Abstract
Objectives
Having a parent affected with late-onset Alzheimer's disease (LOAD) is a major risk factor for developing the disease among cognitively normal (NL) individuals. This MRI study examines whether NL with a LOAD-affected parent show preclinical brain atrophy, and whether there are parent-of-origin effects.
Methods
Voxel-based morphometry (VBM) on SPM’8 was used to examine volumetric T1-MRI scans of 60 late-middle-aged NL subjects, divided into 3 size-matched, demographically balanced groups of 20 subjects each, including NL with a maternal (FHm), paternal (FHp), or negative family history (FH-) of LOAD.
Results
There were no group differences for clinical and neuropsychological measures, and ApoE status. On VBM, FHm showed reduced gray matter volumes (GMV) in frontal, parietal, temporal cortices and precuneus as compared to FH-, and in precuneus compared to FHp (P<0.05, FWE-corrected). Results remained significant controlling for age, gender, education, ApoE and total intracranial volume. No differences were observed between FHp and FH- in any regions.
Conclusion
NL FHm showed reduced GMV in AD-affected brain regions compared to FH- and FHp, indicating higher risk for AD. Our findings support the use of regional brain atrophy as a preclinical biomarker for LOAD among at risk individuals.
Keywords: Alzheimer's disease, Volumetric MRI, preclinical, family history
1. Introduction
Alzheimer's disease (AD) is the most common cause of dementia in the elderly population, affecting millions of people world-wide (Hebert et al., 2004). Once disease-modifying drugs become available, reliable diagnosis of AD at its earliest stages is increasingly important, because treatment effects may be most beneficial before the pathological progress is well advanced. To accomplish this goal, it is necessary to identify at-risk individuals, and to develop biological markers that predict and correlate with clinical change, in order to track disease progression and monitor treatment effects.
While genetic mutations have been identified in the early-onset familial forms of AD, the genetic mechanisms involved in the more common late-onset AD (LOAD) remain largely unknown. Several studies have shown that having a family history of LOAD is a major risk factor for developing the disease among cognitively normal (NL) individuals (Farrer et al., 1997). The risk for developing LOAD is 4- to 10-fold higher in 1st degree relatives of LOAD patients, and is highest in children of parents affected by LOAD (Cupples et al., 2004; Green et al., 2002; Silverman et al., 2005). Epidemiological studies have shown both maternal (Edland et al., 1996) and paternal transmission of LOAD (Ehrenkrantz et al., 1999). However, having an AD-affected mother appears to confer greater risk to the offspring than having an AD-affected father, was associated with poorer cognitive performance in late life and with a more predictable age at onset of dementia (Debette et al., 2009; Duara et al., 1993; Edland et al., 1996; Gomez-Tortosa et al., 2007; Locke et al., 1995).
We and others have been using brain imaging to provide biological endophenotypes related to maternally- and paternally-transmitted risk of LOAD in NL individuals. Using [18F]fluorodeoxyglucose positron emission tomography (FDG-PET), we demonstrated that NL with a maternal family history of LOAD (FHm) show progressive reductions in cerebral metabolic rates of glucose (CMRglc) in AD-vulnerable regions compared to NL with a paternal history (FHp) and to NL with negative family history (FH-) (Mosconi et al., 2007, 2009). In contrast, NL FHp showed no metabolic abnormalities (Mosconi et al., 2007, 2009). Additionally, a recent cross-sectional MRI study showed reduced gray matter volumes, an indicator of brain atrophy, in NL with a family history of LOAD compared to controls, with the reductions being more severe in FHm than in FHp (Honea et al., 2010). However, this study was based on retrospective examination of existing MRI scans, which resulted in relatively small and uneven groups, and subjects were fairly old, with a mean age of 74 years (Honea et al., 2010). Additionally, the ApoE-4 genotype was found in over 60% FHm while a minority of FHp and FH- were ApoE-4 carriers. Since ApoE-4 genotype is a major risk factor for LOAD, and is associated with increased atrophy (Chen et al., 2007), it remains unclear whether GMV reductions in FHm were due to the relatively high proportion of ApoE-4 carriers or more specifically to maternal history of LOAD.
The present MRI study used voxel-based morphometry to map regional gray matter volume reductions in a cohort of 60 NL individuals (mean age 63 years, 30% ApoE-4 carriers), divided into 3 size-matched, demographically balanced groups based on their family history of LOAD. We examined whether late-middle-aged NL with LOAD-parents, particularly FHm, showed reduced gray matter volumes several years before the possible onset of dementia.
2. Methods
2.1. Subjects
We examined 60 clinically and cognitively normal (NL) subjects, divided into 3 size-matched, demographically balanced groups of 20 subjects each, based on their parental family history of LOAD (see below). All subjects were prospectively recruited at NYU School of Medicine to participate in ongoing longitudinal brain MRI studies. These included individuals interested in research participation and risk consultation, spouses, family members, and caregivers of patients participating in our studies. All subjects received a standard diagnostic evaluation that included medical, neuropsychological, and MRI examinations. IRB-approved informed consent was obtained from all subjects. Individuals with medical conditions or history of conditions that may affect brain structure or function, i.e., stroke, diabetes, head trauma, any neurodegenerative diseases, depression, hydrocephalus, intracranial mass, and infarcts on MRI, and use of psychoactive medications were excluded.
Subjects had age ≥45 years, education≥12 years, Clinical Dementia Rating (CDR)=0, Global Deterioration Scale (GDS)≤2, Modified Hachinski Ischemia Scale scores <4, and Mini-Mental State Examination (MMSE)≥28. All subjects had normal cognitive test performance relative to normative values on the immediate and delayed recall of a paragraph and of paired associates, the digit-symbol substitution test, the designs, object naming, and WAIS-vocabulary tests (De Santi et al., 2008). ApoE genotype was determined using standard PCR procedures.
A family history (FH) of LOAD that included at least one first-degree relative with dementia onset between 65 and 80 years was elicited by using the NYU Brain Aging Family History questionnaire (available upon request) (Mosconi et al., 2007). Participants were asked to fill in information of affected family members, which was confirmed by other family members in the interview with the examining neurologist. Only NL subjects whose parents’ diagnosis of AD was reportedly clinician certified were included in this study. Subjects were not included if their parents had not lived to at least age 65, or if the diagnosis of AD was not documented. Subjects with FHm (i.e., only the mother was affected with AD), FHp (i.e., only the father was affected with AD), and FH-(i.e., negative FH) were included in the study (Mosconi et al., 2007, 2009). From a larger groups of possible recruits, we created 3 groups of 20 subjects each, balanced for demographical characteristics, for a total N=60. This was done as follows: first, 11 of the available FH- and FHp subjects were individually matched to 11 FHm subjects for (in order of importance): gender, age (within 3 years), and educational level. Second, 6 of remaining FH- and FHp subjects were matched to 6 FHm subjects based on gender and educational level. Finally, 3 subjects were matched for their mean age and educational level to the FHm group. Demographic characteristics of the subjects under study are shown in Table 1. Age, gender, education and ApoE status were included as covariates in all analyses, as described below.
Table 1.
Subjects characteristics by family history groups.
| FH- | FHp | FHm | |
|---|---|---|---|
| N | 20 | 20 | 20 |
| Age, years [range] | 65.5±8.9 [50-83] | 62.9±12.1 [46-85] | 63.0±7.8 [46-83] |
| Gender (female/male) | 12/8 | 10/10 | 13/7 |
| Education, years | 16.4±2.2 | 16.9±2.0 | 17.2±1.6 |
| ApoE-4 status (carriers/non-carriers) | 5/15 | 7/13 | 6/14 |
| MMSE | 29.4±0.8 | 29.6±0.8 | 29.6±0.8 |
| PARD | 9.7±3.4 | 10.4±2.9 | 10.1±2.4 |
| PRDD | 6.0±3.0 | 6.8±3.1 | 6.1±2.6 |
| Designs | 7.3±2.6 | 7.2±2.7 | 7.4±2.6 |
| DSST | 55.0±11.4 | 60.2±11.0 | 56.5±13.1 |
| Digit Span Forward | 7.5±1.0 | 7.2±0.8 | 7.0±1.1 |
| Digit Span Backward | 5.5±1.3 | 5.8±1.1 | 5.5±1.3 |
Values are mean ± SD.
Abbreviations. FH: family history of LOAD, FH-: negative FH, FHp: paternal FH, FHm: maternal FH, MMSE: Mini-Mental State Examination; PARD: Paragraph Recall Delayed; PRDD: Paired Associates Delayed Recall; DSST: Digit Symbol Substitution Test.
2.2. MRI acquisition protocol and pre-processing
All subjects received a diagnostic and a research MRI study on a 1.5 T GE Signa imager (General Electric, Milwaukee, USA). The diagnostic study was performed using contiguous 3 mm axial T2-weighted images. The research scan was a 124 slice T1-weighted Fast-Gradient-Echo acquired in a sagittal orientation as 1.2 mm thick sections (field of view=25 cm, NEX=1, matrix=256×128, repetition time= 35 ms, echo time= 9 ms and flip angle=60 0, no interslice gaps). These scans were used to rule out MRI evidence of hydrocephalus, intracranial mass, strokes, subcortical gray matter lacunes, non specific white matter disease, and focal white matter hyperintensities (George et al., 1986).
MRI images were analyzed with voxel-based morphometry (VBM) using MATLAB 7.8 (the MathWorks, Natick, MA) and Statistical Parametric Mapping (SPM’8) (Ashburner et al., 2000; Good et al., 2001). For all subjects, MRI images were spatially normalized to the SPM template in the stadardized anatomical space, which conforms to the MNI space, by estimating the optimum (least squares) 12-parameter affine transformation, followed by 7×8×7 discrete cosine functions (Ashburner et al., 2000; Good et al., 2001). A masking procedure was used to weight the normalization to brain rather than non-brain tissue. The spatially normalized MRI images were segmented into gray (GM), white matter, and cerebrospinal fluid images using SPM8's unified tissue segmentation procedure which rely on an iterative process to determine proportions of GM and white matter in each voxel, after removal of all non-brain voxels and application of image intensity non-uniformity correction (Ashburner et al., 2000). The GM images were retained for analyses and normalized to an a priori GM template (Good et al., 2001) by using the same normalization parameters described above. To preserve gray matter volumes (GMV) within each voxel, the images were modulated by the Jacobian determinants derived from the spatial normalization, and then smoothed using an 8-mm FWHM isotropic Gaussian kernel (Ashburner et al., 2000; Good et al., 2001). GM, white matter, and cerebrospinal fluid segmentations for each image were used to calculate the total-intracranial volume (TIV) for each subject. The TIV was computed in cubic centimeters and examined as a covariate in subsequent analyses, as done in previous VBM papers in similar study groups (Honea et al 2009).
2.3. Statistical analysis
SPSS (version 12.0; Chicago, IL), and SPM’8 (Ashburner et al., 2000; Good et al., 2001) were used for data analyses. Differences in clinical and neuropsychological measures between FH groups were examined with Chi-square tests and the General Linear Model (GLM) with post-hoc LSD tests, as appropriate. Results were considered significant at P<0.05. For SPM’8 analysis, the GLM/univariate analysis with post-hoc t-tests was used to test for GMV differences across FH groups. First, we compared NL groups with (FH+) and without (FH-) a FH of AD, and second, we examined parent gender effects [FH- vs FHp vs FHm]. All analyses were performed controlling for TIV as a covariate (Whitwell et al., 2001; Honea et al 2009). Additionally, analyses were repeated correcting for other potential risk factors for LOAD, such as age, gender, education and ApoE status, by including these variables as nuisance variables (i.e., covariates) in the GLM. GMV measures were extracted from clusters of voxels reaching statistical significance in these analyses using the SPM-compatible Marsbar toolbox [http://www.mrc-cbu.cam.ac.uk/Imaging/marsbar.html] (Brett et al., 2002), and used to calculate percent differences in GMV across FH groups for descriptive purposes.
As a secondary analysis, we tested whether there were associations between gender and FH status on GMV using SPM’8, correcting for TIV. First, we used two-sample T-tests to compare females and males in the entire group. We then examined FH effects (FH- vs FHp vs FHm) within female and male groups using the GLM/univariate analysis with post-hoc t-tests to perform two separate comparisons: first, we compared the female FH groups, and second the male FH groups to assess whether decreased GMV were found in FHm irrespective of subjects’ gender.
We used WFU Pick-atlas package (Maldjian et al., 2003) to create a masking image from a set of predefined AD-related regions, including posterior cingulate cortex/precuneus, inferior parietal lobule, superior and middle temporal gyri, medial and prefrontal cortex, and medial temporal lobes. Results were considered significant at P<0.05 after family-wise error (FWE) correction for multiple comparisons within the search volume defined by the masking image, which was set as an exclusive mask in the analysis. Only clusters exceeding an extent threshold of 100 voxels were considered significant. Anatomical location of brain regions showing significant effects was described using the Talairach and Tournoux coordinates (Talairach and Tournoux, 1988), after coordinates conversion from the MNI to the Talairach space [http://www.mrccbu.cam.ac.uk/Imaging/].
3. Results
3.1. Subjects’ characteristics
Demographic and neuropsychological measures of the subjects are shown in Table 1. There were no differences across FH groups for age, gender, education, ApoE status, MMSE scores and neuropsycological test performance (Table 1).
3.2. Voxel-based morphometry
After correcting for TIV, as compared to FH- subjects, FH+ subjects showed significantly decreased GMV in middle and inferior frontal gyri of the right hemisphere, and in inferior parietal lobule of the left hemisphere (P<0.05, FWE-corrected) (Table 2). No regions showed GMV reductions in FH- subjects compared to FH+.
Table 2.
Brain regions showing significant differences in gray matter volume between FH groups.
| Cluster extent | Coordinates* | Z value** | Functional area | Brodmann Area | ||
|---|---|---|---|---|---|---|
| Reduced gray matter volume in FH+ compared to FH- | ||||||
| 325 | 48 | 36 | 22 | 4.52 | right Middle Frontal Gyrus | 46 |
| 45 | 44 | -7 | 4.43 | right Middle Frontal Gyrus | 47 | |
| 40 | 49 | -1 | 4.42 | right Inferior Frontal Gyrus | 10 | |
| 354 | -56 | -39 | 27 | 4.41 | left Inferior Parietal Lobule | 40 |
| Reduced gray matter volume in FHm compared to FH- | ||||||
| 649 | -57 | -47 | 38 | 4.86 | left Inferior Parietal Lobule | 40 |
| 508 | -32 | -60 | 47 | 4.46 | left Precuneus | 7 |
| -40 | -64 | 42 | 4.42 | left Inferior Parietal Lobule | 39 | |
| 105 | 59 | -32 | 20 | 4.50 | right Superior Temporal Gyrus | 42 |
| 297 | 40 | 50 | -1 | 4.71 | right Inferior Frontal Gyrus | 10 |
| Reduced gray matter volume in FHm compared to FHp | ||||||
| 113 | -33 | -77 | 40 | 4.52 | left Precuneus | 19 |
Coordinates (x, y, z) from the atlas of Talairach and Tournoux (Talairach and Tournoux, 1988)
Z value at the peak of statistical significance, P≤0.05, FWE-corrected, after correction for total intracranial volume.
Comparison of the three FH groups showed that the GMV reductions in the frontal, temporal and parietal cortices were driven by the FHm group, which showed reduced GMV compared to both FH- and FHp (Table 2). As compared to FH-, FHm subjects showed significant GMV reductions in the inferior parietal cortex and precuneus of the left hemisphere, and in superior temporal cortex and inferior frontal gyrus of the right hemisphere (P<0.05, FWE-corrected) (Table 2, Figure 1). As compared to FHp, FHm subjects showed reduced GMV in the left precuneus (P<0.05, FWE-corrected) (Table 2, Figure 1). In these regions, FHm had on average 15% reduced GMV compared to FH- (ranging from 13% in superior temporal to 16% in inferior frontal gyrus), and 15% reduced GMV in precuneus compared to FHp (Figure 2).
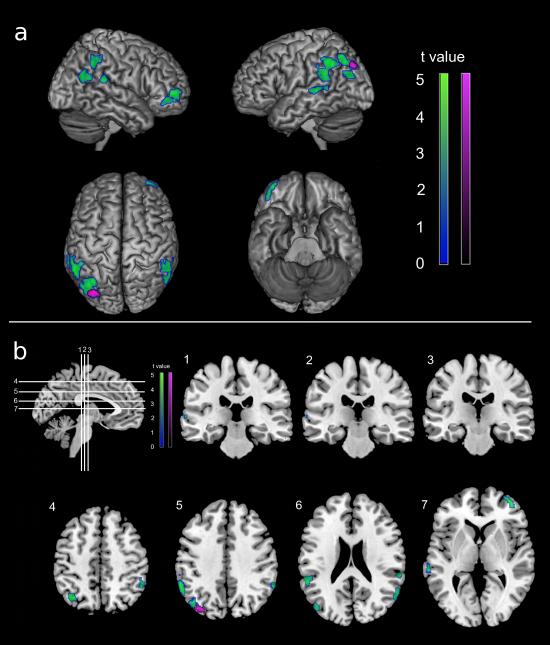
Figure 1.
Statistical parametric maps showing GMV reductions in NL FHm compared to FH- (in blue-green) and FHp (in violet), after correcting for total intracranial volume. Results are shown at P<0.05, FWE-corrected.
a) Areas of GMV reductions are displayed onto the left and right lateral, superior and inferior views of a standardized, spatially normalized, 3D-rendered MRI.
b) Areas of GMV reductions are displayed on coronal (1-3) and axial (4-7) slices of a standardized, spatially normalized MRI.
Figure 2.
Quantitative GMV differences in FH groups.
a. Percent difference in GMV in NL FHm vs FH- (in blue-green) and in FHm vs FHp (in violet) within the brain regions showing significant group effects at P<0.05, FWE-corrected, as depicted in Figure 1b and listed in Table 2. Areas of % difference in GMV across FH groups are displayed on axial (1-4) slices of a standardized, spatially normalized MRI.
b. Selected volume parameter estimates from VBM analyses. The graphs show the corresponding parameter estimates for key regions identified in Figure 1 and listed in Table 2, representing group differences in GMV. Error bars are SEM. GMV was significantly reduced in NL FHm compared to both FHp and FH- in the precuneus (A), and compared to FH- in inferior parietal lobule (B), superior temporal gyrus (C) and inferior frontal gyrus (D).
FHp subjects showed no regions of reduced GMV as compared to FH- and to FHm. No GMV decreases were found in FH- subjects compared to FHm or FHp subjects. All results remained significant by further controlling for age, gender, education, and ApoE genotype (P<0.05, FWE-corrected).
On secondary analysis of gender effects, there were no regions showing GMV differences between males and females for the entire group. When the analysis was restricted to gender groups, there were no clusters that showed FH effects after correction for multiple comparisons. For exploratory purposes and because of the smaller number of subjects per gender group than in the entire sample (Table 1), we repeated the analysis at P<0.001, uncorrected, and found that the FHm group still showed GMV reductions compared with FH- and FHp in areas overlapping with the larger analysis of FH effects. Among females, FHm showed GMV reductions in precuneus and temporal cortex compared to FH- and FHp, and additionally in parietal, temporal, and frontal areas compared to FH-. Among males, FHm showed reduced GMV in temporal cortex and precuneus compared to FH- and FHp. Overall, GMV were reduced in FHm irrespective of subjects’ gender, as shown in Supplemental Figure 1.
4. Discussion
The present VBM MRI study shows GMV reductions in AD-vulnerable regions of adult children of parents affected with LOAD, which were related to the affected parent's gender. Specifically, NL FHm showed reduced GMV in AD-vulnerable brain regions as compared to FH- and FHp subjects of similar demographic charateristics. Specifically, NL FHm showed reduced GMV in the precuneus compared to both FH- and FHp groups, and additional regional GMV reductions in frontal and parieto-temporal cortices compared to FH-. In contrast, there were no significant differences between FHp and FH.
Previous MRI studies have shown that precuneus/posterior cingulate, parieto-temporal and frontal regions are affected in AD patients compared to controls (Convit et al., 2000; De Toledo-Morrell et al., 1997; Hirata et al., 2005). Reduced GMV in these AD-vulnerable regions is associated with increased risk for developing AD among NL individuals (Chen et al., 2007; Chetelat et al., 2005; Hamalainen et al., 2007; Jack et al., 2005; Reiman et al., 1998; Schott et al., 2003). A characteristic pattern of temporo-parietal atrophy was observed in presymptomatic individuals carrying genetic mutations responsible for early-onset familial AD (Ginestroni et al., 2009; Schott et al., 2003), as well as in NL carriers of the ApoE-4 allele, a major genetic risk factor for LOAD (Chen et al., 2007; Reiman et al., 1998). Previous studies have shown volume reductions of 15-20% in AD-vulnerable regions of MCI patients and individuals with subjective memory complaints compared to controls, which have been interepreted as a possible biological correlate of clinically increased risk for AD in these individuals (Holland et al., 2009; Jessen et al., 2006; Besga et al., 2010). GMV in AD-regions of our NL FHm were reduced by up to 16% relative to FH- and 15% relative to FHp, possibly reflecting increased risk for LOAD.
Overall, our data are consistent with volumetric studies characterizing regions vulnerable to progressive atrophy in the earliest stages of AD (Karas et al., 2007; Desikan et al., 2010). Atrophy in cortical parieto-temporal and precuneus/posterior cingulate areas accurately predicted decline from mild cognitive impairment (MCI) to AD (Chetelat et al., 2005; Hamalainen et al., 2007), as recently confirmed in several analyses of the Alzheimer Disease Neuroimaging Initiative (ADNI) cohort (Risacher et al., 2010; Querbes et al., 2009). Together with previous findings, our results of GMV reductions in AD-regions of NL FHm compared to FH- and FHp suggest that having a mother affected by LOAD may be associated with increased risk for preclinical brain volume loss, and associated risk for developing AD, compared to having a father with LOAD. Longitudinal studies are needed to determine whether the observed GMV abnormalities in NL FHm are predictive of future cognitive impairment.
Our data are consistent with a recent MRI study showing more pronounced GMV reductions in AD-regions of NL FHm compared to FH- and FHp (Honea et al., 2010). However, the previous study found additional GMV reductions in FHp compared to FH- in frontal regions and precuneus (Honea et al., 2010). In our study, NL FHp had no regional GMV reductions compared to FH-. A relevant difference between the two studies is that our NL subjects were on average 11 years younger than those in the previous study (mean age 63 vs 74 years). Therefore, the GMV reductions in our younger cohort likely reflect structural changes antecedent to those reported by Honea et al. (2010). Additionally, while the previous study was based on retrospective examination of relatively small FH groups of different size (i.e., 8 FHp, 16 FHm and 43 controls) (Honea et al., 2010), our study focused on 3 groups of 20 subjects each, matched by age, gender and education. This procedure was chosen to minimize possible confounding effects of age and other factors on detection of GMV group differences. Finally, in the previous paper, a high proportion of FHm subjects (63%) were ApoE-4 carriers, while the ApoE-4 genotype was found in only 25% of FHp and FH- (Honea et al., 2010). Given the well established relationship between ApoE-4 genotype and increased brain atrophy (Chen et al., 2007; Reiman et al., 1998), studies with a comparable distribution of ApoE-4 carriers across groups are better suited to rule out possible ApoE-4 effects on GMV group differences. In our study, the frequency of ApoE-4 carriers was not different across FH groups, and only 30% of our subjects were ApoE-4 carriers (25% FH-, 35% FHp and 30% FHm, see Table 1).
Collectively, the results from both studies suggest that NL FHm may develop significant GMV reductions in AD-regions when they are in their early 60's, while FHp may develop significant structural changes at an older age. Given the known relationship between brain atrophy and age of onset in AD (Convit et al., 2000), these data suggest that the pathological AD process leading to neuronal loss and subsequent brain atrophy evolves at a younger age in FHm than in FHp individuals, and is relatively independent of other possible risk factors for LOAD such as age, gender, education and ApoE status. While FHm showed widespread GMV reductions compared to FH-, involving the precuneus and the parieto-temporal cortices, only the precuneus showed atrophic changes in FHm compared to FHp. This data suggests that FHp may be showing early signs of atrophy, not yet severe enough to reach statistical significance in our cohort. However, cross-sectional studies do not provide direct evidence of volumetric shrinkage or reductions over time, but rather show relative differences across groups at a given time point, limiting potential for drawing inferences on longitudinal progression of brain damage. A recent longitudinal MRI study in over 200 MCI patients provided evidence for greater atrophy rates in MCI with a maternal history of dementia than in those with a paternal or negative family history (Andrawis et al., 2010). Other longitudinal studies are needed to establish whether the observed cross-sectional GMV reductions in NL FHm are progressive, and correlate with the AD process.
Present findings of increased risk in NL FHm than FHp are consistent with epidemiological data showing that maternal transmission of LOAD, besides being more frequent than paternal transmission (Edland et al., 1996), is associated with higher risk of developing the disease, poorer cognitive performance and a more predictable age at onset in the offspring (Debette et al., 2009; Gomez-Tortosa et al., 2007; Silverman et al., 2005).
We previously used FDG-PET to investigate differences in CMRglc in NL individuals with similar FH and demographic characteristics to those in the current study. FDG-PET data showed that NL FHm had progressive CMRglc reductions in AD-regions compared with FHp and FH-, whereas NL FHp did not show hypometabolic changes (Mosconi et al., 2007, 2009). Additionally, on N-methyl-[11C]2-(4’-methylaminophenyl)-6-hydroxybenzothiazole (Pittsburgh Compound-B, PIB)-PET imaging, NL FHm showed increased amyloid-beta (Aβ) burden, a hallmark of AD pathology, compared to FHp and controls (Mosconi et al., 2010a). Together with previous studies, the present MRI findings of selective GMV reductions in FHm compared to FHp and FH- indicate that NL FHm may be a group of individuals at particularly high risk for LOAD by virtue of developing brain hypometabolism, atrophy and Aβ pathology prior to their age-matched peers with different family histories. Longitudinal studies are needed to determine whether the observed preclinical GMV abnormalities in NL FHm are predictive of future cognitive impairment.
While not discounting the influence of possible environmental variables, our data suggests the presence of genetic, maternally inherited risk factors that predispose the offspring of LOAD-affected mothers to AD-like brain changes. Among possible explanatory genetic mechanisms, evidence for an inherited predisposition to brain metabolic reductions and atrophy in FHm suggests alterations of mitochondrial DNA (mtDNA), which is entirely maternally inherited in humans (Lin and Beal, 2006). Defective mtDNA would lead to mitochondrial dysfunction, increased oxidative damage, reduced metabolism, and changes in calcium homeostasis, which ultimately trigger neuronal apoptosis and subsequent tissue atrophy (Lin and Beal, 2006; Swerdlow and Khan, 2004). The role of maternally inherited mitochondrial genome in the pathogenesis of AD needs further clarification, and future studies are needed to unravel the molecular mechanisms involved in parental transmission of structural and functional damage in LOAD (for review, see Mosconi et al., 2010b).
Due to unequal sample sizes and an underpowered sample when split, there were not enough subjects to specifically test for interactions between gender and FH status. Nonetheless, analysis of FH effects within gender groups confirmed results from the entire sample by showing GMV reductions in FHm compared with FH- and FHp in areas overlapping with the larger analysis of FH effects. Overall, in the current study, we both controlled for gender and analyzed gender groups separately, and demonstrated FHm-related GMV reductions are present in females as well as males (Supplemental figure 1). Lack of gender effects on brain GMV reductions is consistent with transmission via mitochondrial as well as imprinted genes, which are transmitted equally to male and female offspring. Other studies with larger samples are necessary to replicate these preliminary data by specifically examining the interaction between FH and gender on brain structure and function, as presence of gender effects in conjunction with FHm status would unravel important insights on the genetic mechanisms involved in maternal transmission of LOAD.
Our determination of FH was performed in the absence of neuropathological confirmation. However, diagnoses were based on established clinical diagnostic criteria for AD (McKhann et al., 1984), and questionnaires used to elicit FH information are known to have good agreement with clinical and neuropathological findings (Kawas et al., 1994). Nonetheless, our FH cohort may have included subjects whose parents did not have AD but another dementia, leading to erroneous inclusion of subjects in FH groups, which would conservatively reduce the power to detect group differences.
In the present study, we used VBM as implemented in SPM’8 to examine differences in GMV across FH groups. There are limitations to the use of VBM. First, VBM involves numerous pre-processing steps, which may reduce sensitivity to reveal subtle GMV differences within our group of NL subjects. Second, while the regional pattern of GMV reductions observed in our subjects is consistent with the known distribution of AD-related cortical atrophy, we did not observe GMV reductions in the medial temporal lobes (MTL) in FHm, consistent with a previous VBM study in older NL FHm (Honea et al 2009). The MTL are an early site of neurodegeneration and of atrophic changes in AD (Chetelat et al., 2005; De Leon et al., 1993). The majority of MRI studies that reported MTL atrophy in AD used a region of interest (ROI) approach, which is more anatomically accurate than VBM, and therefore more sensitive for detection of volume changes in small brain structures like the MTL. The MTL have a complex shape and undergo severe morphological changes during AD, which make this structure highly variable between subjects (Whitwell and Jack, 2005). MRI studies using ROI are needed to examine whether NL FHm have MTL GMV reductions compared to the other groups. Finally, results were assessed after FWE correction for multiple comparisons, which is a well established procedure in VBM, but may be over-conservative for analysis of late-middle-aged NL individuals. Future studies are needed to compare VBM to other image analysis techniques to fully characterize the extent of GMV reductions and atrophy in NL with a family history of LOAD.
Present results were independent of ApoE genotype, and only 30% of our subjects were ApoE-4 carriers, indicating that other factors contribute to the etiology and phenotypic expression of disease. As previous reports showed reduced GMV in non-demented ApoE-4 carriers (Chen et al., 2007; Debette et al., 2009; Reiman et al., 1998), other studies with larger samples are needed to test for interaction between FH and ApoE status, and determine whether FHm ApoE-4 carriers show more severe atrophy than the other groups.
In conclusion, this study shows that late-middle-aged NL FHm show GMV reductions in AD-affected regions compared to FH- and FHp. Our findings indicate that maternal transmission may lead to increased risk for LOAD than paternal transmission, support the use of regional brain atrophy as a preclinical biomarker for LOAD among at risk individuals, and may motivate further epidemiology and genetic research on parent of origin effects in LOAD.
Supplementary Material
5. Acknowledgments
This study was supported by NIH-NIA AG13616, AG12101, AG08051, AG022374, NIH-NCRR MO1RR0096, the Alzheimer's Association, and an Anonymous Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.1. Disclosure statement
Drs. Berti, Murray, Pupi, and Tsui have no actual or potential conflicts of interest. Drs. Mosconi, Li and de Leon have received compensation for consulting services from Abiant Imaging Inc. Dr. Glodzik is PI on an investigator initiated clinical trial supported by Forrest Labs. Dr. de Leon has received honoraria from the French Alzheimer's Foundation, and is PI on an investigator initiated clinical trial supported by Neuroptix. Dr. De Santi is an employee at Bayer Healthcare Pharmaceuticals.
6. References
- Andrawis JP, Hwang KS, Green AE, Kotlerman J, Elashoff D, Morra JH, Cummings JL, Toga AW, Thompson PM, Apostolova LG. Effects of ApoE4 and maternal history of dementia on hippocampal atrophy. Neurobiol. Aging. 2010 Sep 10; doi: 10.1016/j.neurobiolaging.2010.07.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Besga A, Ortiz L, Fernández A, Maestu F, Arrazola J, Gil-Gregorio P, Fuentes M, Ortiz T. Structural and functional patterns in healthy aging, mild cognitive impairment, and Alzheimer disease. Alzheimer. Dis. Assoc. Disord. 2010;24:1–10. doi: 10.1097/WAD.0b013e3181aba730. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(Suppl 1) [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Caselli RJ, Gerkin R, Bandy D, Domb A, Osborne D, Fox N, Crum VN, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am. J. Psychiatry. 2007;164:916–921. doi: 10.1176/ajp.2007.164.6.916. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Zenge F, Viader F, De La Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: A longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Convit A, De Asis J, De Leon MJ, Tarshish C, De Santi S, Rusinek H. Atrophy of the Medial Occipitotemporal, Inferior, and Middle Temporal Gyri in non-demented elderly predict decline to Alzheimer's Disease. Neurobiol. Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Farrer LA, Sadovnik AD, Relkin N, Whitehouse P, Green P. Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: The REVEAL study. Genet. Med. 2004;6:192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- De Leon MJ, Golomb J, Convit A, De Santi S, McRae TD, George AE. Measurement of medial temporal lobe atrophy in diagnosis of Alzheimer's disease. Lancet. 1993;341:125–126. doi: 10.1016/0140-6736(93)92610-6. [DOI] [PubMed] [Google Scholar]
- De Santi S, Pirraglia E, Barr WB, Babb J, Williams S, Rogers K, Glodzik L, Brys M, Mosconi L, Reisberg B, Ferris S, De Leon MJ. Robust and conventional neuropsychological norms: Diagnosis and prediction of age-related cognitive decline. Neuropsychology. 2008;22:469–484. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Toledo-Morrell L, Sullivan MP, Morrell F, Wilson RS, Bennett DA, Spencer S. Alzheimer's disease: In vivo detection of differential vulnerability of brain regions. Neurobiol. Aging. 1997;18:463–468. doi: 10.1016/s0197-4580(97)00114-0. [DOI] [PubMed] [Google Scholar]
- Debette S, Wolf PA, Beiser A, Au R, Himali JJ, Pikula A, Auerbach S, Decarli C, Seshadri S. Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology. 2009;73:2071–2078. doi: 10.1212/WNL.0b013e3181c67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Sabuncu MR, Schmansky NJ, Reuter M, Cabral HJ, Hess CP, Weiner MW, Biffi A, Anderson CD, Rosand J, Salat DH, Kemper TL, Dale AM, Sperling RA, Fischl B, Alzheimer's Disease Neuroimaging Initiative Selective disruption of the cerebral neocortex in Alzheimer's disease. PLoS One. 2010;5:e12853. doi: 10.1371/journal.pone.0012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duara R, Lopez-Alberola RF, Barker WW, Loewenstein DA, Zatinsky M, Eisdorfer CE, Weinberg GB. A comparison of familial and sporadic Alzheimer's disease. Neurology. 1993;43:1377–1384. doi: 10.1212/wnl.43.7.1377. [DOI] [PubMed] [Google Scholar]
- Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer's disease cases: evidence for maternal inheritance. Neurology. 1996;47:254–256. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- Ehrenkrantz D, Silverman JM, Smith CJ, Birstein S, Marin D, Mohs RC, Davis KL. Genetic epidemiological study of maternal and paternal transmission of Alzheimer's disease. Am. J. Med. Genet. 1999;88:378–382. doi: 10.1002/(sici)1096-8628(19990820)88:4<378::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- George AE, De Leon MJ, Kalnin A, Rosner L, Goodgold A, Chase N. Leukoencephalopathy in normal and pathologic aging: 2. MRI and brain lucencies. Am. J. Neuroradiol. 1986;7:567–570. [PMC free article] [PubMed] [Google Scholar]
- Ginestroni A, Battaglini M, Della Nave R, Moretti M, Tessa C, Giannelli M, Caffarra P, Nacmias B, Bessi V, Sorbi S, Bracco L, De Stefano N, Mascalchi M. Early structural changes in individuals at risk of familial Alzheimer's disease: a volumetry and magnetization transfer MR imaging study. J. Neurol. 2009;256:925–932. doi: 10.1007/s00415-009-5044-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Tortosa E, Barquero MS, Baron M, Sainz MJ, Manzano S, Payno M, Ros R, Almaraz C, Gomez-Garré P, Jimenez-Escrig A. Variability of age at onset in siblings with familial Alzheimer disease. Arch. Neurol. 2007;64:1743–1748. doi: 10.1001/archneur.64.12.1743. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, Williams M, Hipps Y, Graff-Radford N, Bachman D, Farrer LA, MIRAGE Study Group. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- Hamalainen A, Tervo S, Grau-Olivares M, Niskanen E, Pennanen C, Huuskonen J, Kivipelto M, Hanninen T, Tapiola M, Vanhanen M, Hallikanen M, Helkala EL, Nissinen A, Vanninen R, Soininen H. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage. 2007;37:1122–1131. doi: 10.1016/j.neuroimage.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. State-specific Projections Through 2025 of Alzheimer Disease Prevalence. Neurology. 2004;62:1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Matsuda H, Nemoto K, Ohnishi T, Hirao K, Yamashita F, Asada T, Iwabuchi S, Samejima H. Voxel-based morphometry to discriminate early Alzheimer's disease from controls. Neurosci. Lett. 2005;382:269–274. doi: 10.1016/j.neulet.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Holland D, Brewer JB, Hagler DJ, Fenema-Notestine C, Dale AM, Alzheimer's Disease Neuroimaging Initiative Subregional neuroanatomical change as a biomarker for Alzheimer's disease. Proc. Natl. Acad. Sci. 2009;106:20954–20959. doi: 10.1073/pnas.0906053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni E, Goodwin J, Burns JM. Reduced Gray Matter Volume in Normal Adults with a Maternal Family History of Alzheimer Disease. Neurology. 2010;74:113–120. doi: 10.1212/WNL.0b013e3181c918cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol. Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Karas G, Scheltens P, Rombouts S, van Schijndel R, Klein M, Jones B, van der Flier W, Vrenken H, Barkhof F. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch. Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Locke PA, Conneally PM, Tanzi RE, Gusella JF, Haines JL. Apolipoprotein E4 allele and Alzheimer disease: examination of allelic association and effect on age at onset in both early- and late-onset cases. Genet. Epidemiol. 1995;12:83–92. doi: 10.1002/gepi.1370120108. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik-Sobanska L, Pirraglia E, Tsui W, De Santi S, De Leon MJ. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc. Natl. Acad. Sci. USA. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, De Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer's. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Rinne JO, Tsui W, Berti V, Li Y, Wang H, Murray J, Scheinin N, Nagren K, Williams S, Glodzik L, De Santi S, Vallabhajosula S, De Leon MJ. Increased fibrillar amyloid-β burden in normal individuals with a family history of late-onset Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2010a;107:5949–5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, De Leon MJ. Maternal transmission of Alzheimer's disease: prodromal metabolic phenotype and the search for genes. Hum. Genomics. 2010b;4:170–193. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querbes O, Aubry F, Pariente J, Lotterie JA, Démonet JF, Duret V, Puel M, Berry I, Fort JC, Celsis P, Alzheimer's Disease Neuroimaging Initiative Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, De Leon MJ, De Santi S, Convit A, Osborne D, Weaver A, Thibodeau SN. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer's disease. Ann. Neurol. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, Harvey DJ, Jack CR, Jr., Weiner MW, Saykin AJ, Alzheimer's Disease Neuroimaging Initiative (ADNI) Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol. Aging. 2010;31:1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott JM, Fox NC, Frost C, Scahill RI, Janssen JC, Chan D, Jenkins R, Rossor MN. Assessing the onset of structural change in familial Alzheimer's disease. Ann. Neurol. 2003;53:181–188. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Ciresi G, Smith CJ, Marin DB, Schnaider-Beeri M. Variability of familial risk of Alzheimer disease across the late life span. Arch. Gen. Psychiat. 2005;62:565–573. doi: 10.1001/archpsyc.62.5.565. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med. Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: impications for londitudinal quantitative MR imaging. Am. J. Neuroradiol. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR. Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Top. Magn. Reson. Imag. 2005;16:409–425. doi: 10.1097/01.rmr.0000245457.98029.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.