Abstract
We conducted secondary analyses to determine the relationship between longstanding personality traits and risk for Alzheimer’s disease (AD) among 767 participants 72 years of age or older who were followed for more than 6 years. Personality was assessed with the NEO-FFI. We hypothesized that elevated Neuroticism, lower Openness, and lower Conscientiousness would be independently associated with risk of AD. Hypotheses were supported. The finding that AD risk is associated with elevated Neuroticism and lower Conscientiousness can be added to the accumulating literature documenting the pathogenic effects of these two traits. The link between lower Openness and AD risk is consistent with recent findings on cognitive activity and AD risk. Findings have implications for prevention research and for the conceptualization of the etiology of Alzheimer’s Disease.
Keywords: personality, Alzheimer’s Disease, Openness to Experience, prospective study, prevention
Introduction
Societal costs of Alzheimer’s disease (AD) are undeniable. Patients suffer and families are burdened by the physical, emotional, and financial demands of providing care (Monin & Schulz, 2009; Pinquart & Sorensen, 2003; Vitaliano, Zhang, & Scanlan, 2003). Risk for AD and other dementias emerges in mid-life and increases exponentially with age (Plassman, Langa, Fisher, et al., 2007; Ritchie & Lovestone, 2002). As most industrialized countries are experiencing rapid growth in the age-segment of the at-risk population, public health officials and researchers are preparing for dramatic increases in AD incidence (Cummings, Doody, & Clark, 2007). Research aimed at more precisely defining the phenotype at risk for AD can lead to insights into disease etiology that could inform the design of preventive trials (Cummings et al., 2007). This study examines the risk associated with personality traits, clusters of dispositional tendencies that capture the major axes of phenotypic behavioral variation in humans.
Over the last several years, randomized controlled trials have examined the preventive effects of an array of traditional and alternative treatments. The results have largely been disappointing. No intervention substantially mitigates risk, although some might delay disease onset or slow its course (Breitner, 2007; Cummings et al., 2007). A benefit of the existing controlled trials is their capacity to yield insights about risk factors, including personality traits, for AD in a cohort of individuals who are likely to enter a trial or seek preventive treatment. Prior research on personality and risk for cognitive impairment (Crowe, Andel, Pedersen, & Gatz, 2007), dementia (Persson, Berg, Nilsson, & Svanborg, 1991; Wang, Karp, Herlitz, Crowe, Kareholt, Winblad, & Fratiglioni, 2009), and AD (Wilson, Arnold, Schneider, Kelly, Tang, & Bennett, 2006; Wilson, Barnes, Bennett, Li, Bienias, Mendes de Leon, & Evans, 2005; Wilson, Evans, Bienias, Mendes de Leon, Schneider, & Bennett, 2003; Wilson, Schneider, Arnold, Bienias, & Bennett, 2007) has yielded some promising leads. All domains of the Five Factor Taxonomy (FFT; Digman, 1990; Goldberg, 1993; McRae & Costa, 1997) were examined in only one study (Wilson et al., 2007b). Its sample was constituted solely of Catholic nuns, brothers, and priests, a relatively homogeneous group both with respect to education and lifestyle. Given the putative role in AD risk of education (Gatz et al., 2007; Stern, 2006) and lifestyle (e.g., Fritsch, Smyth, Debanne, Petot, & Friedland, 2005; Gatz, Prescott, & Pedersen, 2006; Wang et al., 2009), particularly cognitive activities (Crowe, Andel, Pedersen, Johansson, & Gatz, 2003; Hertzog, Kramer, Wilson, & Lindenberger, 2009; Wilson, Scherr, Schneider, Li, & Bennett, 2007a), the authors called for research in more representative, less homogeneous cohorts (Wilson et al., 2007b).
In the present paper, we sought to determine the relationship between longstanding personality traits and AD risk. We report secondary analyses of data collected on a sample of participants enrolled in the Ginkgo Evaluation of Memory (GEM) study, a randomized placebo-controlled trial of the effect of Ginkgo biloba in the prevention of dementia in individuals aged 72 and older with normal cognition or mild cognitive impairment (Dekosky et al., 2008). Although the trial revealed no impact of Gingko biloba impact on dementia risk (Dekoskey et al., 2008), its sampling strategy and inclusion of a comprehensive assessment of personality makes it particularly well-suited to examine the influence on AD risk of personality traits.
The Five Factor Taxonomy (FFT) as a Hypothesis-Testing and Hypothesis-Generating Tool
Based on seven decades of factor-analytic research on personality in the natural lexicon and questionnaires, there is considerable (Costa & McCrae, 1995; Digman, 1990; John & Srivastava, 1999; McCrae & Costa, 1997), but not complete (Block, 1995; Cloninger, Svrakic, & Przybeck, 1993; Tellegen, 1985), agreement that personality traits can be grouped along five major domains: Neuroticism, Extraversion, Openness to Experience, Agreeableness, and Conscientiousness. (Definitions are provided in the next section.) The FFT specifies the minimum number of traits required for a comprehensive yet parsimonious description of personality phenotypes across cultures (McCrae & Costa, 1997) using natural, everyday language. Traits are generally stable across the adult lifespan (Allemand, Zimprich, & Hertzog, 2007; Roberts & DelVecchio, 2000).
Personality traits are believed to have biological bases (McCrae et al., 2000), reflecting underlying neurophysiological processes, some of which are themselves mediated by genetic processes The anlage of adult personality is evident in infant behavior and temperament (Evans & Rothbart, 2006; Kagan, 1994; McCrae et al., 2000)–individual differences in emotional, motor, and attentional reactivity that reflect underlying neural processes (Rothbart, 2007). Given substantial evidence for associations between personality domains and neurophysiologic processes involved in the body’s response to perturbations (Eysenck, 1990; Segerstrom, 2000), relations between personality and diseases involving personality-linked neurophysiologic processes are to be expected. As a consequence of proactive interaction (Caspi & Bem, 1990) traits are also associated with environmental exposures. Some people, on account of underlying traits may be more likely to select themselves into environments that provide greater opportunity for healthful behavior or cognitive stimulation, decreasing risk for certain diseases. Beyond conferring risk for AD, traits may also influence its symptomatic manifestation. AD frequently involves behavioral changes, some of which have been construed as personality alterations (Balsis, Carpenter, & Storandt, 2005; Chatterjee, Strauss, Smyth, & Whitehouse, 1992; Ducheck, Balota, Storandt, Larsen, 2007; Siegler et al., 1991; Strauss, Pasupathi, & Chatterjee, 1993). It is likely that these behavioral changes are influenced by premorbid traits, reflecting pathoplastic processes (Widiger, Verheul, & van den Brink, 1999). In other words, premorbid personality might affect the symptomatic expression of the disease, including behavioral changes. To date, the most rigorous study on this issue suggests that changes in Neuroticism and Conscientiousness constitute preclinical signs of disease expression in AD (Ducheck et al., 2007).
Despite strong associations between personality and health (Bogg & Roberts, 2004; Kern & Friedman, 2008; Smith & Spiro, 2000), surprisingly few epidemiologic studies include personality assessments. Assessing personality in epidemiologic research confers two scientific advantages. First, statistical adjustment for personality traits in the epidemiologic context yields more precise risk estimates for other so-called risk factors that may be related to personality (e.g., depression, cognition). Second, studying the risk conferred by personality in its own right can yield insights about the etiology of disease and social pathology (Krueger et al., 2001).
The assessment of personality in the GEM trial enables both hypothesis-testing research and hypothesis generation concerning risk for AD. The capacity to generate hypotheses is particularly important when conducting research in areas that have been relatively underinvestigated or about which there is relatively little theorizing. Few theories of AD pay heed to personality traits. Use of an omnibus personality questionnaire grounded in the FFT increases the likelihood that traits central to AD risk are not overlooked. Despite suggestive research that began more than two decades ago (e.g., Petry, Cummings, Hill, & Shapira, 1988), the established role of Openness to Experience in cognitive function (McCrae, 1993/1994), and the replicated finding that AD risk is tied to lower levels of education and cognitive activity (Gatz et al., 2007; Stern, 2006; Hertzog et al., 2009), only one study has examined whether lower levels of Openness confer risk (Wilson et al., 2007b). The authors reported lower levels of Openness scores in subjects who later developed AD, but multivariate analyses revealed that the independent effect of Openness on AD risk was not significant. This does not mean that lower Openness was not associated with AD risk, just that the effect was no longer statistically significant in the presence of other variables, some of which may have been mediators.
An important advantage of the FFT in risk factor research is that it provides a fixed reference point from which to assess a variety of different scales (Costa & McCrae, 1992; Marshall, Wortman, Vickers, Kusulas, & Hervig, 1994). It therefore overcomes a vexing problem in personality research: scales with different labels measure the same personality construct, while those with the same label measure different personality constructs. The provision of a fixed reference point is also advantageous in epidemiologic research. Many psychosocial “risk factors” --- social support (Pierce, Lakey, Sarason, & Sarason, 1997), educational and occupational attainment (Borghans, Duckworth, Heckman, & ter Weel, 2008; Roberts, Kuncel, Shiner, Caspi, & Goldberg, 2007) and health-relevant lifestyle variables (Bogg & Roberts, 2004)—are inextricably related to personality. Often, personality and its underlying neural and physiologic processes (Cacioppo, 1993; Rothbart, 2007) precede the psychosocial risk factors investigated in risk factor epidemiology. Some so-called psychosocial risk factors studied in the epidemiologic context could thus be construed as proxy indicators or epiphenomena of personality.
We would be remiss if we did not acknowledge that the FFT has its detractors (Kagan, 1994; McAdams, 1994; Block, 1995). Block (1995) critiqued the high intercorrelations among the ostensibly uncorrelated five factors, a point raised by others (Donellan, Oswald, Baird, & Lucas, 2006). In the present study, we conducted analyses designed to determine whether simultaneous entry of all five traits in the regression equation yields findings that are comparable to those observed when only one trait is entered.
Rationale for Specific Hypotheses
No theory makes explicit predictions about the contributions to AD risk of each of the five trait domains. Our hypotheses were based primarily on current knowledge of the five trait domains and prior studies of personality and dementia (Persson et al., 1991; Wang et al., 2009), cognitive impairment (Crowe et al., 2007) and AD (Wilson et al., 2003; 2005a; 2007). Given that personality is known to influence lifestyle, prior research on lifestyle factors and AD (Fritsch et al., 2005; Gatz et al., 2006; Wang et al., 2009) also informed our hypotheses.
Neuroticism
Neuroticism is characterized by the tendency to experience distress and anxiety, along with difficulty managing stress and controlling impulses (Costa & McCrae, 1992). It is rooted in the basic temperament dimension encompassing negative affectivity (Eysenck, 1960) and is marked by elevated autonomic reactivity and dysregulations in the hypothalamic-pituitary-adrenal (HPA) axis (Mangold & Wand, 2006). Neuroticism has also been associated with inflammatory activity (Bouhys, Flentge, Oldehinkel, & van den Berg, 2004), which is believed to be associated with risk for depression and chronic diseases of aging, including AD risk (Papassotiropoulos, Hock, & Nitsch, 2001). Over the course of a lifetime, chronic dysregulations in the HPA axis systems could produce hippocampal atrophy (Sapolsky, Krey, & McEwen, 1986), leading to cognitive decline and the erosion of episodic memory (Wilson, Bennett, Mendes de Leon, Bienias, Morris, & Evans, 2005b). Neuroticism scores in older adults could be viewed as indicators of the brain’s cumulative exposure to the stress response (Wilson et sl., 2006). The cumulative toll of chronic HPA-axis dysregulation may also include greater overall disease burden (McEwen, 1999).
The idea that higher Neuroticism increases AD risk even after accounting for covariates has been directly supported in prospective studies (Wilson et al. 2003; 2005a; 2007) and indirectly supported in others (Crowe et al., 2007; Wang et al., 2009). We hypothesize that participants with elevated levels of Neuroticism would be more likely to develop AD over the follow-up period, even after accounting for all covariates.
Extraversion
Extraversion refers to preferences for social interaction and the tendency to experience positive emotion (Costa & McCrae, 1992). It is rooted in the basic temperament dimension encompassing activity, positive anticipation, and pursuit of pleasure; its converse is behavioural inhibition (Eysenck, 1960; Kagan, 1994; Rothbart, 2007). Low Extraversion in adults has been empirically associated with poor social support (Krause, Liang, & Keith, 1990; Von Dras & Siegler, 1997), a characteristic presumed to be associated with AD risk (Seidler, Bernhardt, Nienhaus, & Frölich, 2003). There is some evidence for the putative role of Extraversion in AD (Crowe et al., 2006; Wang et al., 2009; Wilson et al., 2007). The most direct evidence is provided by Wilson et al. (2007) who reported that participants who developed AD scored lower on Extraversion; however, Extraversion did not emerge as an independent predictor of AD risk in multivariate analyses, perhaps because of its associations with Neuroticism or Conscientiousness. We expected the same pattern: Extraversion will be associated with AD risk in analyses that controlled solely for demographics, but not after controlling for important covariates.
Openness to Experience
Most languages, including English, do not have simple terms that connote Openness to Experience. Single-term synonyms are nonexistent (McCrae, 1990); definitions cannot be pithy. Open people have an interest in the pursuit of novelty -- ideas, art, fantasy, emotions, and sensations. They tend to be cognitively flexible, score higher on intelligence tests, and pursue higher levels of education (McCrae, 1993/1994). Open people are characterized by higher levels of attunement to surroundings (Evans & Rothbart, 2006), are more likely to become absorbed in activities, and more likely to perceive stimuli at comparatively low levels of intensity. Openness may lead to lifelong patterns of cognitive activity that have been shown to be associated with decreasing dementia risk (Andel, Kareholt, Parker, Thorslund, & Gatz, 2007; Wilson, Scherr, Schneider, Li, & Bennett, 2007; Hertzog et al., 2009), possibly because it is associated with greater cognitive reserve (Boyle, Wilson, Schneider, Bienias, & Bennett, 2008; Stern, 2006; Tucker-Drob, Johnson, & Jones, 2009).
Just as Neuroticism scores in older adults could be conceptualized as indicators of cumulative exposure to stress and stress hormones, Openness scores in a cohort of older adults may be viewed as proxy indicators for the brain’s capacity to exercise and cumulative exposure to cognitive activity and associated neurophysiologic processes. Indirect evidence for the role of Openness in AD risk comes from relatively early studies using caregiver ratings of personality (Chatterjee, Strauss, Smyth, & Whitehouse, 1992; Strauss, Pasupathi, & Chatterjee, 1993; Siegler, Dawson, & Welsh, 1994; Siegler et al., 1991). These studies showed that people who develop Alzheimer’s disease are perceived to be low in Openness prior to disease onset. Wilson et al. (2007b) reported that participants who developed AD scored lower on Openness, but multivariate analyses suggested that Openness is not an independent predictor of disease risk. Assuming that the effect of Openness on AD risk is driven in part by lifestyle- or education-related cognitive activity, the homogeneity of that sample may have obscured its effect. We hypothesized that, in this presumably more heterogeneous sample, Openness will emerge as a significant predictor of AD risk both in unadjusted analyses and after controlling for important covariates.
Conscientiousness
Conscientiousness refers to the capacity to plan ahead, delay gratification, and work steadfastly toward attaining goals (Costa & McCrae, 1992). It is rooted in the basic temperament dimension encompassing effortful control, attentional focusing, the capacity to shift attention when desired, plan future actions, and suppress inappropriate responses (Rothbart, 2006). Conscientious people are focused, task-oriented, reliable, and dependable. A large body of research has now established that Conscientious people live longer (Kern & Friedman, 2008) and are more likely to engage in health-promoting behaviors (Bogg & Roberts, 2004). It is generally believed that their longevity can be explained by their dutiful and focused adherence to prescribed medical and preventive (e.g., exercise, diet) regimen, but there are no definitive supporting studies. Indeed, prior research suggests that health behaviors cannot fully explain the association between Conscientiousness and health (Lodi-Smith et al., in press) or longevity (Martin, 2007).
Wilson et al. (2007) reported that lower Conscientiousness increases AD risk, even after accounting for covariates. We hypothesized that participants with lower levels of Conscientiousness would be more likely to develop AD over the follow-up period, even after accounting for relevant covariates.
Agreeableness
Agreeableness has not been found to be associated with increased risk of other diseases, though it has been associated with smoking (Terraciano & Costa, 2004). Only one study has reported data bearing on Agreeableness and AD risk (Wilson et al., 2007). Wilson et al.’s (2007) bivariate analyses suggested that people who developed AD were lower in Agreeableness, but Agreebleness did not emerge as an independent predictor in multivariate analyses. We expected the same. There is no a priori reason to expect an independent association between Agreeableness and Alzheimer’s Disease.
Conceptual Approach to Covariate Coverage
Our goal was to examine the relationship between personality traits and AD risk; no attempt was made to build a comprehensive risk model for AD. In considering the effects of personality on disease risk, it is important to adjust for demographic correlates of AD that are unlikely to be associated with personality. Given age and gender differences in risk for AD (Cummings et al., 2007), our base model adjusted for these variables. We adjusted for race (non-Hispanic white vs. other) because there is some evidence of race differences in dementia risk (Husaini, Sherkat, Moonis, Levine, Holzer, & Cain, 2003; Plassman, Langa, Fisher, et al., 2007). We conservatively adjusted for education in the base model even though educational attainment may reflect underlying personality traits (Borghans et al., 2008; Roberts et al., 2007), particularly Openness to Experience. To determine whether personality traits are independently associated with AD, we controlled for baseline (time invariant) levels of other known correlates of AD (Brommelhoff et al., 2009; Cummings et al., 2007) including depressive symptoms, cognitive function, and common chronic diseases of aging (e.g., hypertension, diabetes, osteoporosis). These variables were construed not as causal mediators but as potential confounders that could affect both the assessment of personality at baseline as well as risk for AD. As adjustment for these potential influences could lead to an underestimate of personality’s contribution to AD risk, we also examined the influence of personality without covariate adjustment.
Summary of Hypotheses
Based on prior AD research and personality theory, we hypothesized that higher Neuroticism and lower Extraversion, Conscientiousness, and Openness would be independently associated with the onset of Alzheimer’s disease over the roughly six year study period after controlling for key demographic covariates. We further hypothesized that only higher Neuroticism and lower Openness and Conscientiousness would emerge as significant predictors in multivariate analyses that controlled for the covariates that were conceptualized as potential confounders. With respect to the comparability of findings when only one trait is entered vs. simultaneous entry of all five traits, we anticipated that findings for Openness would be relatively unaffected by simultaneous entry. We based this hypothesis on prior research that has shown that the other four traits form a non-specific distressed personality type (Chapman, Duberstein, & Lyness, 2007), suggesting that, beyond certain pathogenic thresholds, they are more intercorrelated with each other than with Openness. Given the paucity of prior research, we did not offer an a priori hypothesis about whether Neuroticism would be more affected than Conscientiousness by inclusion of other traits in the model.
Methods
Sample
Details of the Ginkgo Evaluation of Memory (GEM) study have been published elsewhere (Dekosky et al., 2006; 2008). The study was conducted under an investigational new drug application with the Food and Drug Administration under the auspices of the National Center for Complementary and Alternative Medicine (NCCAM) and the National Institute on Aging (NIA) and registered at clinicaltrials.gov (identifier number NCT00010803). Eligible subjects for the trial were recruited at 4 sites between 2000 and 2002. Volunteers were recruited using voter registration records and purchased mailing lists. For this prospective cohort sub-study, subjects were enrolled exclusively at the University of California, Davis (UCD) site. All subjects in this sub-study consented to the additional data collection. Key enrollment criteria in GEM included: age 72 years or older, availability of a proxy respondent, English as usual language, and absence of significant morbidity. The initial age cut-off was 75, but this was modified during the course of the study to increase minority enrollment. Exclusion criteria included but were not limited to: current treatment with cholinesterase inhibitors, anti-Parkinson medications, tricyclic antidepressants, antipsychotics, or other medications with significant psychotropic or central cholinergic effects. Those who had been hospitalized for depression within the last year or received electroconvulsive therapy within the prior decade were also excluded. At the UCD site, 2234 potential subjects participated in an initial screening telephone interview; 551 (24.7%) were ineligible, 761 (34.1%) refused to participate, and 922 (41.3%) attended the baseline study visit. Of the latter, 916 (99.3%) were randomized. Subjects at the UCD site had characteristics similar to those at the other sites. Because this sub-study was initiated after the start of the main GEM study, only 767 (83.7%) of the 916 enrolled subjects completed the personality inventory by the first 6-month assessment and are included in the current analyses. Compared with those not completing the personality inventory, those completing the inventory were slightly younger (mean [SD] 78.6 [3.1] vs. 79.5 [4.0], t = 2.4, p = 0.02), as a consequence of the change in the age cut-off over the course of the study. The groups did not differ statistically by gender, race, or education level. Subjects were examined every 6 months until study conclusion, a maximum of 7.3 years (median 6.1 years).
Measures
Predictor Variables
The NEO-Five Factor Inventory (NEO-FFI; Costa & McCrae, 1992) is a 60-item self-report questionnaire with 12 items measuring each of the five domains that comprise the FFT. These domains are Neuroticism (e.g., “I often feel inferior to others”), Extraversion (e.g., “I like to have a lot of people around me”), Openness to Experience (e.g., “I have a lot of intellectual curiosity”), Agreeableness (e.g., “I try to be courteous to everyone I meet”) and Conscientiousness (e.g., “I keep my belongings clean and neat”). Response options comprise a 5-point Likert scale from Strongly Disagree to Strongly Agree. Internal consistency (Cronbach’s coefficient alpha) for the five scales ranged from .75 (Openness) to .82 (Conscientiousness) in the current study. The NEO-FFI has been validated (Costa & McCrae, 1992), and its use in gerontologic research (e.g. Duberstein et al., 2003; Hooker et al., 1998, Schmutte & Ryff, 1997) attests to its reliability and applicability to samples of older adults. For descriptive purposes, NEO-FFI scores are reported in terms of T-scores standardized to values provided in the manual (Costa & McCrae, 1992. For analytic purposes, scores were standardized so a unit change in the parameter estimate corresponded to a 1 standard deviation difference in the personality trait.
Outcome Variable
The diagnosis of dementia was established by an expert panel of clinicians using a previously validated adjudication process (Lopez, Kuller, Fitzpatrick, Ives Becker, & Beauchamp, 2003). Details of the diagnostic process have been previously published (DeKosky, et al 2008). Briefly, participants were required to complete the full GEM Study neuropsychological battery, a complete neurological exam, and undergo brain magnetic resonance imaging (MRI) or computed tomographic (CT) scan if their (1) scores declined a prespecified number of points from their entry scores on 2 of 3 cognitive tests, (2) proxy reported the onset of a new memory or other cognitive problem or (3) personal physician diagnosed dementia subsequent to the last research assessment or prescribed a medication for dementia. An expert panel blinded to treatment assignment then reviewed the results from the full GEM battery and all clinical assessments. Diagnosis of dementia was made using criteria from the DSM-IV (American Psychiatric Association, 1994). Classification of dementia type was made using the National Institute of Neurological and Communication Disorders and Stroke, Alzheimer’s Disease and Related Disorders Association (McKhann, et al., 1984), the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences (Erkinjuntti, 1994; Rockwood et al., 1994), and the Alzheimer’s Disease Diagnostic and Treatment Centers (Chui et al., 2000). All participants with dementia were assigned to 1 of the following, possible or probable: (1) Alzheimer’s dementia; (2) Alzheimer’s dementia and vascular dementia (3) vascular dementia; or (4) dementia, other etiology. These analyses examined the relationship between personality and possible or probable Alzheimer’s dementias (i.e. [1] or [2]).
Baseline Covariates
The 10-item version (Irwin, Artin, & Oxman, 1991) of the CES-D (Radloff, 1974) was used to assess depressive symptoms. The Modified Mini-Mental Status Exam [3MSE] (Teng & Chui, 1987) was used to assess cognitive function. The 3MSE expands on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1974) and offers more graded scoring. To adjust for chronic diseases of aging, dichotomous indicators were created for hypertension, diabetes, osteoporosis, cancer within the past five years, and cardio-vascular disease (includes coronary heart disease, angina, stroke, transient ischemic attack, bypass surgery, or angioplasty).
Analyses
Data were analyzed using Stata (Version 10.1, StataCorp, College Station, Texas). Key analyses were conducted using a series of Cox proportional hazards survival analyses. The end point was time to AD (date of randomization to AD onset defined as mid-point between last visit at which participant was not diagnosed with possible or probable AD and visit at which AD was diagnosed). Participants were censored at study termination, time of death, diagnosis of non-Alzheimer’s dementia, or follow-up refusal. The key independent variables were the scores on the NEO-FFI; analyses were conducted with each factor separately and with all 5 together. To facilitate interpretation, FFT factors were standardized when included in the survival analyses so a unit change in the parameter estimate corresponded to a 1 standard deviation change in the FFT factor. Our base model adjusted for age, gender, education (years of schooling, coded as <12, 12, 13-15, ≥ 16, and race). The second model added the 3MSE score to the base model, the third added the CES-D score to the base, and the fourth added the dichotomous chronic disease indicators. A fully adjusted variable added all covariates simultaneously. All analyses also adjusted for study group assignment. Graphical (log-log plots) and analytical (Schoenfeld residuals) methods were used to test the proportional hazards assumption for each analysis; the assumption was supported in every case.
A series of sensitivity and supplementary analyses were conducted to explore possible additional confounding and effect modification. Analyses also examined interactions between each of the FFT factors and other covariates in the model. Finally, analyses explored curvilinear relationships between the FFT factors and Alzheimer’s risk, by including each factor together with a squared term for each factor.
Results
The analytic sample of 767 persons completed 4410.6 person-years of follow-up with 116 (15.1%) cases of possible or probable AD. Table 1 reports descriptive and demographic statistics by final AD status. Subjects who subsequently developed AD (compared to those who did not) were older (t=7.3, p < 0.001) and were more likely to have less than 12 years or 16 or more years of schooling (chi-square = 11.5, d f = 3, p < 0.001), but did not differ statistically by gender or race. Rates of disease at study entry were as follows: hypertension (54.1%), diabetes (8.0%), osteoporosis (12.0%) cancer within the past five years (21.8%), and cardio-vascular disease (25.8%); 51.3% self-rated their health as excellent or very good (vs. good, fair or poor). Table 2 reports the descriptive statistics for the personality traits and key covariates along with the intercorrelations among variables. Those with a final diagnosis of AD compared to those without had lower initial 3MSE (mean [SD] 91.2 [4.9] vs. SD94.7 [4.1], t = 8.3, p < 0.001) and had more depressive symptoms (mean [SD] 4.9 [4.4] vs. 3.6 [3.4], t = 3.5, p < 0.001).
Table 1.
Baseline Demographic Characteristics Of Participants, By Final Dementia Status
| Overall | AD | No AD | |
|---|---|---|---|
| n=767 | N=116 | N=651 | |
| Age, years [M (S.D.)]= | 78.6 (3.1) | 80.5 (3.2) | 78.3 (3.0) |
| Male (%) | 58.1 | 57.8 | 58.1 |
| White, non-Hispanic (%) | 91.8 | 94.0 | 91.6 |
| Education, years(%)= | |||
| • <12 | 6.7 | 10.3 | 5.8 |
| • 12 | 24.0 | 19.8 | 24.9 |
| • 13-15 | 25.6 | 16.4 | 27.2 |
| • ≥ 16 | 43.7 | 53.4 | 42.1 |
Note. M = Mean; S.D. = Standard Deviation. A.D. = Alzheimer’s Disease. = Statistically significant difference. See text for details.
Table 2.
Descriptive Statistics And Intercorrelations Among Key Variables (N=767)
| M (S.D) | N | O | C | A | E | 3MSE | CES-D | |
|---|---|---|---|---|---|---|---|---|
| Neuroticism | 44.8 (8.2) | 1.00 | ||||||
| Openness | 53.6 (11.5) | -0.15 | 1.00 | |||||
| Conscientiousness | 46.7 (9.5) | -0.38 | 0.15 | 1.00 | ||||
| Agreeableness | 46.7 (9.5) | -0.35 | 0.15 | 0.27 | 1.00 | |||
| Extraversion | 49.0 (11.3) | -0.32 | 0.25 | 0.38 | 0.29 | 1.00 | ||
| 3MSE | 94.2 (4.4) | -0.05 | 0.19 | -0.05 | 0.08 | 0.05 | 1.00 | |
| CES-D-10 | 3.8 (3.6) | 0.43 | -0.06 | -0.20 | -0.15 | -0.19 | -0.12 | 1.00 |
Note. Personality traits are reported here in terms of T-scores standardized to values provided in the manual (Costa & McCrae, 1992), with a Mean of 50 and Standard Deviation of 10. In the multivariate analyses (Table 3), scores were standardized so a unit change in the parameter estimate corresponded to a 1 standard deviation difference in the personality trait score. N = Neuroticism; E = Extraversion; O = Openness to Experience; A = Agreeableness; C = Conscientiousness; 3MSE = Mini Mental Status Exam Revised, range of scores: 0 to 100; CES-D- 10 = Center for Epidemiologic Studies Depression Scale—10 item version, range of scores: 0-30. Correlations with an absolute value above 0.07 are statistically significant at p <.05 or lower.
Table 3 reports the adjusted hazard ratios (AHR) and 95% confidence intervals (CI) for possible and probable AD for each of the personality traits. Each trait is reported in two rows. The top row shows the AHR for that trait when no other personality trait is entered in the model. The bottom row shows the AHR for that trait when the other four traits are entered in the model. Of the four base model covariates (age, gender, education, and race), only age was independently associated with AD risk. Older participants [AHR (95% CI) = 1.20 (1.14, 1.26)] were at greater risk of developing AD over the follow-up period.
Table 3.
Adjusted Hazard Ratios (AHR) For Probable Or Possible Alzheimer’s Disease (N=767)
| BASE | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Predictor | Age, Gender, Race, Education | Base + 3MSE | Base + CES-D-10 | Base + Morbidity |
| N | 1.39 (1.16,1.67) | 1.49 (1.23,1.80) | 1.22 (1.00,1.50) | 1.35(1.11,1.64) |
| N-FFT | 1.30 (1.05, 1.60) | 1.34 (1.08, 1.67) | 1.14 (0.91, 1.44) | 1.25 (1.00, 1.58) |
| E | .90 (0.74 1.08) | .86 (0.70 1.05) | .95 (0.78, 1.15) | .89 (0.73, 1.09) |
| E-FFT | 1.05 (0.85, 1.30) | 1.02 (0.82, 1.27) | 1.07 (0.86, 1.32) | 1.04 (0.83, 1.31) |
| O | .78 (0.64, 0.95) | .81 (0.67, 0.98) | .80(0.66, 0.97) | .76(0.62, 0.93) |
| O-FFT | .82 (0.67, 1.00) | .87 (0.71, 1.07) | .82 (0.67,0 .99) | .79 (0.64, .98) |
| A | .86 (0.71, 1.04) | .84 (0.70, 1.01) | .91(0.75, 1.11) | .84 (0.70, 1.01) |
| A-FFT | 1.00 (0.80, 1.24) | 1.03 (0.83, 1.28) | .99 (0.80, 1.32) | .98 (0.78, 1.24) |
| C | .76 (0.63, 0.93) | .65 (0.53, 0.80) | .81 (0.66, 0.99) | .76 (0.62, 0.94) |
| C-FFT | .86 (0.69, 1.08) | .86 (0.69, 1.08) | .86 (0.69, 1.08) | .88 (0.69, 1.13) |
Note. There were 116 cases of probable or possible Alzheimer’s disease. N = Neuroticism; E = Extraversion; O = Openness to Experience; A = Agreeableness; C = Conscientiousness. For all five models, two sets of adjusted hazard ratios (AHR) are reported. For each personality trait (N, E, O, A, C) the top row (trait alone) reports the AHR when only that trait is entered. The bottom row (trait-FFT) reports the AHR for that trait when all five traits are entered simultaneously. The AHRs reflect the hazard associated with a 1 standard deviation change in the personality trait. Clinical conditions included: hypertension, diabetes, osteoporosis, cancer within the past five years, and cardio-vascular disease (includes coronary heart disease, angina, stroke, transient ischemic attack, bypass surgery, or angioplasty). All analyses also adjusted for study group assignment.
Analyses of the base model revealed that, over and above the effects of age, gender, race, and education, AD risk was greater among participants higher in Neuroticism and lower in Openness and Conscientiousness. Whereas the substantive findings for Neuroticism and Openness were unaffected by the addition of other personality traits to the model, the effect of Conscientiousness on AD risk was no longer statistically significant in the presence of other traits. The AHR (95 % CI) changed from 0.76 (0.63, 0.93) to 0.86 (0.69, 1.08).
In a model that added the 3MSE score at study entry to the base model, participants higher in Neuroticism and lower in Openness and Conscientiousness remained at greater risk. The AHRs for Neuroticism and Conscientiousness were slightly larger than corresponding figures for the base model. Whereas the substantive finding for Neuroticism was unaffected by the addition of other personality traits to the model, the effects of Openness and Conscientiousness were no longer statistically significant in the presence of other traits. People who scored lower on the 3MSE at study entry were more likely to develop AD, AHR (95%CI) = 0.84 (0.81, 0.88).
In a model that added the CES-D-10 score at study entry to the base model, higher Neuroticism and lower Openness and Conscientiousness were still associated with AD risk. The AHRs for Neuroticism and Conscientiousness were slightly smaller than the base model AHRs. Whereas the substantive finding for Openness was unaffected by the addition of other personality traits to this model, the effects of Neuroticism and Conscientiousness were no longer significant in the presence of other traits. There was a positive association between CES-D-10 scores and AD risk, though it was not as large as the effect of 3MSE [AHR (95%CI) =1.08 (1.02, 1.13)].
Adding the five morbidity indicators did not substantially alter the overall pattern of findings from the base model, but the AHRs for Neuroticism and Openness were slightly attenuated. For example, the AHR (95% CI) for the former decreased from 1.39 (1.16, 1.67) to 1.35(1.11, 1.64). Corresponding figures for the latter decreased from 0.78 (0.64, 0.95) to 0.76 (0.62, 0.93). Cardiovascular disease [AHR (95% CI) = 1.77 (1.18, 2.65)] and osteoporosis [AHR (95% CI) = 1.09 (1.00, 1.18)] were both associated with increased AD risk, but diabetes, cancer over the past 5 years, and hypertension were not.
In a model that added the disease indicators, CES-D-10, and 3MSE to the base model covariates (data not shown), the overall pattern was retained. AD risk was tied to higher Neuroticism [AHR (95% CI) =1.36 (1.08,1.71)] and lower Openness [AHR (95% CI) =.80 (0.65, 0.98)] and Conscientiousness [AHR (95% CI)=.71 (0.57, 0.89)].
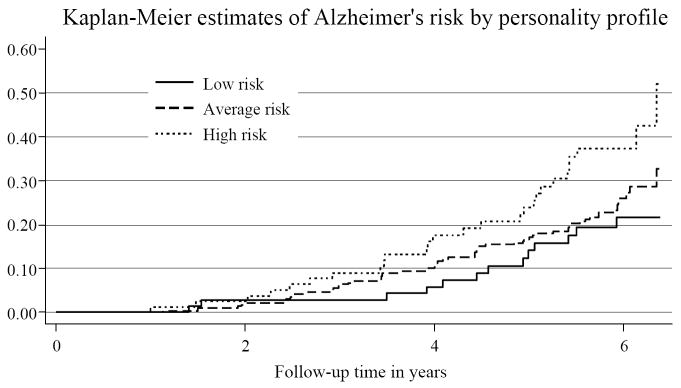
To provide a graphic illustration of the findings, three personality profiles were constructed. Figure 1 presents the Kaplan-Meier probability of developing AD over the study duration for those with differing personality profiles.
Figure 1.
Kaplan-Meier probability of developing AD over the study duration. High risk: Scored above mean on Neuroticism and below mean on Openness and Conscientiousness. Low risk: Scored below mean on Neuroticism and above mean on Openness and Conscientiousness. Average risk: the remainder of the sample. Participants in the high risk group accumulated an AD risk of greater than 50% by the end of the study.
Relative to the low risk group, the AD HR for high risk group was 2.64 (95% CI = 1.46, 4.77) and was 1.41 (95% CI = 0.83, 2.40) for the average risk group; with full adjustment (age, gender, education, CES-D, 3MSE, morbidity indicators) the respective HRs were 2.11 (95% CI = 1.07, 4.16) and 1.29 (95% CI = 0.74, 2.27).
Finally, the supplementary and sensitivity analyses revealed no significant interactions between the personality variables and any of the other covariates. There were no gender differences in the relationships between personality and AD risk. None of the squared trait terms were significant.
Discussion
We observed that persons 72 years of age or older who were higher in Neuroticism, lower in Conscientiousness, and lower in Openness were at greater risk for AD. For Neuroticism and Openness, the findings were roughly comparable with and without simultaneous entry of all five traits. The predictive value of Neuroticism was attenuated in the simultaneous entry model after accounting for depressive symptoms, and the predictive value of Openness was similarly attenuated after accounting for baseline cognitive function. The predictive value of Conscientiousness was not apparent when all five traits were entered simultaneously, suggesting that shared variance with Neuroticism may have influenced the findings. Findings for Neuroticism appear quite robust, and our data probably under-estimate its effect: Neuroticism confers risk for depression in older adults (Duberstein et al., 2008), yet patients on tricyclics or hospitalized for depression were excluded from the GEM trial. Findings for Openness are similarly robust. It was rendered nonsignificant only after statistical adjustment for the other four personality traits and a variable to which it might be causally related, baseline cognitive function. Adjustment for variables on the intervening pathway from personality to AD spuriously deflates total risk estimates for personality.
The findings for elevated Neuroticism and lower Conscientiousness can be added to the accumulating literature documenting their pathogenic effects. HPA axis dysregulation has been invoked in mechanistic explanations of the link between Neuroticism and cognitive decline (Wilson et al., 2005b), including AD (Wilson et al., 2003; 2005a; 2007). It is also likely that Neuroticism-related poor decision-making capacities (Denburg et al., 2009) and compromised self-care (diet, exercise, medication adherence) increase risk nonspecifically not only for AD but for other adverse health outcomes. Similarly, relationships between low Conscientiousness and disease risk could potentially be explained by deficits in self-care (Bogg & Roberts, 2004), but evidence for this is scant (Lodi-Smith et al., in press; Martin et al., 2007) and other pathways, including neurocognitive ones (MacDonald, 2008) could be entertained.
Novel findings concerned the role of Openness, a trait that is associated with cognitive activity and engagement (McCrae, 1993/1994). The activity does not have to be effortful or “intellectual” per se. Openness also encompasses heightened spontaneous and decidedly nonintellectual cognitive content. Fantasies, daydreams, and other cognitive activities associated with low intensity stimuli, such as experiencing visual images when one’s eyes are closed, are more common in open people (Evans & Rothbart, 2006). It is important to emphasize that Openness is associated with AD risk even after accounting for education. We controlled for education in the base model because personality does not become reasonably stable until the 4th decade (McCrae & Costa,1990; Roberts & Delvecchio, 2000), yet most people complete standard education by age 18; the few who pursue graduate education typically complete their degrees before age 30. Still, there is reason to believe that childhood and adolescent personality – perhaps especially Openness – predicts educational attainment (Borghans et al., 2008).
The Openness findings are consistent with the emerging literature on cognitive activity and AD risk (Crowe et al., 2003; Wilson et al., 2007a), but the data do not allow us to draw any inferences about whether any specific type of cognitive activity - intellectual or emotional, controlled or uncontrolled – is particularly protective. The most parsimonious hypothesis is that it is the amount of cognitive activity, rather than the particular form (daydreaming, reading, meditating, listening to music, etc.) that mediates the link between Openness and AD risk. By exposing themselves to a greater array of sundry life experiences, open people have allowed their brains to “get more exercise.” It is tempting to speculate that this greater exposure, over the course of a lifetime, contributes to the decreased risk of AD, perhaps by enhancing plasticity (Greenwood, 2007).
Implications, Caveats, Future Directions
Our findings have implications for the conceptualization of prevention and prevention trials. Whereas universal or primary prevention initiatives are not dependent on the identification of at-risk individuals, the success of secondary prevention trials depends on adequate risk-identification. One cannot demonstrate the success of a preventive treatment without first adequately defining the phenotype or identifying the proper at-risk cohort. A central task in the design of prevention trials is the identification of inclusion and exclusion criteria. Massive trials appear to “fail” largely because the risk level in the inception cohort was too low to yield a sufficient number of cases over the (often too brief) follow-up period. If risk is too low at study outset, then the probability of identifying a significant intervention effect is diminished. By more carefully defining the at-risk phenotype in future studies, prevention scientists increase the likelihood of detecting an effect when it is present. As well, such studies will be more cost-effective because prevention efforts are limited to those most likely to need them. Our findings suggest that future clinical trials on the prevention of AD might benefit by crafting inclusion criteria that consider personality traits, particularly Neuroticism, Openness, and Conscientiousness. The assessment of personality at study entry will allow researchers to enrich the inception cohort with participants who are more likely to develop AD.
With respect to the public health significance of our findings, if further research shows that the relationship between personality and AD risk is robust, then interventions could be crafted that target personality-defined risk populations. Moreover, if future research identifies modifiable mediators, then interventions could focus on particular mechanisms. Even without the identification of mediators, the applied public health significance is still potentially profound, as interventions could be offered that shape and change personality earlier in the lifecourse and thereby prevent or mitigate an array of adverse health outcomes (Borghans, Duckworth, Heckman, & ter Weel, 2008). Although the findings do not have immediate, practical implications for the contemporary diagnostic process, they may have indirect consequences to the extent that cognitive change is predictable from baseline personality or personality change (e.g., Duchek et al., 2007).
Our goal was to document the presence and strength of associations between personality traits and risk for AD. Influenced by epidemiologic personology (Krueger et al., 2000), our driving premise was that many of the health behaviors and lifestyle variables examined in risk factor epidemiology are themselves influenced by personality. Investigation of mediators and mechanisms of action linking AD to Neuroticism, Conscientiousness, and Openness is warranted. The effects on AD risk of low Openness may be more specific than the effects of Neuroticism and Conscientiousness; the latter two traits have been associated with a wider array of adverse health outcomes, ranging from medication nonadherence to suicide. Moreover, multiple putative pathways have been identified (Smith & Spiro, 2000). In contrast, few health effects of Openness have been documented. Given the association between this trait and cognitive function, we contend that its relationship dementia or cognitive disorders is relatively specific. If that is indeed the case, we suspect that fewer mediators will be identified. Moreover, multiple putative pathways have been identified linking these traits and health outcomes (Smith & Spiro, 2000). In contrast, few health effects of Openness have been documented. Given the association between Openness and cognitive function, we contend that its relationship to dementia or cognitive disorders is relatively specific. If that is indeed the case, we suspect that fewer mediators will be identified. Whereas physiologic, neurocognitive, behavioural, and social mechanisms (Smith & Spiro, 2000) may drive the relatively nonspecific health effects of Neuroticism and Conscientiousness, we speculate that the links between Openness and dementing disorders will be mediated primarily by neurocognitive mechanisms.
Several caveats and future research directions must be entertained before concluding. First, selective loss of subjects with specific personality profiles may have influenced the observed relationships. As both elevated Neuroticism and lower Conscientiousness are associated with mortality (Chapman, Fiscella, Kawachi, & Duberstein, 2010), healthy survivor effects would likely lead to an underestimate of the associations between these traits and dementia. Moreover, Neuroticism has also been shown to be associated with a younger age of AD onset (Archer, Brown, Reeves, Nicholas, Boothby, & Lovestone, 2009) and the youngest participants in the present study were 72 years old. Second, given that personality change (Balsis et al., 2005; Ducheck et al., 2007) may precede the clinical diagnosis of dementia, it is possible that the assessment of personality upon study entry was influenced by the presence of incipient disease. Although this might have led to pathoplastic shifts in Neuroticism and Conscientiousness at study entry, effects on Openness, if present, would be trivial (Duchek et al., 2007).
Fourth, the availability of proxy respondents was a criterion for participation in GEMS, which may have had implications for the cohort’s personality scores. For example, the Openness scores in this cohort of volunteers were slightly higher (T=54) than might have been expected (Costa & McCrae, 1992) of a sample with a mean (S.D.) age of 78.6 (3.1) years. The effects of Openness in this study may be under-estimated on account of the approach to covariate adjustment (education was included in the base model) and the sampling approach (passive recruitment strategy, need for proxy respondent). It is ironic that those who have the most to benefit from preventive agents may also be the least likely to participate in randomized trials (Cummings et al., 2007). The designs of future clinical trials must anticipate that participants nonrandomly volunteer for research. Incentives may need to be offered that are specifically tailored to those who are especially difficult to recruit into research.
In conclusion, our findings suggest that elevated Neuroticism and lower levels of Conscientiousness and Openness may be important risk markers for dementia. These findings have implications for the design of subsequent preventive trials, and for the conceptualization of the etiology of Alzheimer’s disease.
Acknowledgments
This research was supported in part by grants T32 MH073452 and K24MH072712 from the National Institute of Mental Health; by U01 AT000162 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements, and support from the National Institute on Aging, National Heart, Lung, and Blood Institute, the University of Pittsburgh Alzheimer’s Disease Research Center (P50AG05133), K08AG031328, the Roena Kulynych Center for Memory and Cognition Research, and National Institute of Neurological Disorders and Stroke. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health
APPENDIX
GEM Study Personnel
Project Office
Richard L. Nahin, PhD, MPH, Barbara C. Sorkin, PhD, National Center for Complementary and Alternative Medicine
Clinical Centers
Linda Fried, MD, MPH, Michelle Carlson, PhD, Pat Crowley, MS, Claudia Kawas, MD, Paulo Chaves, MD, Joyce Chabot, John Hopkins University; John Robbins, MD, MHS, Katherine Gundling, MD, Sharene Theroux, CCRP, Lisa Pastore, CCRP, University of California-Davis; Lewis Kuller, MD, DrPH, Roberta Moyer, CMA, Cheryl Albig, CMA, University of Pittsburgh; Gregory Burke, MD, Steve Rapp, PhD, Dee Posey, Margie Lamb, RN, Wake Forest University School of Medicine
Schwabe Pharmaceuticals
Robert Hörr, MD, Joachim Herrmann, PhD.
Data Coordinating Center
Richard A. Kronmal, PhD, Annette L. Fitzpatrick, PhD, Fumei Lin, PhD, Cam Solomon, PhD, Alice Arnold, PhD, University of Washington
Cognitive Diagnostic Center
Steven DeKosky, MD, Judith Saxton, PhD, Oscar Lopez, MD, Beth Snitz PhD, M. Ilyas Kamboh PhD, Diane Ives, MPH, Leslie Dunn, MPH, University of Pittsburgh
Clinical Coordinating Center
Curt Furberg, MD, PhD, Jeff Williamson, MD, MHS; Nancy Woolard, Kathryn Bender, Pharm.D., Susan Margitiæ, MS, Wake Forest University School of Medicine
Central Laboratory
Russell Tracy, PhD, Elaine Cornell, University of Vermont
MRI Reading Center
William Rothfus MD, Charles Lee MD, Rose Jarosz, University of Pittsburgh
Data Safety Monitoring Board
Richard Grimm, MD, PhD (Chair), University of Minnesota; Jonathan Berman, MD, PhD (Executive Secretary), National Center for Complementary and Alternative Medicine; Hannah Bradford, M.Ac., L.Ac., MBA, Carlo Calabrese, ND MPH, Bastyr University Research Institute; Rick Chappell, PhD, University of Wisconsin Medical School; Kathryn Connor, MD, Duke University Medical Center; Gail Geller, ScD, Johns Hopkins Medical Institute; Boris Iglewicz, Ph.D, Temple University; Richard S. Panush, MD, Department of Medicine Saint Barnabas Medical Center; Richard Shader, PhD, Tufts University.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/PAG
Contributor Information
Paul R. Duberstein, Laboratory of Personality and Development, Department of Psychiatry, University of Rochester Medical Center
Benjamin P. Chapman, Laboratory of Personality and Development, Department of Psychiatry, University of Rochester Medical Center
Hilary A. Tindle, University of Pittsburgh
Kaycee M. Sink, Wake Forest University
Patricia Bamonti, Laboratory of Personality and Development, Department of Psychiatry, University of Rochester Medical Center.
John Robbins, Department of Internal Medicine, University of California Davis School of Medicine.
Anthony F. Jerant, Center for Healthcare Policy and Research, and Department of Family and Community Medicine, University of California Davis School of Medicine
Peter Franks, Center for Healthcare Policy and Research, and Department of Family and Community Medicine, University of California Davis School of Medicine.
References
- Allemand M, Zimprich D, Hertzog C. Cross-sectional age differences and longitudinal age changes of personality in middle adulthood and old age. Journal of Personality. 2007;73:323–358. doi: 10.1111/j.1467-6494.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Andel R, Kareholt I, Parker MG, Thorslund M, Gatz M. Complexity of primary lifetime occupation and cognition in advanced old age. Journal of Aging and Health. 2007;19:397–415. doi: 10.1177/0898264307300171. [DOI] [PubMed] [Google Scholar]
- Archer N, Brown RG, Reeves S, Nicholas H, Boothby H, Lovestone S. Midlife neuroticism and the age of onset of Alzheimer’s Disease. Psychological Medicine. 2009;39:665–673. doi: 10.1017/S003329170800408X. [DOI] [PubMed] [Google Scholar]
- Balsis S, Carpenter BD, Storandt M. Personality change precedes clinical diagnosis of dementia of the Alzheimer type. Journals of Gerontology B: Psychological Sciences and Social Sciences. 2005;60:P98–P101. doi: 10.1093/geronb/60.2.p98. [DOI] [PubMed] [Google Scholar]
- Block J. A contrarian view of the five-factor approach to personality description. Psychological Bulletin. 1995;117:187–215. doi: 10.1037/0033-2909.117.2.187. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: a meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Bouhys AL, Flentge F, Oldehinkel AJ, van den Berg MD. Potential psychosocial mechanisms linking depression to immune function in elderly subjects. Psychiatry Research. 2004;127:237–245. doi: 10.1016/j.psychres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Borghans L, Duckworth AL, Heckman JJ, ter Weel B. The economics and psychology of personality traits. Journal of Human Resources. 2008;43:972–1059. [Google Scholar]
- Boyle PA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology. 2008;70:1534–1542. doi: 10.1212/01.wnl.0000304345.14212.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JCS. Prevention of Alzheimer’s Disease: Principles and prospects. In: Tsuang MT, Stone WS, Lyons MJ, editors. The Recognition and Prevention of Major Mental and Substance Use Disorders. Washington: American Psychiatric Publishing; 2007. [Google Scholar]
- Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, Pedersen NL. Depression as a risk factor or prodromal feature for dementia? Findings in a population based sample of Swedish twins. Psychology and Aging. 2009;24:373–84. doi: 10.1037/a0015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT. Social neuroscience: autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31:113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Bem DJ. Personality continuity and change across the lifecourse. In: Pervin LA, editor. Handbook of Personality Theory and Research. New York: The Guilford Press; 1990. pp. 549–575. [Google Scholar]
- Chapman BP, Duberstein P, Lyness JM. Replicability of the distressed personality type in older primary care patients and associations with general health indicators. European Journal of Personality. 2007;21:911–929. [Google Scholar]
- Chapman BP, Fiscella K, Kawachi I, Duberstein PR. Personality, socioeconomic status, and all cause mortality in the United States. American Journal of Epidemiology. 2010;171:83–92. doi: 10.1093/aje/kwp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Lyness JM, Duberstein PR. Personality and medical illness burden among older adults in primary care. Psychosomatic Medicine. 2007;69:277–282. doi: 10.1097/PSY.0b013e3180313975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Strauss ME, Smyth KA, Whitehouse PJ. Personality change in Alzheimer’s Disease. Archives of Neurology. 1992;49:486–491. doi: 10.1001/archneur.1992.00530290070014. [DOI] [PubMed] [Google Scholar]
- Chui HC, Mack W, Jackson JE, Mungas D, Reed BR, Tinklenberg J, Chang F-LK, Skinner K, Tasaki C, Jagus WJ. Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Archives of Neurology. 2000;57:191–196. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory and NEO Five Factor Inventory: Professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Costa PT, Jr, McCrae RR. Solid ground in the wetlands of personality: A reply to Block. Psychological Bulletin. 1995;117:216–220. doi: 10.1037/0033-2909.117.2.216. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Fratiglioni L, Gatz M. Personality and risk of cognitive impairment 25 years later. Psychology and Aging. 2006;21:573–580. doi: 10.1037/0882-7974.21.3.573. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Gatz M. Do work-related stress and reactivity to stress predict dementia more than 30 years later? Alzheimer Disease & Associated Disorders. 2007;21:205–209. doi: 10.1097/WAD.0b013e31811ec10a. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. Journals of Gerontology: Psychological Sciences. 2003;58B:P249–P255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Doody R, Clark C. Disease-modifying therapies for Alzheimer Disease: Challenges to early intervention. Neurology. 2007;69:1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- Dekosky ST, Fitzpatrick A, Ives DG, Saxton J, Williamson J, Lopez OL, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemporary Clinical Trials. 2006;27(3):238–253. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Weller JA, Yamada TH, Shivapour DM, Kaup AR, LaLoggia M, et al. Poor decision making among older adults is related to elevated levels of neuroticism. Annals of Behavioral Medicine. 2009;37:164–172. doi: 10.1007/s12160-009-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman JM. Personality structure: Emergence of the five-factor model. Annual Review of Psychology. 1990;41:417–440. [Google Scholar]
- Donellan MB, Oswald FL, Baird BM, Lucas RE. The Mini-IPIP Scales. Tiny-yet-effective measures of the Big Five Factors of personality. Psychological Assessment. 2006;18:192–203. doi: 10.1037/1040-3590.18.2.192. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Pálsson S, Waern M, Skoog I. Personality and risk for depression in a birth cohort of 70 year olds followed for 15 years. Psychological Medicine. 2008;38:663–671. doi: 10.1017/S0033291707002620. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Sörensen S, Lyness JM, King DA, Conwell Y, Seidlitz L, Caine ED. Personality is associated with perceived health and functional status in older primary care patients. Psychology and Aging. 2003;18:25–37. doi: 10.1037/0882-7974.18.1.25. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Storandt M, Larsen R. The power of personality in discriminating between healthy aging and early-stage Alzheimer’s disease. Journals of Gerontology B: Psychological Science. 2007;62:P353–P361. doi: 10.1093/geronb/62.6.p353. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T. Clinical criteria for vascular dementia: the NINDS-AIREN criteria. Dementia. 1994;5:189–192. doi: 10.1159/000106721. [DOI] [PubMed] [Google Scholar]
- Evans D, Rothbart MK. Developing a model for adult temperament. Journal of Research in Personality. 2007;41:868–888. [Google Scholar]
- Eysenck HJ. Biological dimensions of personality. In: Pervin LA, editor. Handbook of personality: Theory and research. New York: Guilford Press; 1990. pp. 244–276. [Google Scholar]
- Eysenck HJ. The Structure of Human Personality. 2. London: Methuen; 1960. [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fritsch T, Smyth KA, Debanne SM, Petot GJ, Friedland RP. Participation in novelty-seeking leisure activities and Alzheimer’s disease. Journal of Geriatric Psychiatry and Neurology. 2005;18:134–141. doi: 10.1177/0891988705277537. [DOI] [PubMed] [Google Scholar]
- Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Andel R, Crowe M, Fiske A, Reynolds CA, Pedersen NL. Accounting for the relationship between low education and dementia: A twin study. Physiology & Behavior. 2007;92:232–237. doi: 10.1016/j.physbeh.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Prescott CA, Pedersen NL. Lifestyle risk and delaying factors. Alzheimer Disease & Associated Disorders. 2006;20:S84–88. doi: 10.1097/00002093-200607001-00013. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. The structure of phenotypic personality traits. American Psychologist. 1993;48:26–34. doi: 10.1037//0003-066x.48.1.26. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology. 2007;21(6):657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hooker K, Monahan D, Bowman SR, Frazier LD, Shifren K. Personality counts for a lot: Predictors of mental and physical health of spouse caregivers in two disease groups. Journal of Gerontology: Psychological Sciences. 1998;53B:P73–P85. doi: 10.1093/geronb/53b.2.p73. [DOI] [PubMed] [Google Scholar]
- Husaini BA, Sherkat D, Moonis M, Levine R, Holzer C, Cain VA. Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services. Psychiatric Services. 2003;54:92–96. doi: 10.1176/appi.ps.54.1.92. [DOI] [PubMed] [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: Criterion validity of the 10-item Center for Epidemiologic Studies Depression Scale (CES-D) Archives of Internal Medicine. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- John OP, Srivastava S. The Big Five trait taxonomy: History, measurement and theoretical perspectives. In: Pervin LA, John OP, editors. Handbook of personality: theory and research. 2. New York: The Guilford Press; 1999. [Google Scholar]
- Kagan J, Snidman N, Arcus D, Reznick JS. Galen’s prophecy: Temperament in human nature. New York: Basic Books; 1994. [Google Scholar]
- Kern ML, Friedman HS. Do conscientious individuals live longer? A quantitative review. Health Psychology. 2008;27:505–512. doi: 10.1037/0278-6133.27.5.505. [DOI] [PubMed] [Google Scholar]
- Krause N, Liang J, Keith V. Personality, social support, and psychological distress in later life. Psychology and Aging. 1990;5:315–326. doi: 10.1037//0882-7974.5.3.315. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: the unifying role of personality in population-based research on problem behaviors. Journal of Personality. 2000;68:967–998. doi: 10.1111/1467-6494.00123. [DOI] [PubMed] [Google Scholar]
- Lodi-Smith JL, Jackson JJ, Bogg T, Walton K, Wood D, Harris PD, Roberts BW. The interplay of conscientiousness, social environmental factors, and health behaviors on health. submitted. [Google Scholar]
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- MacDonald KB. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychological Review. 2008;115:1012–1031. doi: 10.1037/a0013327. [DOI] [PubMed] [Google Scholar]
- Mangold DL, Wand GS. Cortisol and adrenocorticotropic hormone responses to naloxone in subjects with high and low neuroticism. Biological Psychiatry. 2006;60:850–855. doi: 10.1016/j.biopsych.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Marshall GN, Wortman CB, Vickers RR, Jr, Kusulas JW, Hervig LK. The five-factor model of personality as a framework for personality-health research. Journal of Personality and Social Psychology. 1994;67:278–286. doi: 10.1037//0022-3514.67.2.278. [DOI] [PubMed] [Google Scholar]
- Martin LR, Friedman HS, Schwartz JE. Personality and mortality risk across the life span: the importance of conscientiousness as a biopsychosocial attribute. Health Psychology. 2007;26:428–436. doi: 10.1037/0278-6133.26.4.428. [DOI] [PubMed] [Google Scholar]
- McAdams DP. Can personality change? Levels of stability and growth in personality across the life span. In: Heatherton TF, Weinberger JL, editors. Can personality change? Washington, DC: American Psychological Association; 1994. pp. 299–314. [Google Scholar]
- McCrae RR. Traits and trait names. How well is Openness represented in natural language? European Journal of Personality. 1990;4:119–129. [Google Scholar]
- McCrae RR. Openness to experience as a basic dimension of personality. Imagination, Cognition and Personality. 1993/1994;13:39–55. [Google Scholar]
- McCrae RR, Costa PT., Jr . Personality in adulthood. New York: Guilford Press; 1990. [Google Scholar]
- McCrae RR, Costa PT., Jr Personality trait structure as a human universal. American Psychologist. 1997;52:509–516. doi: 10.1037//0003-066x.52.5.509. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT, Jr, Ostendorf F, Angleitner A, Hrebícková M, Avia MD, Sanz J, Sánchez-Bernardos ML, Kusdil ME, Woodfield R, Saunders PR, Smith PB. Nature over nurture: temperament, personality, and life span development. Journal of Personality and Social Psychology. 2000;78:173–186. doi: 10.1037//0022-3514.78.1.173. [DOI] [PubMed] [Google Scholar]
- McEwen B. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1999;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Monin JK, Schulz RS. Interpersonal effects of suffering in older adult caregiving relationships. Psychology and Aging. 2009;24:681–695. doi: 10.1037/a0016355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papassotiropoulos A, Hock C, Nitsch RM. Genetics of interleukin 6: Implications for Alzheimer’s Disease. Neurobiology of Aging. 2001;22:863–871. doi: 10.1016/s0197-4580(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Persson G, Berg S, Nilsson L, Svanborg A. Subclinical dementia. relation to cognition, personality and psychopathology: a nine-year prospective study. International Journal of Geriatric Psychiatry. 1991;6:239–247. [Google Scholar]
- Petry S, Cummings JL, Hill MA, Shapira J. Personality alternations in dementia of the Alzheimer’s type. Archives of Neurology. 1988;45:1187–1190. doi: 10.1001/archneur.1988.00520350025009. [DOI] [PubMed] [Google Scholar]
- Pierce GR, Lakey B, Sarason IG, Sarason BR, editors. Sourcebook of social support and personality. New York, NY: Plenum Press; 1997. [Google Scholar]
- Pinquart M, Sörensen S. Differences between caregivers and non-caregivers in psychological health: a meta-analysis. Psychology and Aging. 2003;18:250–2677. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa K, Fisher GG, et al. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ritchie K, Lovestone S. The dementias. Lancet. 2002;360:1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [DOI] [PubMed] [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age - a quantitative review of longitudinal studies. Psychological Bulletin. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Kuncel N, Shiner RN, Caspi A, Goldberg L. The power of personality: A comparative analysis of the predictive validity of personality traits, SES, and IQ. Perspectives in Psychological Science. 2007;4:313–346. doi: 10.1111/j.1745-6916.2007.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Parhad I, Hachinski V, Erkinjuntti T, Rewcastle B, Kertesz A, Eastwood MR, Phillips S. Diagnosis of vascular dementia: Consortium of Canadian Centres for Clinical Cognitive Research consensus statement. The Canadian Journal of Neurological Sciences. 1994;21:358–364. doi: 10.1017/s0317167100040968. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Temperament, development, and personality. Current Directions in Psychological Science. 2007;16:207–212. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schmutte PS, Ryff CD. Personality and well-being: Reexamining methods and meanings. Journal of Personality and Social Psychology. 1997;73:549–559. doi: 10.1037//0022-3514.73.3.549. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Personality and the immune system: Models, methods, and mechanisms. Annals of Behavioral Medicine. 2000;22:180–190. doi: 10.1007/BF02895112. [DOI] [PubMed] [Google Scholar]
- Seidler A, Bernhardt T, Nienhaus A, Frölich L. Association between the psychosocial network and dementia—a case-control study. Journal of Psychiatric Research. 2003;37:89–98. doi: 10.1016/s0022-3956(02)00065-1. [DOI] [PubMed] [Google Scholar]
- Siegler IC, Dawson DV, Welsh KA. Caregiver ratings of personality change in Alzheimer’s Disease patients: a replication. Psychology and Aging. 1994;9:464–466. doi: 10.1037//0882-7974.9.3.464. [DOI] [PubMed] [Google Scholar]
- Siegler IC, Welsh KA, Dawson DV, Fillenbaum GG, Earl NL, Kaplan EB, Clark CM. Ratings of personality change in patients being evaluated for memory disorders. Alzheimer’s Disease & Associated Disorders. 1991;5:240–250. doi: 10.1097/00002093-199100540-00003. [DOI] [PubMed] [Google Scholar]
- Smith TW, Spiro A., III Personality, health, and aging: Prolegomenon for the next generation. Journal of Research in Personality. 2002;36:363–394. [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Strauss ME, Pasupathi M, Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer’s disease. Psychology and Aging. 1993;8:475–480. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Mason J, editors. Anxiety and the Anxiety Disorders. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1985. pp. 681–706. [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Terracciano A, Costa PT., Jr Smoking and the Five-Factor Model of personality. Addiction. 2004;99:472–481. doi: 10.1111/j.1360-0443.2004.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: a longitudinal examination of age-associated declines in reasoning and processing speed. Developmental Psychology. 2009;45:431–446. doi: 10.1037/a0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Von Dras DD, Siegler IC. Stability in extraversion and aspects of social support at midlife. Journal of Personality and Social Psvchologv. 1997;72:233–241. doi: 10.1037//0022-3514.72.1.233. [DOI] [PubMed] [Google Scholar]
- Wang HX, Karp A, Herlitz A, Crowe M, Kåreholt I, Winblad B, Fratiglioni L. Personality and lifestyle in relation to dementia incidence. Neurology. 2009;72:253–259. doi: 10.1212/01.wnl.0000339485.39246.87. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Verheul R, van den Brink W. Personality and psychopathology. In: Pervin LA, John OP, editors. Handbook of personality: Theory and research. 2. New York: Guilford Press; 1999. pp. 347–366. [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005a;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology. 2005b;30:11–17. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Li Y, Bennett DA. The relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology. 2007a;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of General Psychiatry. 2007b;64:1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]