nighttime symptoms (p=0.65) at a greater frequency than Whites (Table 3). We also found that subjects with a lower estimated household income were 2.8 times more likely to report the child had activity limitations than those with higher estimated incomes (p=0.022). No other socioeconomic factor studied was found to be associated with activity limitations, nor was income status associated with any of the other outcomes studied.
Secondary Outcomes (Major Treatment Decisions)
Socio-economic status, sex, and race/ethnicity were not associated with disparities of care for home oxygen, gastrostomy tubes, and initial length of stay. In terms of secondary outcomes, at the first clinic visit, 40 subjects were receiving supplemental oxygen alone (30%), 6 subjects were receiving gastrostomy tube feeds alone (4%), 13 were receiving both (10%), and 76 were receiving neither (56%). The mean age at initial discharge to home from either the NICU or a subacute facility was 4.0 ± 2.7 months. Using adjusted logistic regression, none of the socio-economic factors, sex, or race/ethnicity was associated with the prescription of home oxygen or gastrostomy tube placement (Table 3). Using linear regression in a similar manner, none of these factors were associated with differences in the length of initial hospital stay.
Post-Hoc Analysis (Inhaled Corticosteroid Use)
Based on the increased use of systemic steroids and rescue medications among Non-whites, we wished to assess whether a disparity in the use of daily inhaled corticosteroids (ICS) was present between Non-whites and Whites that could explain the increased systemic steroids and rescue medication use in Non-whites. Post-hoc chart review revealed 68% of subjects (n=135) had ever been prescribed an ICS, and subjects had been prescribed an ICS at 69% of visits (n=328). There was no difference by race/ethnicity in terms of ever having been prescribed an ICS (Non-whites: 69% ever prescribed an ICS; Whites: 67%; p=0.81).
Discussion
In contrast to other chronic respiratory diseases of childhood, we found that socio-economic status, sex, and race/ethnicity were generally not associated with acute care outcomes or recent respiratory symptoms. We also did not observe any disparities in care for the selected therapies that we studied (i.e., home oxygen, gastrostomy tubes, and initial length of stay). There are several possible explanations for our findings. First, it may be that the infants with CLDP that we studied are at such significant risk for acute care usage that their disease severity outweighs any socio-economic factors. Alternatively, the services provided by NICU nurses, case managers, and social workers may provide a protective cushion for the first several years of life in the form of education, 14 arranging home nursing, and developmental services such as Infants and Toddlers. Additionally, disparities may be more subtle as these infants are being seen by a limited number of subspecialists. Lastly, it is possible that other confounding factors not measured in this study reduce the effects of socio-economic status, such as increased risks for in vitro fertilization (IVF) babies or advanced maternal age.15
One of our key findings was that self-reported Non-whites were more likely to report the use of a respiratory rescue medication in the past week than Whites. A similar finding has been reported previously with an increased use of inhaled or oral β-agonist use in black infants compared to other racial and ethnic groups independent of outpatient visits for respiratory symptoms.16 Given that the frequency of respiratory symptoms reported in the past week between Whites and Non-whites in our study was not statistically different, this implies that Whites and Non-whites likely do not differ in the frequency of a “reactive airway” or “wheezing” phenotype for CLDP. However, it is unclear (i) whether Non-white children with CLDP have a better response to inhaled β-agonists, thus prompting their increased use, (ii) whether Non-white children with CLDP have a worse response to inhaled β-agonists, thus prompting their increased use to obtain the same level of symptom relief, or (iii) whether Nonwhite children with CLDP have more severe symptoms when symptomatic, prompting increased use. These explanations may have a genetic cause with differing frequencies of genetic polymorphisms in Non-whites compared to Whites. Although candidate gene studies of the β2-adrenergic receptor gene (ADRB2) have not conclusively proved its role in the development of asthma,17-19 polymorphisms may play a role in β-agonist response,20 and racial differences in polymorphism frequencies have been observed;21 similar studies have not been conducted in CLDP. Alternatively, Non-whites may have different perceptions regarding the severity of symptoms that require intervention. Finally, it may be that Non-whites are more frequently exposed to specific environmental agents that lead to more severe symptoms; although perhaps not daycare or secondhand smoke, where no differences in ever having been exposed to daycare (p=0.90) or secondhand smoke (p=0.93) were observed by race/ethnicity.
Non-whites were also more likely to report systemic steroid use since the last clinic visit. This may be a function of the reported increased use of rescue inhaled β-agonist therapy leading clinicians to prescribe systemic steroids more frequently. The increased reported use of systemic steroids and rescue medications by Non-whites in our study does not appear to be related to a disparity in being prescribed inhaled corticosteroids which may prevent the symptoms that prompt the use of systemic steroids and rescue medications. However, it is possible that adherence rates to inhaled corticosteroids may differ between Whites and Non-whites, which has been reported in asthma.22, 23 It is also possible that caregiver health beliefs regarding the efficacy of inhaled corticosteroids, as is seen in asthma,24-27 may lead to differing levels of use among Non-whites and Whites. Ultimately, it may be that Non-whites may need to be more aggressively prescribed inhaled corticosteroids to prevent increased rescue medication and systemic steroid use, but further research will be required to determine this.
Additionally, we had hypothesized that differences in prescribed therapies and length of hospital stays differed by groups; we did not identify any disparities in care by socio-economic status or the other factors we examined. More specifically, insurance status does not appear to be impacting the initiation of these therapies or length of initial hospital stay. However, we did find that public insurance was associated with a decreased risk of hospitalization since last clinic visit. This is in contrast to a previous cohort study of extremely low birth weight infants which did demonstrate that Medicaid was a predictor for re-hospitalization for respiratory causes (OR: 2.0; p<0.05).14 Reasons for this difference may include our population being drawn from a single catchment area, which may have differences in how patients on Medicaid access care relative to other regions in the U.S.
Although our study was limited to patients attending a single tertiary care center's clinic, the treatment decisions (i.e., gastrostomy tube placement, initiation of supplemental oxygen, and initial length of stay) were made in the NICU setting and our population is derived from tertiary and community Maryland NICUs, both rural and urban. Nevertheless, it is possible that our study may not reflect disparities in CLDP outcomes that may exist in other regions of the U.S. Our study has other several limitations, including data collection via questionnaires, which may be subject to recall bias, and possibly being underpowered to detect subtle disparities (OR<2.0) or for SES factors where the number of completed questionnaires in our study is substantially less than 250. For example, although we attempted to correct for daycare exposure, the types of daycare that children attend may be stratified by SES. While we had family-specific data regarding caregiver education and insurance status, we were unable to obtain such data on income, which was census tract-based for our analyses. The increased uncertainty with a census tract-based measure may limit the power to detect differences in outcomes based on income. We may also have a selection bias present as subjects who were excluded tended to have caregivers with a lower completed education level and were more likely to be covered by public insurance than subjects who were included, thus the effects of caregiver education and insurance status on outcomes may be underestimated. Although we adjusted for at least one confounding factor that tends to be associated with higher SES (i.e., daycare attendance),26 there are other potentially confounding factors that have not been adjusted for (e.g. IVF) that may be associated with both low and high SES. Finally, CLDP is subject to physician diagnosis and not a homogenous disease process as different patients may have differing combinations of vascular, airway, and interstitial disease, thus stratification of disease phenotype by SES could bias our results.
In summary, we found an association between race/ethnicity and systemic steroid use as well as rescue inhaled β-agonist use, which may be biologically mediated. Further research will be necessary to confirm our finding and test for possible etiologies. Most importantly, we did not identify any substantial links between SES and acute care outcomes, recent symptoms, and disparities in care, suggesting that disease severity or protective effects from medical, educational, and psychosocial services provided in the NICU may last at least as long as 3 years after discharge, thus blunting the effects of SES. Future longitudinal research is needed to see if disparities arise over time following discharge.
Supplementary Material
Acknowledgments
The authors wish to thank the families who participated in the Johns Hopkins Pediatric Pulmonary Registry, its research coordinator Beth Stewart, the research assistants who interacted with the families, and Drs. Brian McGinley and Maureen Lefton-Greif for their helpful comments. This work was supported by the Thomas Wilson Foundation (S.A.M. & J.M.C.) and NIH HL089410 (S.O.O.).
Thomas Wilson Foundation; NIH, Number HL089410.
Footnotes
Disclosure: All authors disclose that they have no financial interests in the subject of this manuscript.
Reference List
- 1.Mathews TJ, Macdorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008;57(2):1–32. [PubMed] [Google Scholar]
- 2.Shankaran S, Fanaroff AA, Wright LL, et al. Risk factors for early death among extremely low-birthweight infants. Am J Obstet Gynecol. 2002;186(4):796–802. doi: 10.1067/mob.2002.121652. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor GT, Quinton HB, Kneeland T, et al. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111(4 Pt 1):e333–e339. doi: 10.1542/peds.111.4.e333. [DOI] [PubMed] [Google Scholar]
- 4.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 5.Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS. Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr. 2009;155(5):634–639. doi: 10.1016/j.jpeds.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in the United States. Ambul Pediatr. 2002;2(5):382–387. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Hamosh A, Fitzsimmons SC, Macek M, Jr, Knowles MR, Rosenstein BJ, Cutting GR. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr. 1998;132(2):255259. doi: 10.1016/s0022-3476(98)70441-x. [DOI] [PubMed] [Google Scholar]
- 8.Zeitlin PL. Cystic fibrosis and estrogens: a perfect storm. J Clin Invest. 2008;118(12):3841–3844. doi: 10.1172/JCI37778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics. 2006;117(5):e868e877. doi: 10.1542/peds.2005-1721. [DOI] [PubMed] [Google Scholar]
- 10.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123 3:S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine. Unequal Treatment. Washington, DC: National Academies Press; 2002. [Google Scholar]
- 12.Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182(2):237–245. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demidenko E. Mixed Models: Theory and Applications. Internet. 2010 Nov 15; 2010. [Google Scholar]
- 14.Morris BH, Gard CC, Kennedy K. Rehospitalization of extremely low birth weight (ELBW) infants: are there racial/ethnic disparities? J Perinatol. 2005;25(10):656–663. doi: 10.1038/sj.jp.7211361. [DOI] [PubMed] [Google Scholar]
- 15.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Lorch SA, Wade KC, Bakewell-Sachs S, Medoff-Cooper B, Escobar GJ, Silber JH. Racial differences in the use of respiratory medications in premature infants after discharge from the neonatal intensive care unit. J Pediatr. 2007;151(6):604–10. 610. doi: 10.1016/j.jpeds.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Contopoulos-Ioannidis DG, Manoli EN, Ioannidis JP. Meta-analysis of the association of beta2adrenergic receptor polymorphisms with asthma phenotypes. J Allergy Clin Immunol. 2005;115(5):963–972. doi: 10.1016/j.jaci.2004.12.1119. [DOI] [PubMed] [Google Scholar]
- 18.Migita O, Noguchi E, Jian Z, et al. ADRB2 polymorphisms and asthma susceptibility: transmission disequilibrium test and meta-analysis. Int Arch Allergy Immunol. 2004;134(2):150–157. doi: 10.1159/000078648. [DOI] [PubMed] [Google Scholar]
- 19.Thakkinstian A, McEvoy M, Minelli C, et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol. 2005;162(3):201–211. doi: 10.1093/aje/kwi184. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta-agonists in children with asthma: a meta-analysis. J Asthma. 2009;46(9):900–905. doi: 10.3109/02770900903199961. [DOI] [PubMed] [Google Scholar]
- 21.Drysdale CM, McGraw DW, Stack CB, et al. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000;97(19):10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams LK, Joseph CL, Peterson EL, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120(5):1153–1159. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it's not just black and white. J Allergy Clin Immunol. 2003;111(6):1219–1226. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 24.Wells K, Pladevall M, Peterson EL, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med. 2008;178(12):11941201. doi: 10.1164/rccm.200808-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le TT, Bilderback A, Bender B, et al. Do asthma medication beliefs mediate the relationship between minority status and adherence to therapy? J Asthma. 2008;45(1):33–37. doi: 10.1080/02770900701815552. [DOI] [PubMed] [Google Scholar]
- 26.Panettieri RA, Jr, Spector SL, Tringale M, Mintz ML. Patients' and primary care physicians' beliefs about asthma control and risk. Allergy Asthma Proc. 2009;30(5):519–528. doi: 10.2500/aap.2009.30.3281. [DOI] [PubMed] [Google Scholar]
- 27.Wu AC, Smith L, Bokhour B, Hohman KH, Lieu TA. Racial/Ethnic variation in parent perceptions of asthma. Ambul Pediatr. 2008;8(2):89–97. doi: 10.1016/j.ambp.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.