Abstract
The O2 requirements of contracting skeletal muscle may increase 100-fold above rest. In 1919 August Krogh’s brilliant insights recognized the capillary as the principal site for this increased blood-myocyte O2 flux. Based on the premise that most capillaries did not sustain RBC flux at rest Krogh proposed that capillary recruitment (i.e., initiation of red blood cell (RBC) flux in previously non-flowing capillaries) increased the capillary surface area available for O2 flux and reduced mean capillary-to-mitochondrial diffusion distances. More modern experimental approaches reveal that most muscle capillaries may support RBC flux at rest. Thus, rather than contraction-induced capillary recruitment per se, increased RBC flux and hematocrit within already-flowing capillaries likely elevate perfusive and diffusive O2 conductances and hence blood-myocyte O2 flux. Additional surface area for O2 exchange is recruited but, crucially, this may occur along the length of already-flowing capillaries (i.e. longitudinal recruitment). Today, the capillary is still considered the principal site for O2 and substrate delivery to contracting skeletal muscle. Indeed, the presence of very low intramyocyte O2 partial pressures (PO2’s) and the absence of PO2 gradients, whilst refuting the relevance of diffusion distances, place an even greater importance on capillary hemodynamics. This emergent picture calls for a paradigm-shift in our understanding of the function of capillaries by de-emphasizing de novo ‘capillary recruitment.’ Diseases such as heart failure impair blood-myocyte O2 flux, in part, by decreasing the proportion of RBC-flowing capillaries. Knowledge of capillary function in healthy muscle is requisite for identification of pathology and efficient design of therapeutic treatments.
Keywords: blood flow, capillary, exercise, microcirculation, oxygen uptake, skeletal muscle
Introduction
August Krogh was not the first to discover the presence of capillaries, observe red blood cells (RBCs) passing through them or recognize the role of oxygen in the respiration that occurred in peripheral tissue. That story begins possibly with Galen (c. 130–199 CE) who considered that blood passed from the right to the left ventricle through minute pores in the interventricular septum and the notion of these ‘pores of Galen’ was still held by Sir William Harvey (1578–1657) in his seminal work, De Motu Cordis, published in 1628 (cited in West, 1996). It was Marcello Malpighi (1628–1694) in 1661 (Duae Epistolae Pulmonibus) who was perhaps the first person to observe the motion of red blood cells (RBCs) in capillaries in the frog lung, but, because of their lack of color, he thought that they were fat globules. Later, Anton Loewenhoeck (1632–1723) would recognize RBCs flowing in capillaries for what they were. The discovery of molecular O2 has been attributed to Joseph Priestley (1733–1804), Carl Wilhelm Scheele (1742–1786) and the chemist Antoine Lavoisier (1743–1794) who named it “oxygine” for its acid-forming properties and studied its rate of use and CO2 production during exercise. Also, well before Krogh’s time, Gustav Magnus (1802–1870) had developed blood gas analyses which, together with von Helmholtz’s (1821–1894) measurements of heat production by single twitches of isolated muscle in 1848 (von Helmholtz, 1848; see also Bassett, 2001), had helped ‘move’ the site of respiration (i.e., V̇O2 leading to CO2 production) from the lungs to the peripheral tissues (rev. West, 1996; Jones and Poole, 2005).
In the early 1900’s Krogh’s brilliance, and the unique ability to measure respiratory gas concentrations accurately (to one thousandth of a percent, Krogh, 1920a), combined with his wife, Dr. Marie Krogh’s, measurements of pulmonary diffusing capacity (Krogh, 1915) was central to their proposing that gas exchange across the blood-gas barrier in the lung occurred solely by means of physical forces, specifically, diffusion. In this regard he courageously opposed his mentor Christian Bohr, the eminent British physiologist John Scott Haldane and other scientific luminaries of the day. The concept of O2 diffusion across biological barriers, applied to muscle, emplaced the capillaries center-stage in the processes of blood-muscle O2 flux and muscle energetics (Krogh, 1919a,b, 1920b); the rest of the vascular system was thus relegated to a largely supportive role (though see Pittman, 2010 for a review of arteriolar-tissue O2 fluxes). Once that was accomplished his painstaking measurements of muscle capillary bed structure and function together with his understanding of metabolism would lay the foundation for his and Ehrlang’s elegant mathematical models of muscle oxygenation that remain so widely accepted today. Sentinel among the precepts of these models are the notions that: 1. Intramuscular PO2 decreases systematically with increased distance from the RBC in the capillary. Thus, large intramyocyte PO2 gradients were hypothesized to exist producing anoxic loci and denying mitochondria within those loci the opportunity to contribute to ATP production. 2. The importance of diffusion distances, together with Krogh’s measurements of more “open” capillaries either during or after muscle contraction, supported the theory that, at low metabolic rates (i.e., rest), most capillaries did not support RBC flux and that they were therefore available to be “recruited” (defined as the initiation of RBC flux in previously non-flowing capillaries) and reduce intramyocyte diffusion distances during contractions. Key to this recruitment during contractions was Krogh’s belief in the presence of a contractile capability that could close-off individual capillaries.
Krogh himself recognized many problems with his techniques (Krogh, 1919a,b). Paramount amongst which were: 1. The extreme surgical interventions often necessary to achieve good perfusions (Krogh, 1919a,b, 1920). Specifically, tying off multiple vessels to prevent leakage, non-physiologically high perfusion pressures, and sometimes, waiting several days post-mortem to achieve good perfusion. 2. The clumping together of India ink particles that blocked ink entry to some capillaries. This clumping effect would be reduced at higher flow rates and may artifactually have increased the opportunity for more capillaries to contain ink, and therefore be considered to support RBC flux, during the hyperemic conditions present during or after contractions.
Thanks, in large part, to the stimulus provided by Krogh and his contemporaries in this field, the nine intervening decades since his 1919 papers and Nobel prize (1920), have witnessed impressive technological advances in our ability to study the microcirculation. These advances have focused acute scientific attention on capillary hemodynamics, vascular and muscle oxygenation and blood-myocyte O2 flux. For instance: a range of skeletal muscles (e.g., rat: spinotrapezius, cremaster, diaphragm, extensor digitorum longus; hamster: retractor, cremaster, sartorius; rabbit: tenuissimus; cat: sartorius) suitable for transmitted and incident light microscopy has been developed and techniques are available for determining microvascular (phosphorescence quenching, Rumsey et al. 1988; Poole et al. 1995; Golub and Pittman, 2002, 2008; Golub et al. 2007; Pittman et al. 2010) and muscle (hemoglobin + myoglobin, Near-infrared Spectroscopy, NIRS, Lutjemeier et al. 2008) oxygenation with high temporal and spatial (phosphorescence quenching) fidelity. In addition, cryomicrospectrophotometric techniques (Gayeski et al. 1985; Gayeski and Honig, 1986a,b; Gayeski et al. 1987; Honig et al. 1997; Voter and Gayeski, 1999) and Proton Nuclear Magnetic Resonance Spectroscopy (MRS, Richardson et al. 1995, 1998; Molè et al. 1999) have permited ex-vivo and in vivo determination of intramyocyte PO2. Finally, methods have also been developed for resolving O2 flux across (constant-infusion thermodilution, Andersen and Saltin, 1985; Rowell et al. 1986; Savard et al. 1988; Grassi et al. 1996; Roca et al. 1992; Richardson et al. 1993; Volianitis et al. 2003; vascular isolation, Hogan et al. 1989) and within (radiolabeled microspheres, Laughlin et al. 1982; Armstrong and Laughlin, 1983; Musch and Terrell, 1992; Musch et al. 2004; Bailey et al. 2000; Poole et al. 2000) contracting muscles and the muscle microcirculation (Behnke et al. 2002; Kindig et al. 2002).
This paper highlights how observations made using these latter techniques have challenged our understanding of the microcirculatory function and its role in matching O2 delivery (Q̇O2)-to-V̇O2 during muscle contractions in health and disease. Specifically, under the auspices of resolving the physiological behavior of microcirculatory hemodynamics and O2 flux in healthy contracting muscle and dysfunction in disease, the necessity of capillary recruitment and the presence of intramyocyte PO2 gradients will be examined critically. As epitomized by Krogh, intravital microscopy observations of the capillary bed take center stage and are supported by powerful modern technologies which provide crucial new insights into the control of intravascular and intramyocyte O2 pressures and fluxes within contracting skeletal muscle.
Is capillary recruitment obligatory for increased blood-myocyte O2 flux in contracting muscle?
In the pentobarbital-anesthetized rat spinotrapezius muscle at rest ≥80% of capillaries support RBC flux at any given time (Kindig and Poole, 1998, 2001; Kindig et al. 1999, 2002; Poole et al. 1997). This is true also for the rat diaphragm (Kindig and Poole, 1998), extensor digitorum longus (Anderson et al. 1997; Ellis et al. 2002), hamster sartorius and cremaster (Damon and Duling, 1984), rabbit tenuissimus (Vrielink et al. 1987), and cat sartorius (Burton and Johnson, 1972). When stimulated to contract at 1 Hz, spinotrapezius capillary RBC velocity and flux begin to increase within the first contraction cycle (i.e., 1 s) generally reaching steady-state values within 30–60 s (Figure 1, top panel, Kindig et al. 2002). With most capillaries “recruited” at rest there appears little room for additional capillaries to initiate flow and indeed no significant increase in % flowing was found. Given that this result departs from conventional wisdom and established dogma it is crucial to consider whether this response is accompanied by an appropriate increase in blood-myocyte O2 flux. Combining phosphorescence-quenching measurements of microvascular PO2 (PmvO2) (Figure 1, center panel) with those of RBC flux the blood-myocyte V̇O2 kinetics profile can be resolved with high temporal and spatial fidelity (Figure 1, bottom panel, Behnke et al. 2001, 2002). The exponential increase in muscle V̇O2 is initiated from the onset of contractions – as expected from the instantaneous change in high-energy phosphates and current theories of metabolic control – with no discernible delay and kinetics (time constant, τ, 20–30 s) resembling closely that found for in vivo cycling (human leg muscles, Grassi et al. 1996; Bangsbo et al. 2000; Grassi, 2001; Krustrup et al. 2009) in situ (dog gastrocnemius-plantaris complex, Grassi et al. 2002) and isolated single fibers (amphibian lumbrical muscle, Kindig et al. 2003; Poole et al. 2007).
Figure 1.
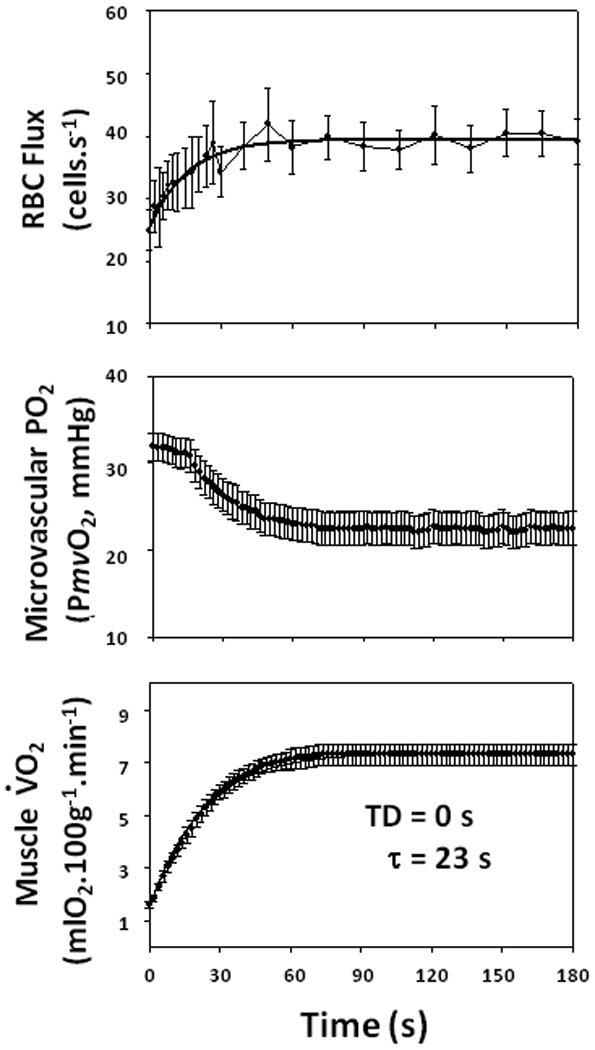
Mean data showing increase in rat spinotrapezius red blood cell (RBC) flux (Top) and microvascular PO2 (Middle) are conflated to estimate V̇O2 (Bottom) in response to 1 Hz electrically-induced contractions (initiated at time = 0 s). Capillary RBC flux was measured by high-resolution videomicroscopy analysis in individual capillaries (Kindig et al. 2002). Microvascular PO2 was determined using phosphorescence quenching techniques (Rumsey et al. 1988; Poole et al. 1995, 2004; Behnke et al. 2001). V̇O2 was calculated using the measured RBC flux and arterial O2 content and microvascular PO2 as an estimate of end-capillary PO2 and O2 dissociation curves established for the rat (Altman and Dittmer, 1974). Model fits are shown. Both RBC flux and V̇O2 (but not PmvO2) were fit by a single exponential with no time delay. TD, time delay. τ, time constant. Redrawn from Behnke et al. (2002). The value for τ resolved is similar to that for human muscle and also Phase II pulmonary V̇O2 kinetics (e.g., Grassi et al. 1996).
In Krogh’s time, the prevailing wisdom was that “…capillaries are passive, (and) that blood is flowing continuously through all of them at rates which are determined by the state of contraction or dilatation of the corresponding arterioles…” (Krogh, 1919b, p. 468). Thus, Krogh’s observations and contention that capillary recruitment was an essential physiological response to increased metabolic rates flew contrary to this opinion. Today, the argument is by no means settled with exemplars of each side listed in Table 1. The commonality of temporal capillary hemodynamic and O2 flux responses across species and preparations and the common observation that, in resting muscle, most capillaries support RBC flux, do not prove, in and of themselves, that in vivo capillary recruitment does not occur; they merely suggest that it may not be obligatory for the normal V̇O2 kinetics. Considered by many as the oldest scientific society in the World, the motto of the British Royal Society, which was founded in 1660 and chartered by King Charles II, is “Nullius in Verba” (Take noboby’s word for it, see it for yourself). This view was certainly espoused by Krogh but, unfortunately, and to the consternation of many scientists who use intravital microscopy to visualize capillary hemodynamics (right hand side of Table 1), it has become convenient and all-too-common to avoid the rigors of observing the capillary bed in vivo and simply presume that capillary recruitment can explain increased blood-myocyte exchange (e.g., Baron et al. 2000; Clark et al. 2001; Rattigan et al. 1997; left hand side of Table 1). Indeed, the eminent microcirculationist, Professor Eugene Renkin, in his Letter to the Editor of the American Journal of Physiology, harshly criticized Dr. Bentzer for entitling his paper “Capillary filtration coefficient is independent of the number of perfused capillaries in cat skeletal muscle” (Bentzer et al. 2001); when that claim was made without making any direct measurements of RBC flowing capillary numbers.
Table 1.
Exemplar references from either side of the ‘capillary recruitment’ debate that have either presented evidence for a substantial proportion of non-RBC/plasma flowing capillaries in skeletal muscle at rest or interpreted their data as evidence for capillary recruitment (left hand side) or provided evidence that most capillaries already support RBC/plasma flow in resting muscle (right hand side). The latter position discounts the possibility of substantial ‘capillary recruitment’ following the onset of contractions.
| Opening of previously “closed” or non- flowing capillaries |
Most capillaries support RBC or plasma flow at rest - with a wide range of flows and transit times |
|---|---|
| Krogh, 1919 | Eriksson and Myrhage, 1972 |
| Gorczynski, Duling, 1978 | Burton and Johnson, 1972 |
| Honig et al. 1980,1982 | Renkin et al. 1981 |
| Klitzman et al. 1982 | Hudlicka et al. 1982 |
| Gray et al. 1983 | Vetterlein et al. 1982 |
| Hargreaves et al. 1990 | Damon and Duling, 1984 |
| Fuglevand and Segal, 1997 | Kayar and Banchero, 1985 |
| Rattigan et al. 1997 | Hudlicka, 1985 |
| Parthasarathi and Lipowsky, 1999 | Dawson et al. 1987 |
| Baron et al. 2000 | Poole et al. 1997 |
| Clark et al. 2001 | Kindig and Poole, 1998,1999,2001 |
| Dawson et al. 2002 | Kindig et al. 2002 |
| Rattigan et al. 2005, 2006 | Russell et al. 2003 |
| Vincent et al. 2006 | Richardson et al. 2003 |
| Womack et al. 2009 | Padilla et al. 2006 |
| Bourdillon et al. 2009 | Copp et al. 2009 |
Notwithstanding the above, several concerns have justifiably been leveled at anesthetized, electrically-stimulated preparations, which are generally necessary to see muscle capillary hemodynamics, and interpretation of data from such. Paramount among these are: 1. Anesthesia relaxes arteriolar smooth muscle and increases capillary perfusion. If this occurred it would be expected that resting muscle blood flow and vascular conductance would increase markedly above those found in the conscious animal. Comparison of blood flows measured under both conditions using radiolabeled microspheres indicates that this certainly does not occur (c.f. Musch and Poole, 1996 with Bailey et al. 2000). Moreover, and even more compelling, if arteriolar smooth muscle function were impaired by anesthesia, it is difficult to conceive how electrical stimulation would increase muscle blood flow in proportion to metabolic rate as seen in vivo. Figure 2 demonstrates the commonality of the slope of the blood flow to V̇O2 relationship across different muscles in anesthetized rats (Ferreira et al. 2006) to that found in conscious man (Andersen and Saltin, 1985; Rowell et al. 1986; Savard et al. 1988; Richardson et al. 1993; Grassi et al. 1996; Poole, 1997; Volianitis et al. 2003). Compelling additional supportive evidence for the presence of flow in most capillaries in resting muscle comes from capillary staining using central Thioflavin-S dye infusions in conscious animals (Kayar and Banchero, 1985). The presence of at least plasma flow was detected in essentially all muscle capillaries investigated within very few seconds of instillation (~1–2 circulation times). 2. Surgical manipulation damages vascular control and increases muscle capillary perfusion. Comparison of in vivo, in situ and exteriorized muscles demonstrates that blood flow is not increased by the surgical exteriorization procedure nor is the blood flow-to-V̇O2 relationship perturbed during contractions as evidenced by the constancy of the PmvO2 profile across in situ and exteriorized conditions (Bailey et al. 2000). 3. Capillaries without flowing RBCs cannot be detected and so, in resting muscle, many capillaries simply are not counted. Measurements of the lineal density of flowing capillaries from rest to contractions and also in pharmacologically-vasodilated conditions do not support the presence of undetected capillaries at rest (Kindig and Poole, 2001).
Figure 2.
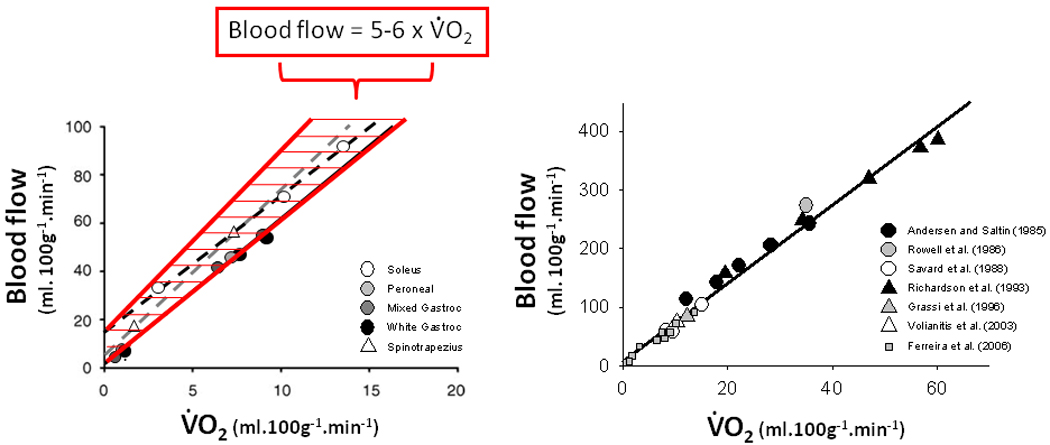
Relation between blood flow and oxygen uptake (V̇O2) during muscle contractions for various muscles in pentobarbital-anesthetized rats (Left Panel) and consciously exercising humans (Right Panel shows relationship in Left Panel expanded to incorporate human data). Rat data are means from Behnke et al. (2002, 2003) as redrawn from Ferreira et al. (2006). Human data are from: Andersen and Saltin, (1985); Rowell et al. (1986); Savard et al. (1988); Richardson et al. (1993); Grassi et al. (1996); Volianitis et al. (2003). This figure demonstrates that anesthetized preparations retain vasomotor control such that blood flow increases in the same proportion with V̇O2 (i.e., 5–6 L.min−1:1 L.min−1) as found in conscious preparations (rev. Poole, 1997) though the range of metabolic rates and blood flows is lower for the animal preparations owing, in part, to the requirement for electrical stimulation.
Another important consideration that can be tested empirically in conscious humans performing voluntary exercise relates to muscle hemoglobin concentration. It is known that, due in part to the endothelial surface layer (often termed the ‘glycocalyx’), there are differential flow rates of plasma and RBCs within and among capillaries such that microvascular hematocrit in resting muscle is far below systemic (e.g., Klitzman and Duling, 1979; Sarelius and Duling, 1982; Desjardins and Duling, 1987,1990; Kindig and Poole, 1998, 2001; Poole et al. 1997; Frisbee and Barclay, 1998; Kindig et al. 2002; Copp et al. 2009). Thus, from rest to exercise an increased microvascular hematocrit towards systemic (Klitzman and Duling, 1979; Kindig et al. 2002; Copp et al. 2009) may therefore, in theory, raise muscle [hemoglobin] as much as 2–3 fold. Accordingly, if we were to accept that most (say 80%, a figure in the range of common usage) of capillaries did not flow at rest and were recruited during exercise, muscle [hemoglobin] would be expected to increase up to four-fold from this effect alone, and with increased microvascular hematocrit, this could be several-fold higher again. However, in human muscles NIRS reveals only a very modest increase in muscle [hemoglobin] of ~20% from rest to exercise (Lutjemeier et al. 2008) which leaves very little room for significant capillary recruitment.
From these studies and a wealth of intravital microscopy observations in the many different muscles (listed above) there is substantial support for the contention that, at least for the muscles investigated, the majority of capillaries sustain plasma and RBC flux even at rest. Very few capillaries are closed, empty of RBCs or contain stagnant RBCs. However, there is substantial heterogeneity of flow rates among capillaries (Erickson and Myrhage, 1972; Duling and Damon, 1987; Kindig et al. 1999; Kindig and Poole, 1998) such that many may be of less relative importance for O2 and substrate delivery. Thus, muscle contractions (and reactive hyperemia, Burton and Johnson, 1972) are accompanied by an increased flow principally within already flowing capillaries (Poole et al. 2008b; right-hand side of Table 1).
Despite the wealth of evidence presented above, the notion of capillary recruitment endures and is commonly invoked to explain increased blood-myocyte O2 and substrate flux during exercise or metabolic stimulation by insulin, for example (Baron et al. 2000; Rattigan et al. 1997; Clark et al. 2001; Vincent et al. 2006; Clark et al. 2008; left-hand side of Table 1). Often, established scientists, who may have never observed the capillary bed in their experiments, interpret their data as evidence for capillary recruitment or, as a self-fulfilling prophecy, explain their results using this phenomenon. In physiology textbooks (e.g., Rowell, 1993; McArdle et al. 2007) and medical schools around the World the notion that O2 diffusion distances within muscle are important and elevated blood-myocyte O2 and substrate fluxes occur by increasing the number of flowing capillaries makes great intuitive sense. Who can forget the visually stunning fluorescence images from Parthasarathi and Lipowsky (1999; Figure 3) purportedly showing hypoxic intervention “recruiting” an enormous number of microvascular units? As the legend explains and scientific reports have substantiated (e.g. Damon and Duling, 1984) what this may really demonstrate is the well-known phenomenon of hyperoxic vasoconstriction – both panels representing supraphysiologic PO2’s (Whalen et al. 1974, 1976)
Figure 3.

Example of how inappropriate boundary conditions may reinforce capillary recruitment notion. Capillaries are visualized in resting cremaster muscle using fluorescently-labeled plasma and the PO2 conditions are 130 mmHg (Left panel) and 35 mmHg (Right panel). Both of these PO2’s are considerably above intramuscular values at rest (~17 mmHg, Whalen et al. 1974) and what is observed is probably not hypoxia-induced capillary recruitment moving from left-to-right (which would support a reserve of non-flowing capillaries that might be ‘recruited’ during exercise) but, rather, hyperoxia-induced vasoconstriction moving from right-to-left. Small arrows denote direction of flow. Images from Parthasarathi and Lipowsky (1999), with permission.
Each experimental observation must be judged on its own merits. However, there are many putative experimental considerations that are often ignored within the capillary recruitment literature and which may have lead to erroneous conclusions. These include: 1. Today capillaries in mammalian skeletal muscles are not thought to have contractile capability as considered by Krogh (1919a,b) and his contemporaries. Neither do they collapse easily under conditions of increased muscle or reduced intraluminal pressure (Poole and Mathieu-Costello, 1990; Kindig and Poole, 1999), possibly due to the presence of Type IV collagen struts that tether their walls to the surrounding myocytes (Caulfield and Borg, 1979; Borg and Caulfield, 1980). This is very different from capillaries in the lung where their thin walls and alveolar gas surround offer little support and, at least under low cardiac output conditions, many may be collapsed - particularly at the lung apex where perfusion pressures are perilously low (Pande and Hughes, 1983) - and thus they are available for recruitment when pulmonary arterial flow increases and perfusion pressure rises (Wearn et al. 1934; Hanson et al. 1989; Wagner, 1997). 2. Capillaries are enormously fragile vessels. Mechanical abuse during muscle surgical preparation – an unfortunately frequent accompaniment to intravital observation – may easily damage their ability to support flow. Also, stretching muscle to sarcomere lengths >3.0 µm narrows their diameter and reduces the proportion supporting flow by increasing their resistance (Poole and Mathieu-Costello, 1992; Kindig and Poole, 2001) as well as invoking a sympathetically-mediated arteriolar vasoconstriction and passively impeding arteriolar and venular flow (Welsh and Segal, 1996; Kindig and Poole, 2001). Few laboratories measure and set muscle sarcomere length and, because stretching thins the muscle and improves the optical quality, this practice may, unfortunately, be common. 3. Some anesthetics lower blood pressure to levels that may invoke skeletal muscle hypoperfusion due to lack of driving pressure which can be exacerbated by a sympathetically-mediated muscle vasoconstriction. 4. The commonly accepted notion that hypoxia leads to capillary recruitment, meaning that sufficient capillaries must be stagnant in resting muscle to elicit this recruitment effect, should be placed in proper context. For example, as mentioned above, the “low O2” condition (~35 mmHg incident PO2) studied by Pathasarathi and Lipowsky (1999, see Figure 3) was actually hyperoxic compared with values established for resting muscle (normoxia ~17 mmHg, Whalen et al. 1974, 1976). It might be argued that what their micrographs demonstrate beautifully may not be hypoxia-induced capillary recruitment but, rather, the opposite - hyperoxia-induced vasoconstriction leading to capillary derecruitment as the incident PO2 is raised from 35 to 130 mmHg.
Given that the human skeletal musculature may contain as many as eight or nine billion capillaries, is it implausible that most may contain at least some flowing RBCs at rest? Figure 4 addresses this concept mathematically using an accepted estimate of 1 L.min−1 for skeletal muscle blood flow (Rowell, 1993, ~5.4 trillion RBC.min−1) and presumes a high average of 300 capillaries per mm2 each of 1,000 µm in length (Andersen and Henriksson, 1977; Brodal et al. 1977; Saltin and Gollnick, 1983; Hoppeler et al. 1985; Coyle et al. 1988; Richardson et al. 1994). If 80% of these capillaries support RBC flux there are sufficient RBCs to supply each with 12.8 RBC.s−1. This value is not far from that measured for rat spinotrapezius muscle (i.e., 15–20 RBC.s−1, Kindig and Poole, 1997; Kindig et al. 2002). Whereas this calculation does not prove that most capillaries support RBC flux in resting muscle – and thus are not available to be ‘recruited’ per se – it demonstrates that, at least, it is feasible.
Figure 4.
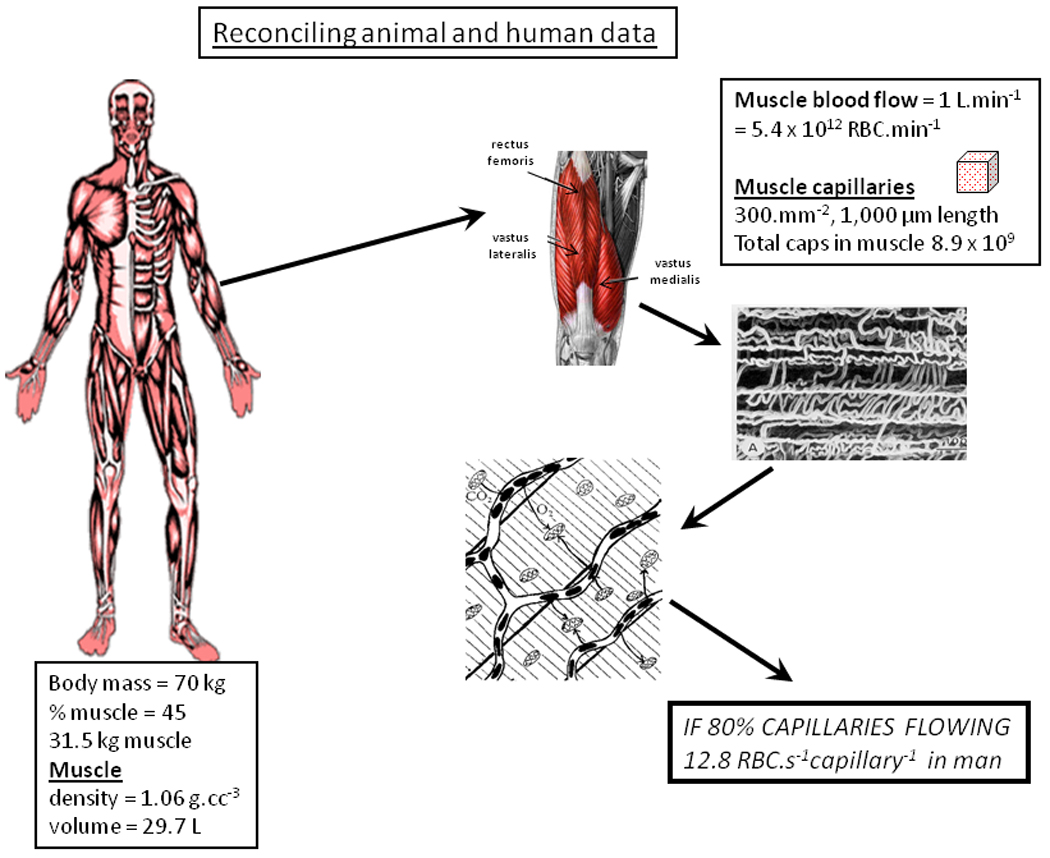
As demonstrated by intravital microscopy (e.g., Kindig et al., 1999) and dye-infusion (Kayar and Banchero, 1995) for anesthetized and conscious animals, is it possible that the majority of capillaries support red blood cell flux in resting muscle in humans? This figure uses established values for muscle blood flow (e.g., Rowell, 1993) combined with good-faith estimates of muscle capillary number (e.g., Saltin and Gollnick, 1983; Hoppeler et al. 1985; Richardson et al. 1994) to demonstrate that, in humans, 80% of total muscle capillaries could, in theory, support an average of nearly 13 RBC.s−1 which is close to that value measured in rat spinotrapezius (15–20 RBC.cap−1.s−1; Kindig et al. 1997). In contrast to the leftmost and center boxes, the italicized capitalized letters in the rightmost box alert the reader that this “80%” value is data taken from the animal literature as such data is not presently available in humans. See text for more details.
Are there pronounced PO2 gradients within contracting muscle fibers?
If the greatest impediment to O2 diffusion occurs close to the RBC and not within the intramyocyte milieu the importance of capillary-to-mitochondrial diffusion distance becomes moot. In the 1980’s Carl Honig, Thomas Gayeski and Richard Connett rapidly froze resting and contracting (electrical stimulation) dog gracilis and other muscles and microscopically analyzed the myoglobin-O2 saturation from which PO2 could be calculated (Gayeski et al. 1985, 1987; Gayeski and Honig, 1986a,b; Honig et al. 1997). Surprisingly, during exercise they found that the intramyocyte PO2 was low (< 3 mmHg) and almost uniform irrespective of the distance from the capillary i.e., the predicted capillary-to-mitochondrial gradients (see Ellis et al. 1983) were absent (Honig et al. 1997). Despite initial uncertainty regarding the size of the myoglobin crystals analyzed and whether the freezing procedure was capable of capturing a PO2 gradient (Voter and Gayeski, 1995; Honig et al. 1997) at least two independent series of experiments using different techniques support that intramyocyte PO2 is very low and that diffusion distance is not an important determinant of muscle O2 diffusing capacity. First, using highly specialized proton MRS techniques, Richardson and colleagues (1995, 1998; see also Molè et al. 1999) found that, even at relatively low levels of exercise, quadriceps intramyocyte PO2 fell close to the levels reported by Honig and colleagues (Gayeski et al. 1985, 1987; Gayeski and Honig, 1986a,b; Honig et al. 1997) in the dog gracilis. Second, in a very elegant experimental design intercapillary diffusion distances in the canine gastrocnemius-plantaris complex were altered chronically by leg immobilization or exercise training in separate groups of dogs (Bebout et al. 1993; Hepple et al. 2000). The results demonstrated that muscle diffusing capacity could be dissociated clearly from capillary density and intercapillary diffusion distances (Figure 5). Collectively, these studies support the elegant modeling papers of Federspiel and Popel (1986) and Groebe and Thews (1990) that muscle O2 diffusing capacity is determined not by the capillary density or intramyocyte O2 diffusion distances per se but by the number of RBCs adjacent to the muscle fibers at any instant. Thus, whereas muscle blood flow and O2 content define convective O2 delivery, the length and hematocrit of RBC-flowing capillaries are sentinel determinants of muscle diffusive O2 delivery and, as such, may be manipulated by perturbations that alter systemic and thus (presumably) capillary hematocrit (Schaffartzik et al. 1993). This concept has been verified empirically in frog skin (Malvin and Wood, 1992).
Figure 5.
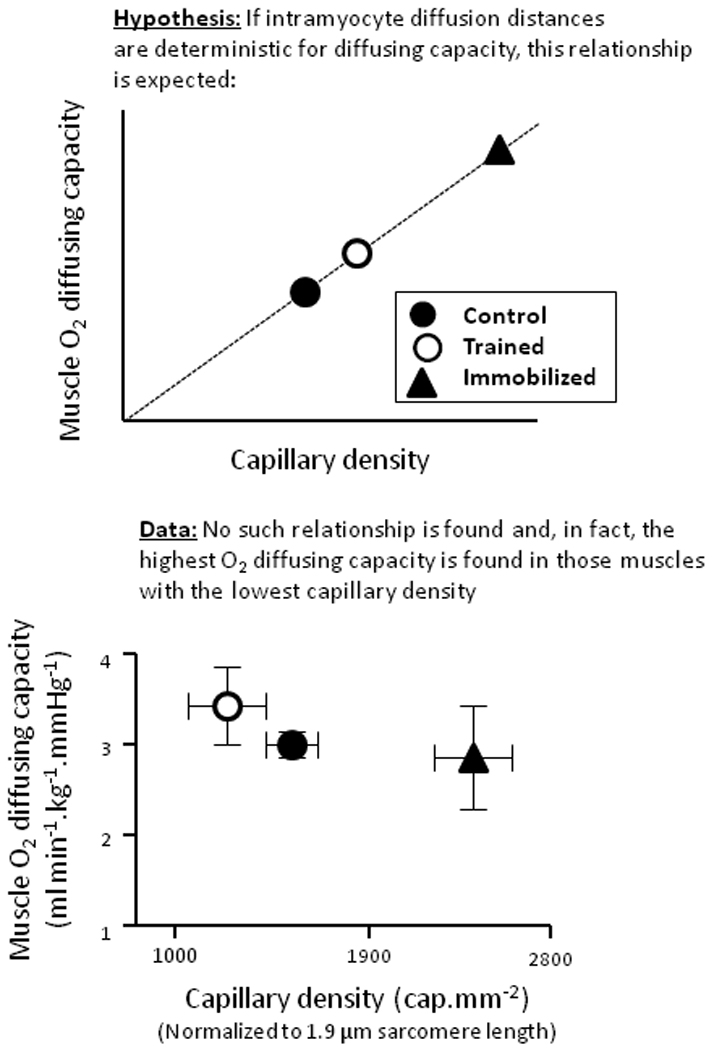
Empirical test of the hypothesis that muscle O2 diffusing capacity is dependent upon diffusion distances per se. Top graph depicts hypothesis tested i.e., if diffusion distances are a key determinant of muscle O2 diffusing capacity then the data should line up as shown with far higher O2 diffusing capacity for the immobilized condition where capillary density is increased substantially as a result of the pronounced fiber atrophy. However, contrary to the hypothesis, bottom graph demonstrates no increased diffusing capacity with reduced fiber cross-sectional area, increased capillary density and reduced O2 diffusion distances with immobilisation. Muscle O2 diffusing capacity is calculated from the mean capillary PO2 which, in turn, is estimated using the measured arterial and effluent PO2’s and the muscle V̇O2 by a forward integration procedure and assumes that all O2 remaining in the venous blood results from diffusional limitation (i.e., the contribution of anatomical and functional shunts and O2 delivery-to-O2 utilization mismatch is negligible) and that intramyocyte PO2 is zero (see Roca et al. 1992). From Hepple et al. (2000), with permission.
Presumptions arising from “capillary recruitment”
The concept of “capillary recruitment” has birthed multiple presumptions (identified in italics below) that conflict with established physiological concepts, empirical observations and current models. These include: 1. Muscle V̇O2 at rest and during submaximal contractions is O2 delivery limited (Clark et al. 1998, 2001). In healthy muscle at rest and during contractions V̇O2 is known to be controlled by intramyocyte high-energy phosphate signaling (Meyer and Foley, 1996) and, other than close to maximal V̇O2 (Richardson et al. 1993; Wagner et al. 1997; Poole, 1997), is not limited by O2 supply. A wealth of measurements in conscious man performing voluntary exercise and animal preparations dissociate both the non-steady-state V̇O2 kinetics and steady-state V̇O2 from changes in O2 supply (Bredle et al. 1989; Arthur et al. 1992; Hogan et al. 1992; Knight et al. 1993; Curtis et al. 1995; Richardson et al. 1995a; Grassi et al. 1998; Grassi, 2001). 2. The exchange capacity of a capillary is determined principally by whether it is flowing (recruited) or not rather than its rate of flow. As seen in Figure 6 increased exchange capacity purported to result from capillary recruitment can be explained by increased flow and substrate delivery through already-recruited capillaries (Hargreaves et al. 1990; Hudlicka et al. 1982; Kindig et al. 2002). This latter notion is in accord with the enormous increase in RBC flux and O2 delivery seen from rest to exercise within already flowing capillaries and also the established wisdom that free fatty acids and glucose are taken up by muscle in proportion to their delivery (i.e., plasma flow × concentration). 3. When the microcirculation in diseased muscles (e.g., in chronic heart failure (CHF) or diabetes) is observed, this may not be distinguishable from the healthy situation. One danger of accepting that most capillaries do not flow in resting muscle is that it is likely to impair the ability to resolve the mechanistic bases for pathologically-reduced muscle function. For instance, in CHF capillaries cease to flow in resting and exercising muscle in proportion to left ventricular dysfunction and left ventricular end-diastolic pressure (Kindig et al. 1999; Richardson et al. 2003), and this might explain, in part, the reduced muscle O2 diffusing capacity and compromised energetic function pathognomonic to this condition. Similarly, in Type II diabetes the Goto-Kakizaki rat spinotrapezius evidences widespread microvascular stagnation and impaired capillary hemodynamics that undoubtedly compromise the opportunity for blood-muscle glucose as well as O2 flux (Padilla et al. 2006, 2007; Bauer et al. 2007). If one accepts that most capillaries should not support RBC flux there will be a failure to recognize these and other pathological dysfunctions (e.g., eccentric exercise-induced muscle damage, Kano et al. 2005) which will likely hamstring attempts to correct the underlying problems therapeutically.
Figure 6.
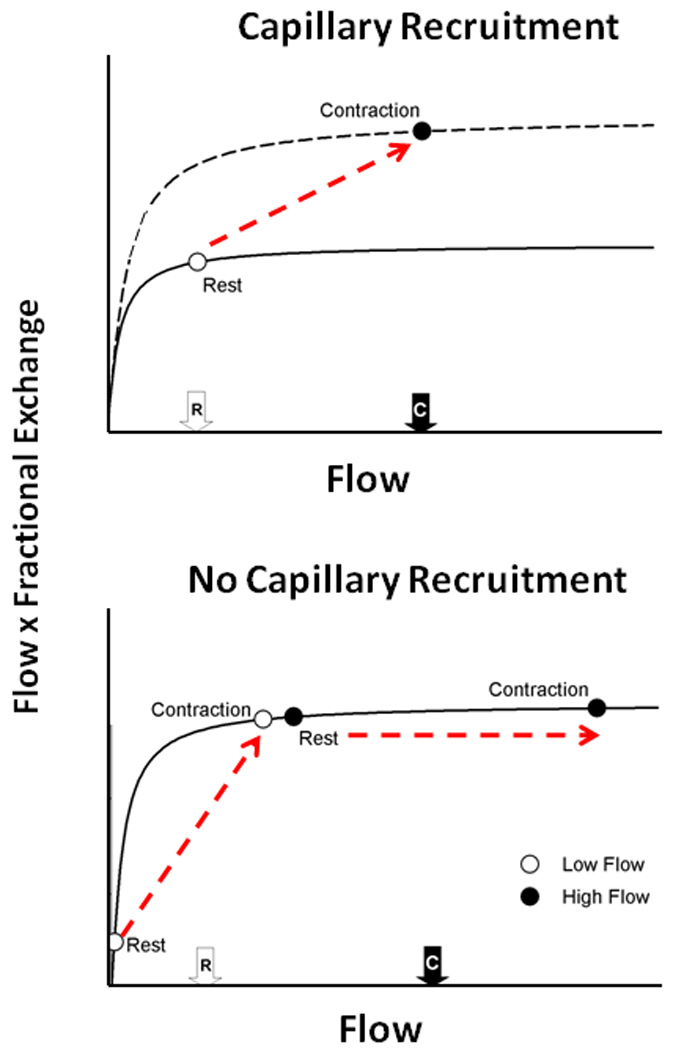
In a simplified manner the relationship between capillary delivery and muscle uptake of a substrate can be described by a close-to-exponential curve (Top Figure). In general, this is the model used by Clark and colleagues employing the 1-methylxanthine metabolism technique (Rattigan et al. 1997a,b; Youd et al. 1999) to estimate capillary recruitment during contractions. The basic assumptions made in this model are: i) that blood flow in the microcirculation (capillaries) is homogeneous, and ii) at rest all capillaries are located in the flat portion of the curve. PS is the permeability-surface area product. Any increase in 1-methylxanthine metabolism from rest-to-contractions is explained by greater surface area, which is assumed to reflect the recruitment of previously non-flowing capillaries. However, as discussed in the text, microvascular blood flow is markedly heterogeneous at rest and during contractions. Thus, the bottom figure considers two groups of capillaries: low-flow and high-flow. In this simulation, low-flow capillaries are located in the flow-limited range of substrate uptake and high-flow capillaries are on the flat portion of the curve (‘diffusion/surface area-limited’ range). The mean resting blood flow is close to the flat portion of the curve (white arrow bottom Figure; same as in Clark et al.’s model; top figure). An increase in blood flow through both groups of capillaries – mean capillary flow during contractions represented by black arrow (Bottom Figure) and similar to that in Clark et al.’s model – will lead to an increase in O2 or substrate uptake that is identical to that seen for the “capillary recruitment” model. Thus, increase in blood flow through capillaries with heterogeneous flow distribution is a viable alternate explanation to Clark and colleague’s data without any capillary recruitment requisite whatsoever.
If capillary recruitment does not occur to any great extent, how does blood-myocyte O2 flux increase during exercise?
As discussed above, the idea that a recruited capillary represents a fixed unit of surface area or exchange capacity appears untenable. Several likely and one speculative mechanism(s) are presented in Figure 7 that may help explain the enhanced blood-myocyte O2 flux in response to elevated muscle metabolic demands which may rise by two orders of magnitude above resting. These mechanisms are consistent with contemporary experimental findings and blood-tissue O2 exchange theory. Note that all panels of Figure 7 may not apply to predominantly plasma- as opposed to RBC-borne substrates as muscle uptake of, for example, free fatty acids or glucose is not dependent upon capillary RBC hemodynamics but rather plasma flow. Key elements in Figure 7 are: 1. Capillaries with very low RBC flow and hence long transit times at rest may have dramatically increased flow during exercise and therefore assume a greater importance for convective O2 delivery. Interestingly, fractional O2 extraction is impacted by the interdependence of O2 diffusing capacity (DO2) and blood flow according to (Roca et al. 1992; see also following section for graphical analysis):
where Q̇O2 is O2 delivery (blood flow, Q̇ × [arterial O2]) and β is the slope of the O2 dissociation curve in the physiologically-relevant range. 2. Capillary hematocrit increases from ~15% towards systemic levels (~45%, Klitzman and Duling, 1979; Kindig et al. 2002; Copp et al. 2009) such that, in the extreme, each capillary may contain up to 2–3 times as many RBCs during exercise compared with rest thereby increasing the instantaneous O2 diffusive conductance (DO2, Federspiel and Popel, 1986; Groebe and Thews, 1990; Malvin and Wood, 1992). An elevated capillary hematocrit increases the likelihood that transverse histological sections will transect an RBC within a given capillary. This observation, combined with the presumption that only capillaries with transected RBCs supported RBC flow in vivo, has contributed to the enduring capillary recruitment concept (Honig et al. 1980). Krogh himself was cognizant of the dangers of presuming capillary hemodynamic behavior from ex-vivo analyses (Krogh, 1919a,b). 3. As considered for the lung by Wiltz Wagner (Wagner, 1997) capillary endothelial surface area available for O2 delivery can be recruited not only by initiating flow in previously non-flowing vessels but also by increasing the length of individual capillaries over which blood-tissue O2 flux occurs. Thus, when RBC velocity is speeded and the fractional extraction of O2 increases (from ~25 up to 80% or more) from rest to exercise, a far greater length of a given capillary is “recruited” for that exchange. The potential importance of this “longitudinal recruitment” of capillary O2 exchange capacity to increased muscle O2 diffusing capacity during exercise cannot be overemphasized. 4. As presented above, measurements in voluntarily contracting human muscle (Richardson et al. 1995b,1998; Molè et al. 1999) and frozen sections of electrically-stimulated canine muscle (Gayeski et al. 1985, 1987; Gayeski and Honig, 1986a,b; Honig et al. 1997) indicate the presence of extremely low intramyocyte PO2’s. The desaturation of myoglobin will enhance its ability to facilitate intramyocyte diffusion and remove what Honig and colleagues have insightfully termed the “functional O2 carrier-depleted region” between the capillary and mitochondrial reticulum (Honig et al. 1997). 5. It is recognized that the endothelial surface layer (‘glycocalyx’) is a crucial regulator of capillary hemodynamics (e.g., Desjardins and Duling, 1990; Zuurbier et al. 2005). Specifically, its removal (Desjardins and Duling, 1990) or modification by hyperglycemia, for example (Zuurbier et al. 2005), alters capillary hematocrit and hemodynamics profoundly. There is thus the strong potential that mechanical alterations of the endothelial surface layer accompanying muscle contraction and exercise hyperemia are important in regulating the capillary hemodynamic response to exercise and thus blood-myocyte O2 flux: though, at present, this remains hypothetical.
Figure 7.
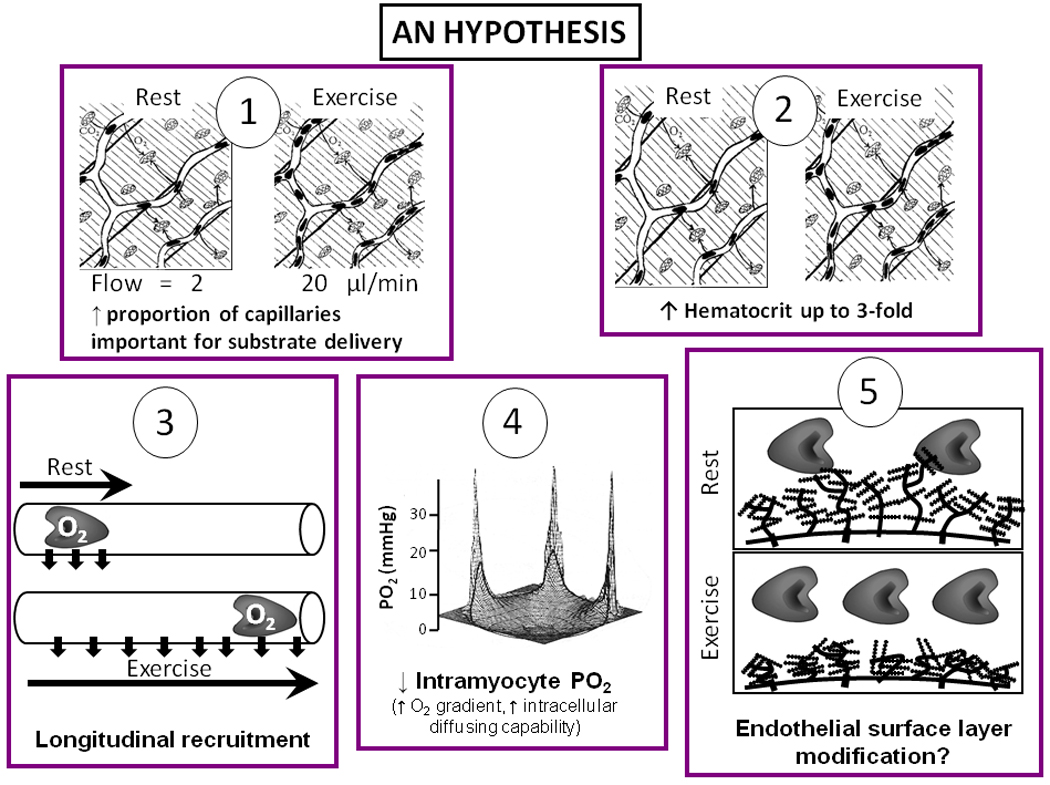
Putative mechanisms that can account for the increase of blood-myocyte O2 (and substrate) flux, from rest-exercise, within existent RBC flowing capillaries without the necessity for ‘recruitment’ of previously non-flowing capillaries. 1. Substantial elevation of blood flow (RBC flux) within originally very low flow capillaries means that capillaries that may have been unimportant for blood-myocyte flux at rest become important during exercise. 2. Elevation of RBC numbers (hematocrit) within capillaries. 3. Increased RBC velocity and greater fractional O2 extraction mean that additional capillary surface area along the length of the capillary becomes important for blood-myocyte flux (i.e., ‘capillary longitudinal recruitment’). 4. In 3 dimensions this graph depicts the precipitous drop in PO2 from capillary to the intramyocyte space (i.e., the area encircled by the thick black line) during muscle contractions. Note the overall very low intramyocyte PO2 and lack of appreciable intramyocyte PO2 gradients. From rest-exercise, the fall in intracellular PO2 increases the blood-myocyte gradient and also desaturates myoglobin removing the ‘O2 carrier functionally-depleted region’ (Honig et al. 1997) and elevating its ability to transport O2 which improves intracellular diffusing capacity for O2. 5. There is the possibility that the endothelial cell surface layer (thought to be crucial in lowering capillary hematocrit below systemic) is modified under high flow conditions.
How does the matching of O2 delivery to V̇O2 change in disease (chronic heart failure, CHF, diabetes) and aging and might this help explain the slowed V̇O2 kinetics in these conditions?
The compelling weight of evidence indicates that, in skeletal muscles of young healthy individuals, the speed of V̇O2 kinetics (expressed, most often, as the time constant, τ, of the response) following the onset of moderate or heavy exercise, is not limited by muscle O2 delivery (Grassi, 2005; Hughson, 2005; Poole et al. 2008a; Nyberg et al. 2010, Figure 8). In marked contrast, V̇O2 kinetics are slowed in many disease conditions such as Type II diabetes (Regensteiner et al. 1998) and heart failure (CHF, Meakins and Long, 1927; Sietsema et al. 1986, 1994; Hepple et al. 1999; rev. Poole et al. 2005) as well as by advanced age (Babcock et al. 1994; Chilibeck et al. 1998; Barstow and Scheuermann, 2005), and there is evidence that this slowing occurs consequent to O2 delivery limitation as schematized in Figure 8. Intravital microscopy of spinotrapezius muscles from Type II diabetic (Padilla et al. 2006), CHF (Kindig et al. 1999; Richardson et al. 2003) and aged (Russell et al. 2003; Poole and Ferreira, 2007; Copp et al. 2009) animals offers mechanistic insights into how deficits in microvascular function contribute to perfusive and diffusive impediments in the O2 transport pathway. One expression of these impediments is the mismatching of O2 delivery-to-V̇O2 which often produces aberrant PmvO2 profiles particularly during the first 30–60 seconds following the onset of contractions and during recovery (Figure 9, Diederich et al. 2002; Behnke et al. 2004; McDonough et al. 2004; Poole et al. 2005; Ferreira et al. 2006; Behnke et al. 2007; Poole and Musch, 2010; Hirai et al. 2009). The consequences of impaired perfusive and diffusive conductances on V̇O2 kinetics in CHF are visualized in Figure 10 using an adaptation of the graphical analyses developed by Peter Wagner (for maximal V̇O2 conditions, Roca et al. 1992; Wagner et al. 1997) which combines the Fick principle (perfusive O2 delivery i.e., V̇O2 = Q̇ (arterial-venous O2 content difference), where Q̇ is blood flow) and Fick’s law (diffusive O2 flux, V̇O2 = DO2(PmvO2-PintramyocyteO2), where DO2 is the apparent O2 diffusing capacity of muscle) to explain how microvascular dysfunction might slow V̇O2 kinetics (and in some cases reduce the steady-state V̇O2) following the onset of muscle contractions. Thus, at any given time during the transient (Figure 10, inset) V̇O2 kinetics will be slowed and V̇O2 decreased by the culmination of perfusive and diffusive O2 transport deficits. The available evidence suggests that the reduced capillary RBC flux and failure of a significant proportion of capillaries to support that flux compromises both perfusive and diffusive O2 transport and contributes significantly to muscle and exercise dysfunction in aging and chronic diseases such as CHF and also Type II diabetes.
Figure 8.
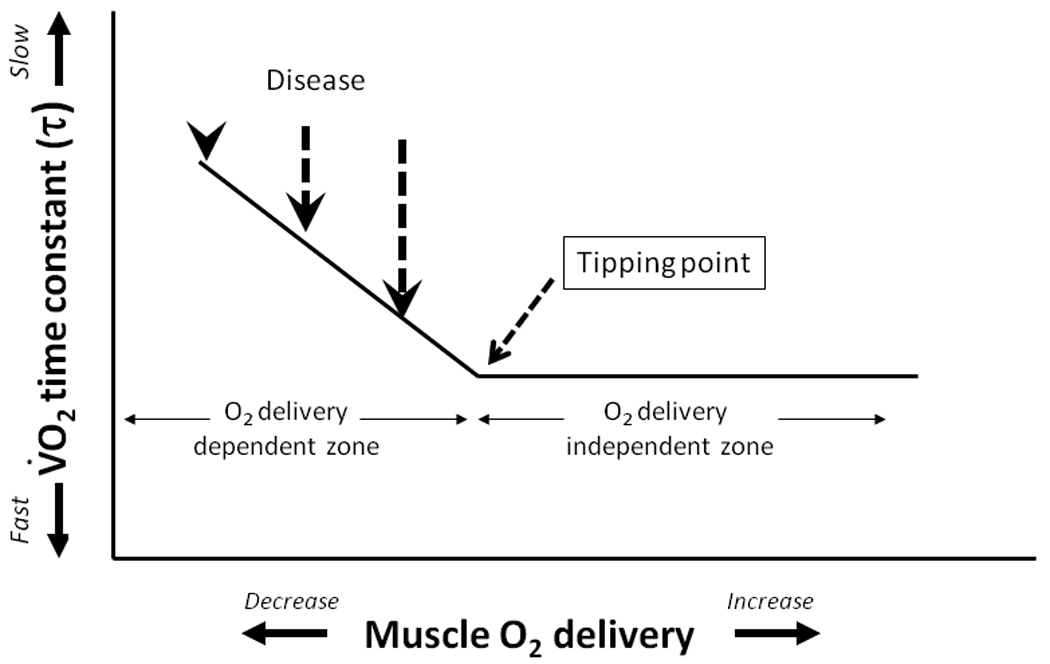
In healthy young individuals the control of O2 uptake (V̇O2) kinetics during moderate intensity exercise is believed to reside within the intramuscular oxidative machinery and is not O2 delivery dependent (i.e., these individuals lie to the right-hand side of the depicted hypothetical ‘tipping point’). Specifically, in healthy young individuals, neither muscle (Grassi et al. 1998) nor pulmonary V̇O2 (MacDonald et al. 1997) is speeded by augmented arterial O2 content or increased muscle O2 delivery. Moreover, strategies designed to decrease muscle O2 delivery do not always slow V̇O2 kinetics (Williamson et al. 1996; MacDonald et al. 1997; Nyberg et al. 2010). These observations suggest that healthy young individuals lie somewhere to the right of the tipping point and somewhere in the “O2 delivery independent zone.” In marked contrast, chronic diseases are associated with very slow V̇O2 kinetics that can be speeded by increasing arterial O2 content (e.g., Palange et al. 1995). Redrawn from Poole et al. (2008a). There is now substantial evidence that microcirculatory impairments likely contribute to these aberrant (slowed) V̇O2 kinetics and exercise intolerance in disease (i.e., heart failure, Kindig et al. 1999; Diederich et al. 2002; Richardson et al. 2003; diabetes, Padilla et al. 2006, 2007).
Figure 9.
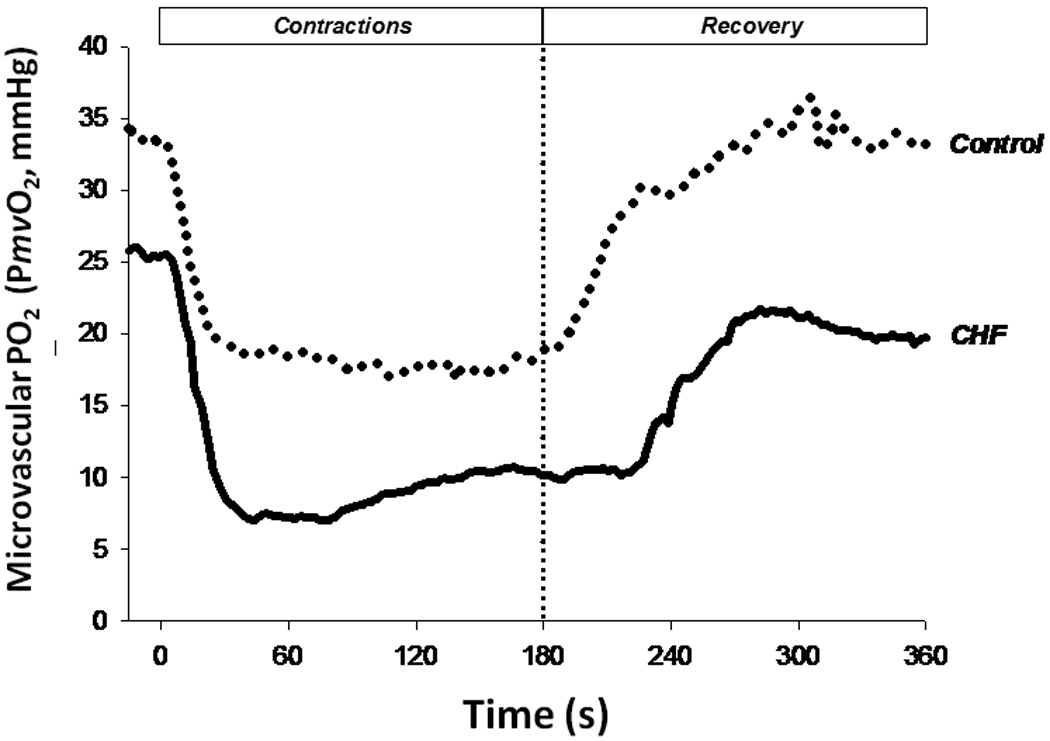
The profile of microvascular PO2 (PmvO2) as measured by phosphorescence quenching (Rumsey et al. 1988; Poole et al. 1995; Behnke et al. 2001) during (0–180 s) and following (181–360 s) muscle contractions in chronic heart failure (CHF) provides clear evidence of profound derangements in the ability to match O2 delivery-to-O2 utilization (O2-to-V̇O2). Impaired microcirculatory dynamics impair both perfusive and diffusive muscle O2 conductance slowing blood-myocyte O2 flux rates and constraining the speed of V̇O2 kinetics thereby forcing the muscle(s) to utilize increased substrate-level phosphorylation to support energetic requirements. Constructed from data set of Copp et al. (2010).
Figure 10.
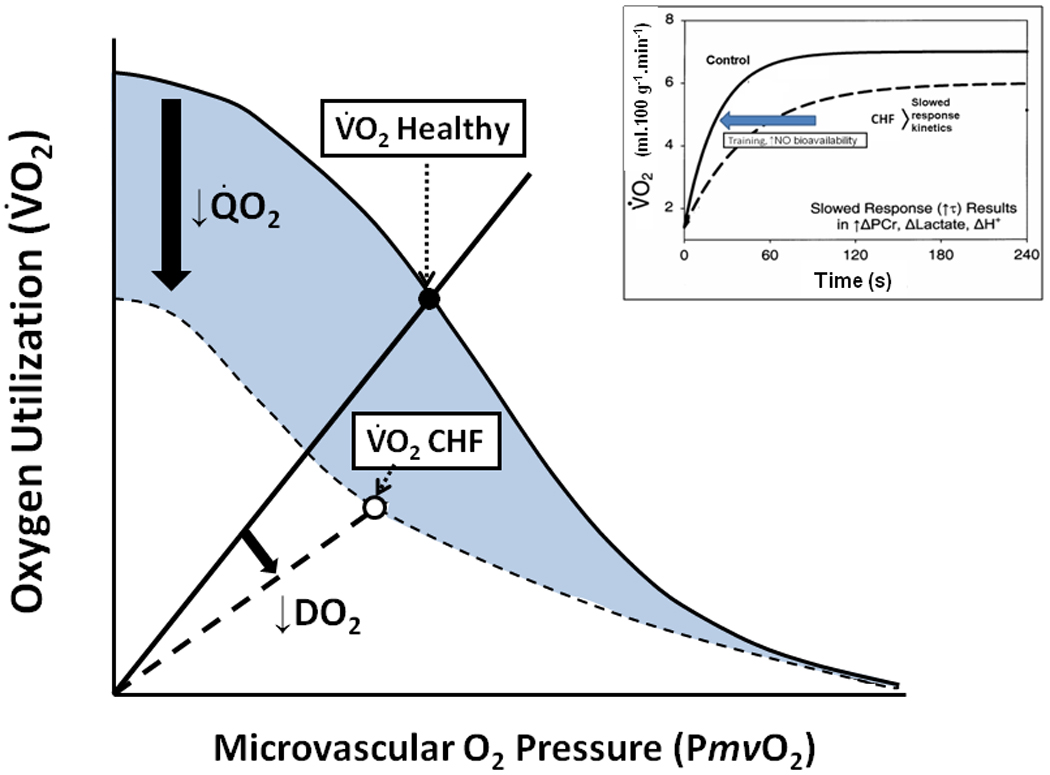
Schematic showing muscle O2 uptake (V̇O2) plotted as a function of microvascular PO2 (PmvO2). Conflation of perfusive (Fick principle, solid curved line) and diffusive (Fick’s law, solid straight line from origin) permits a mechanistic analysis of how these components resolve to achieve a given V̇O2. Note that chronic heart failure (CHF, dashed lines) reduces both perfusive and diffusive O2 fluxes such that, during the transient following the onset of contractions, V̇O2 kinetics are slowed and V̇O2 cannot, and does not, achieve levels seen in healthy individuals (inset). Resolution of the microvascular impairments that reduce perfusive (Q̇O2) and diffusive (DO2) O2 flux constitutes a critical step in the design of effective therapeutic interventions (e.g., exercise training, ↑nitric oxide (NO) bioavailability).
Conclusions
The scientific literature and medical/physiology textbooks consider that, following the onset of contractions, capillary recruitment is obligatory to reduce intramyocyte diffusion distances, increase muscle diffusing capacity and thereby augment blood-myocyte O2 flux. However, the compelling weight of evidence challenges this contention. Specifically, most capillaries may sustain RBC flux at rest and capillary recruitment does not appear to be requisite for the dynamic increase of blood-myocyte O2 flux (i.e., V̇O2) following initiation of contractions. Furthermore, in the absence of detectable intramyocyte PO2 gradients, muscle O2 diffusing capacity and mitochondrial O2 supply do not appear to be dependent on capillary-mitochondrial diffusion distances. These observations mandate a revision of the capillary recruitment theory to consider the likelihood that events within the individual capillaries (i.e., increased RBC flux and hematocrit combined with ‘longitudinal recruitment’ of capillary surface area) are responsible for the contraction-induced increase of muscle O2 diffusing capacity and blood-myocyte O2 flux – genuine homage to August and Marie Krogh’s memory and scientific legacy demands no less. Moreover, such a revision is particularly vital to determining the mechanistic bases for impaired muscle oxidative function in chronic disease.
“The wrong view of science is betrayed by the craving to be right.”
Sir Karl Popper (1902–1994)
Acknowledgements
The authors gratefully acknowledge the expertise of Dr. Leonardo F. Ferreira for construction of Figure 6. Studies presented herein from the authors’ laboratory were supported, in part, by grants from the National Institutes of Health (HL-50306, AG-19228), the American Heart Association (Grants-in-Aid to DCP, TIM), Capes-Brazil Fulbright Fellowship program (to DMH) and Kansas State University (SMILE Award).
Footnotes
Outline
This brief review considers Krogh’s theory of capillary recruitment and consequent reduction of capillary-to-mitochondrial diffusion distances in the light of subsequent empirical observations. Specifically, novel combination of intravital microscopy and microvascular O2 measurements indicate that capillary recruitment is not obligatory for rapid blood-myocyte O2 uptake (V̇O2) kinetics following the onset of contractions. Not only is there compelling evidence supporting that most capillaries sustain RBC flux in resting muscle, but intramyocyte PO2’s during exercise/contractions and measurements of muscle O2 diffusing capacity suggest that diffusion distances may not be limiting for capillary-mitochondrial O2 flux. In the light of these observations the question is posed as to how muscle perfusive and diffusive O2 conductances increase many-fold from rest to contractions and a working hypothesis is proposed. Within this context the mechanistic connection between impaired muscle V̇O2 (i.e., blood-myocyte) kinetics and microcirculatory dysfunction in aging and chronic disease is explored briefly.
Conflict of interest
The authors have no conflict of interest.
References
- Altman PL, Dittmer DS. Biology Data Book. 2nd. vol. III. Bethesda, MD: FASEB; 1974. [Google Scholar]
- Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle in man: Adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SI, Hudlicka O, Brown MD. Capillary red blood cell flow and activation of white blood cells in chronic muscle ischemia in the rat. Am J Physiol. 1997;272:H2757–H2764. doi: 10.1152/ajpheart.1997.272.6.H2757. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur PG, Hogan MC, Bebout DE, Wagner PD, Hochachka PW. Modeling the effects of hypoxia on ATP turnover in exercising muscle. J Appl Physiol. 1992;73:737–742. doi: 10.1152/jappl.1992.73.2.737. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Paterson DH, Cunningham DA, Dickinson JR. Exercise on-transient gas exchange kinetics are slowed as a function of age. Med Sci Sports Exerc. 1994;26:440–446. [PubMed] [Google Scholar]
- Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schoenbein GW, Poole DC. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol. 2000;279:H3131–H3137. doi: 10.1152/ajpheart.2000.279.6.H3131. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R899–R906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes. 2000;49:768–774. doi: 10.2337/diabetes.49.5.768. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Scheuermann BW. V̇O2 kinetics: Effects of maturation and ageing. In: Jones AM, Poole DC, editors. Oxygen uptake kinetics in sport, exercise and medicine. London: Routledge; 2005. [Google Scholar]
- Bassett DR., Jr Scientific contributions of A. V. Hill: exercise physiology pioneer. J Appl Physiol. 2002;93:1567–1582. doi: 10.1152/japplphysiol.01246.2001. [DOI] [PubMed] [Google Scholar]
- Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30:2880–2885. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- Bebout DE, Hogan MC, Hempleman SC, Wagner PD. Effects of training and immobilization on V̇O2 and DO2 in dog gastrocnemius muscle in situ. J Appl Physiol. 1993;74:1697–1703. doi: 10.1152/jappl.1993.74.4.1697. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol. 2002;133:229–239. doi: 10.1016/s1569-9048(02)00183-0. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Delp MD, McDonough P, Spier SA, Poole DC, Musch TI. Effects of chronic heart failure on microvascular oxygen exchange dynamics in muscles of contrasting fiber type. Cardiovasc Res. 2004;61:325–332. doi: 10.1016/j.cardiores.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Delp MD, Poole DC, Musch TI. Aging potentiates the effect of congestive heart failure on muscle microvascular oxygenation. J Appl Physiol. 2007;103:1757–1763. doi: 10.1152/japplphysiol.00487.2007. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol. 2001;126:53–63. doi: 10.1016/s0034-5687(01)00195-5. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzer P, Kongstad L, Grande PO. Capillary filtration coefficient is independent of number of perfused capillaries in cat skeletal muscle. Am J Physiol Heart Circ Physiol. 2001;280:H2697–H2706. doi: 10.1152/ajpheart.2001.280.6.H2697. [DOI] [PubMed] [Google Scholar]
- Borg TK, Caulfield JB. Morphology of connective tissue in skeletal muscle. Tissue Cell. 1980;12:197–207. doi: 10.1016/0040-8166(80)90061-0. [DOI] [PubMed] [Google Scholar]
- Bourdillon N, Mollard P, Letournel M, Beaudry M, Richalet JP. Non-invasive evaluation of the capillary recruitment in the human muscle during exercise in hypoxia. Respir Physiol Neurobiol. 2009;165:237–244. doi: 10.1016/j.resp.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Bredle DL, Samsel RW, Schumacker PT, Cain SM. Critical O2 delivery to skeletal muscle at high and low PO2 in endotoxemic dogs. J Appl Physiol. 1989;66:2553–2558. doi: 10.1152/jappl.1989.66.6.2553. [DOI] [PubMed] [Google Scholar]
- Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol. 1977;232:H705–H712. doi: 10.1152/ajpheart.1977.232.6.H705. [DOI] [PubMed] [Google Scholar]
- Burton KS, Johnson PC. Reactive hyperemia in individual capillaries of skeletal muscle. Am J Physiol. 1972;223:517–524. doi: 10.1152/ajplegacy.1972.223.3.517. [DOI] [PubMed] [Google Scholar]
- Carvalho H, Pittman RN. Longitudinal and radial gradients of PO2 in the hamster cheek pouch microcirculation. Microcirculation. 2008;15:215–224. doi: 10.1080/10739680701616175. [DOI] [PubMed] [Google Scholar]
- Caulfield JB, Borg TK. The collagen network of the heart. Lab Invest. 1979;40:364–372. [PubMed] [Google Scholar]
- Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Thompson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol. 1998;83:107–117. doi: 10.1113/expphysiol.1998.sp004087. [DOI] [PubMed] [Google Scholar]
- Clark AD, Barrett EJ, Rattigan S, Wallis MG, Clark MG. Insulin stimulates laser Doppler signal by rat muscle in vivo, consistent with nutritive flow recruitment. Clin Sci (Lond) 2001;100:283–290. [PubMed] [Google Scholar]
- Clark MG, Rattigan S, Barrett EJ, Vincent MA. Point: There is capillary recruitment in active skeletal muscle during exercise. J Appl Physiol. 2008;104:889–891. doi: 10.1152/japplphysiol.00779.2007. [DOI] [PubMed] [Google Scholar]
- Clark MG, Rattigan S, Newman JM, Eldershaw TP. Vascular control of nutrient delivery by flow redistribution within muscle: implications for exercise and post-exercise muscle metabolism. Int J Sports Med. 1998;19:391–400. doi: 10.1055/s-2007-971935. [DOI] [PubMed] [Google Scholar]
- Copp SW, Ferreira LF, Herspring KF, Musch TI, Poole DC. The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvasc Res. 2009;77:113–119. doi: 10.1016/j.mvr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Ferreira LF, Poole DC, Musch TI. Progressive chronic heart failure slows recovery of microvascular oxygen pressures following contractions in rat spinotrapezius muscle. Am J Physiol: Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00590.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle EF, Coggan AR, Hopper MK, Walter TJ. Determinants of endurance in well-trained cyclists. J Appl Physiol. 1988;64:2622–2630. doi: 10.1152/jappl.1988.64.6.2622. [DOI] [PubMed] [Google Scholar]
- Curtis SE, Vallet B, Winn MJ, Caufield JB, King CE, Chapler CK, Cain SM. Role of the vascular endothelium in O2 extraction during progressive ischemia in canine skeletal muscle. J Appl Physiol. 1995;79:1351–1360. doi: 10.1152/jappl.1995.79.4.1351. [DOI] [PubMed] [Google Scholar]
- Damon DH, Duling BR. Distribution of capillary blood flow in the microcirculation of the hamster: an in vivo study using epifluorescent microscopy. Microvasc Res. 1984;27:81–95. doi: 10.1016/0026-2862(84)90043-8. [DOI] [PubMed] [Google Scholar]
- Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Linder JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab. 2002;282:E714–E720. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- Dawson JM, Tyler KR, Hudlicka O. A comparison of the microcirculation in rat fast glycolytic and slow oxidative muscles at rest and during contractions. Microvasc Res. 1987;33:167–182. doi: 10.1016/0026-2862(87)90015-x. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Duling BR. Microvessel hematocrit: measurement and implications for capillary oxygen transport. Am J Physiol. 1987;252:H494–H503. doi: 10.1152/ajpheart.1987.252.3.H494. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol. 1990;258:H647–H654. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- Diederich ER, Behnke BJ, McDonough P, Kindig CA, Barstow TJ, Poole DC, Musch TI. Dynamics of microvascular oxygen partial pressure in contracting skeletal muscle of rats with chronic heart failure. Cardiovasc Res. 2002;56:479–486. doi: 10.1016/s0008-6363(02)00545-x. [DOI] [PubMed] [Google Scholar]
- Duling BR, Damon DH. An examination of the measurement of flow heterogeneity in striated muscle. Circ Res. 1987;60:1–13. doi: 10.1161/01.res.60.1.1. [DOI] [PubMed] [Google Scholar]
- Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am J Physiol Heart Circ Physiol. 2002;282:H156–H164. doi: 10.1152/ajpheart.2002.282.1.H156. [DOI] [PubMed] [Google Scholar]
- Ellis CG, Potter RF, Groom AC. The Krogh cylinder geometry is not appropriate for modelling O2 transport in contracted skeletal muscle. Adv Exp Med Biol. 1983;159:253–268. doi: 10.1007/978-1-4684-7790-0_23. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Myrhage R. Microvascular dimensions and blood flow in skeletal muscle. Acta Physiol Scand. 1972;86:211–222. doi: 10.1111/j.1748-1716.1972.tb05327.x. [DOI] [PubMed] [Google Scholar]
- Federspiel WJ, Popel AS. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res. 1986;32:164–189. doi: 10.1016/0026-2862(86)90052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 2006a;188:3–13. doi: 10.1111/j.1748-1716.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- Ferreira LF, McDonough P, Behnke BJ, Musch TI, Poole DC. Blood flow and O2 extraction as a function of O2 uptake in muscles composed of different fiber types. Respir Physiol Neurobiol. 2006b;153:237–249. doi: 10.1016/j.resp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Barclay JK. Microvascular hematocrit and permeability-surface area product in contracting canine skeletal muscle in situ. Microvasc Res. 1998;55:153–164. doi: 10.1006/mvre.1997.2067. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Segal SS. Simulation of motor unit recruitment and microvascular unit perfusion: spatial considerations. J Appl Physiol. 1997;83:1223–1234. doi: 10.1152/jappl.1997.83.4.1223. [DOI] [PubMed] [Google Scholar]
- Gayeski TE, Connett RJ, Honig CR. Oxygen transport in rest-work transition illustrates new functions for myoglobin. Am J Physiol. 1985;248:H914–H921. doi: 10.1152/ajpheart.1985.248.6.H914. [DOI] [PubMed] [Google Scholar]
- Gayeski TE, Connett RJ, Honig CR. Minimum intracellular PO2 for maximum cytochrome turnover in red muscle in situ. Am J Physiol. 1987;252:H906–H915. doi: 10.1152/ajpheart.1987.252.5.H906. [DOI] [PubMed] [Google Scholar]
- Gayeski TE, Honig CR. O2 gradients from sarcolemma to cell interior in red muscle at maximal V̇O2. Am J Physiol. 1986a;251:H789–H799. doi: 10.1152/ajpheart.1986.251.4.H789. [DOI] [PubMed] [Google Scholar]
- Gayeski TE, Honig CR. Shallow intracellular O2 gradients and absence of perimitochondrial O2 "wells" in heavily working red muscle. Adv Exp Med Biol. 1986b;200:487–494. doi: 10.1007/978-1-4684-5188-7_61. [DOI] [PubMed] [Google Scholar]
- Golub AS, Barker MC, Pittman RN. PO2 profiles near arterioles and tissue oxygen consumption in rat mesentery. Am J Physiol Heart Circ Physiol. 2007;293:H1097–H1106. doi: 10.1152/ajpheart.00077.2007. [DOI] [PubMed] [Google Scholar]
- Golub AS, Pittman RN. Recovery of radial PO2 profiles from phosphorescence quenching measurements in microvessels. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:169–176. doi: 10.1016/s1095-6433(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Golub AS, Pittman RN. PO2 measurements in the microcirculation using phosphorescence quenching microscopy at high magnification. Am J Physiol Heart Circ Physiol. 2008;294:H2905–H2916. doi: 10.1152/ajpheart.01347.2007. [DOI] [PubMed] [Google Scholar]
- Gorczynski RJ, Duling BR. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol. 1978;235:H505–H515. doi: 10.1152/ajpheart.1978.235.5.H505. [DOI] [PubMed] [Google Scholar]
- Grassi B. Regulation of oxygen consumption at the onset of exercise. Is it really controversial? Exercise and Sports Science Reviews. 2001;29:134–138. doi: 10.1097/00003677-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Grassi B. Limitation of skeletal muscle V̇O2 kinetics by inertia of cellular respiration. In: Jones AM, Poole DC, editors. Oxygen uptake kinetics in sport, exercise and medicine. London: Routledge; 2005. [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect V̇O2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Greenhaff PL, Hamann JJ, Kelley KM, Aschenbach WG, Constantin-Teodosiu D, Gladden LB. Oxygen uptake on-kinetics in dog gastrocnemius in situ following activation of pyruvate dehydrogenase by dichloroacetate. J Physiol. 2002;538:195–207. doi: 10.1113/jphysiol.2001.012984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Gray SD, McDonagh PF, Gore RW. Comparison of functional and total capillary densities in fast and slow muscles of the chicken. Pflugers Arch. 1983;397:209–213. doi: 10.1007/BF00584359. [DOI] [PubMed] [Google Scholar]
- Groebe K, Thews G. Calculated intra- and extracellular PO2 gradients in heavily working red muscle. Am J Physiol. 1990;259:H84–H92. doi: 10.1152/ajpheart.1990.259.1.H84. [DOI] [PubMed] [Google Scholar]
- Hanson WL, Emhardt JD, Bartek JP, Latham LP, Checkley LL, Capen RL, Wagner WW., Jr Site of recruitment in the pulmonary microcirculation. J Appl Physiol. 1989;66:2079–2083. doi: 10.1152/jappl.1989.66.5.2079. [DOI] [PubMed] [Google Scholar]
- Hargreaves D, Egginton S, Hudlicka O. Changes in capillary perfusion induced by different patterns of activity in rat skeletal muscle. Microvasc Res. 1990;40:14–28. doi: 10.1016/0026-2862(90)90003-a. [DOI] [PubMed] [Google Scholar]
- Helmholtz H. Über die Wärmeentwicklung der Muskelaction. Arch f Anat Physiol. 1848;15:144–164. [Google Scholar]
- Helmholtz H. Arch A nat u Physiol. 1848:144–164. [Google Scholar]
- Hepple RT, Hogan MC, Stary C, Bebout DE, Mathieu-Costello O, Wagner PD. Structural basis of muscle O2 diffusing capacity: evidence from muscle function in situ. J Appl Physiol. 2000;88:560–566. doi: 10.1152/jappl.2000.88.2.560. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Liu PP, Plyley MJ, Goodman JM. Oxygen uptake kinetics during exercise in chronic heart failure: influence of peripheral vascular reserve. Clin Sci (Lond) 1999;97:569–577. [PubMed] [Google Scholar]
- Hirai DM, Copp SW, Herspring KF, Ferreira LF, Poole DC, Musch TI. Aging impacts microvascular oxygen pressures during recovery from contractions in rat skeletal muscle. Respir Physiol Neurobiol. 2009;169:315–322. doi: 10.1016/j.resp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Arthur PG, Bebout DE, Hochachka PW, Wagner PD. Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol. 1992;73:728–736. doi: 10.1152/jappl.1992.73.2.728. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Roca J, West JB, Wagner PD. Dissociation of maximal O2 uptake from O2 delivery in canine gastrocnemius in situ. J Appl Physiol. 1989;66:1219–1226. doi: 10.1152/jappl.1989.66.3.1219. [DOI] [PubMed] [Google Scholar]
- Honig CR, Gayeski TE, Groebe K. Myoglobin and oxygen gradients. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung: Scientific Foundations. New York: Raven Press; 1997. [Google Scholar]
- Honig CR, Odoroff CL, Frierson JL. Capillary recruitment in exercise: rate, extent, uniformity, and relation to blood flow. Am J Physiol. 1980;238:H31–H42. doi: 10.1152/ajpheart.1980.238.1.H31. [DOI] [PubMed] [Google Scholar]
- Honig CR, Odoroff CL, Frierson JL. Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol. 1982;243:H196–H206. doi: 10.1152/ajpheart.1982.243.2.H196. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Howald H, Conley K, Lindstedt SL, Claasen H, Vock P, Weibel ER. Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol. 1985;59:320–327. doi: 10.1152/jappl.1985.59.2.320. [DOI] [PubMed] [Google Scholar]
- Hudlicka O. Regulation of muscle blood flow. Clin Physiol. 1985;5:201–229. [PubMed] [Google Scholar]
- Hudlicka O, Zweifach BW, Tyler KR. Capillary recruitment and flow velocity in skeletal muscle after contractions. Microvasc Res. 1982;23:201–213. doi: 10.1016/0026-2862(82)90065-6. [DOI] [PubMed] [Google Scholar]
- Hughson RL. Regulation of V̇O2 on-kinetics by O2 delivery. In: Jones AM, Poole DC, editors. Oxygen uptake kinetics in sport, exercise and medicine. London: Routledge; 2005. [Google Scholar]
- Jones AM, Poole DC. Oxygen uptake kinetics in sport, exercise and medicine. London: Routledge; 2005. [Google Scholar]
- Kano Y, Padilla DJ, Behnke BJ, Hageman KS, Musch TI, Poole DC. Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J Appl Physiol. 2005;99:1516–1522. doi: 10.1152/japplphysiol.00069.2005. [DOI] [PubMed] [Google Scholar]
- Kayar SR, Banchero N. Sequential perfusion of skeletal muscle capillaries. Microvasc Res. 1985;30:298–305. doi: 10.1016/0026-2862(85)90061-5. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Kelley KM, Howlett RA, Stary CM, Hogan MC. Assessment of O2 uptake dynamics in isolated single skeletal myocytes. J Appl Physiol. 2003;94:353–357. doi: 10.1152/japplphysiol.00559.2002. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Musch TI, Basaraba RJ, Poole DC. Impaired capillary hemodynamics in skeletal muscle of rats in chronic heart failure. J Appl Physiol. 1999;87:652–660. doi: 10.1152/jappl.1999.87.2.652. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Poole DC. A comparison of the microcirculation in the rat spinotrapezius and diaphragm muscles. Microvasc Res. 1998;55:249–259. doi: 10.1006/mvre.1998.2075. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Poole DC. Effects of skeletal muscle sarcomere length on in vivo capillary distensibility. Microvasc Res. 1999;57:144–152. doi: 10.1006/mvre.1998.2123. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Poole DC. Sarcomere length-induced alterations of capillary hemodynamics in rat spinotrapezius muscle: vasoactive vs passive control. Microvasc Res. 2001;61:64–74. doi: 10.1006/mvre.2000.2284. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol. 2002;92:2513–2520. doi: 10.1152/japplphysiol.01222.2001. [DOI] [PubMed] [Google Scholar]
- Klitzman B, Damon DN, Gorczynski RJ, Duling BR. Augmented tissue oxygen supply during striated muscle contraction in the hamster. Relative contributions of capillary recruitment, functional dilation, and reduced tissue PO2. Circ Res. 1982;51:711–721. doi: 10.1161/01.res.51.6.711. [DOI] [PubMed] [Google Scholar]
- Klitzman B, Duling BR. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol. 1979;237:H481–H490. doi: 10.1152/ajpheart.1979.237.4.H481. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919a;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol. 1919b;52:457–474. doi: 10.1113/jphysiol.1919.sp001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. A Gas Analysis Apparatus Accurate to 0.001% mainly designed for Respiratory Exchange Work. Biochem J. 1920a;14:267–281. doi: 10.1042/bj0140267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. Nobel Lecture. 1920b [Google Scholar]
- Krogh M. The diffusion of gases through the lungs of man. J Physiol. 1915;49:271–300. doi: 10.1113/jphysiol.1915.sp001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Jones AM, Wilkerson DP, Calbet JA, Bangsbo J. Muscular and pulmonary O2 uptake kinetics during moderate- and high-intensity sub-maximal knee-extensor exercise in humans. J Physiol. 2009;587:1843–1856. doi: 10.1113/jphysiol.2008.166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB, White J, Rouk K. A method for using microspheres to measure muscle blood flow in exercising rats. J Appl Physiol. 1982;52:1629–1635. doi: 10.1152/jappl.1982.52.6.1629. [DOI] [PubMed] [Google Scholar]
- Lutjemeier BJ, Ferreira LF, Poole DC, Townsend D, Barstow TJ. Muscle microvascular hemoglobin concentration and oxygenation within the contraction-relaxation cycle. Respir Physiol Neurobiol. 2008;160:131–138. doi: 10.1016/j.resp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- MacDonald M, Pedersen PK, Hughson RL. Acceleration of V̇O2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol. 1997;83:1318–1325. doi: 10.1152/jappl.1997.83.4.1318. [DOI] [PubMed] [Google Scholar]
- Malvin GM, Wood SC. Effects of capillary red cell density on gas conductance of frog skin. J Appl Physiol. 1992;73:224–233. doi: 10.1152/jappl.1992.73.1.224. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition and Human Performance. 6 ed. London: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- McDonough P, Behnke BJ, Musch TI, Poole DC. Effects of chronic heart failure in rats on the recovery of microvascular PO2 after contractions in muscles of opposing fibre type. Exp Physiol. 2004;89:473–485. doi: 10.1113/expphysiol.2004.027367. [DOI] [PubMed] [Google Scholar]
- Meakins J, Long CN. Oxygen consumption, oxygen debt and lactic acid in circulatory failure. J Clin Invest. 1927;4:273–293. doi: 10.1172/JCI100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Foley JM. Cellular Processes Integrating the Metabolic Response to Exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc.; 1996. [Google Scholar]
- Molè PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol. 1999;277:R173–R180. doi: 10.1152/ajpregu.1999.277.1.R173. [DOI] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol. 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Musch TI, Poole DC. Blood flow response to treadmill running in the rat spinotrapezius muscle. Am J Physiol. 1996;271:H2730–H2734. doi: 10.1152/ajpheart.1996.271.6.H2730. [DOI] [PubMed] [Google Scholar]
- Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol. 1992;262:H411–H419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Saltin B, Hellsten Y, Bangsbo J. Low blood flow at onset of moderate-intensity moderate-intensity exercise does not limit muscle oxygen uptake. Am J Physiol. 2010;298:R843–R848. doi: 10.1152/ajpregu.00730.2009. [DOI] [PubMed] [Google Scholar]
- Oude Vrielink HH, Slaaf DW, Tangelder GJ, Reneman RS. Does capillary recruitment exist in young rabbit skeletal muscle? Int J Microcirc Clin Exp. 1987;6:321–332. [PubMed] [Google Scholar]
- Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;291:H2439–H2444. doi: 10.1152/ajpheart.00290.2006. [DOI] [PubMed] [Google Scholar]
- Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on muscle microvascular oxygen pressures. Respir Physiol Neurobiol. 2007;156:187–195. doi: 10.1016/j.resp.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Palange P, Galassetti P, Mannix ET, Farber MO, Manfredi F, Serra P, Carlone S. Oxygen effect of O2 deficit and V̇O2 kinetics during exercise in chronic obstructive disease. J Appl Physiol. 1995;78:2228–2234. doi: 10.1152/jappl.1995.78.6.2228. [DOI] [PubMed] [Google Scholar]
- Pande JN, Hughes JM. Regional pulmonary clearance of inhaled C15O and C15O2 in man at rest and during exercise. Clin Physiol. 1983;3:491–501. doi: 10.1111/j.1475-097x.1983.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Parthasarathi K, Lipowsky HH. Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am J Physiol. 1999;277:H2145–H2157. doi: 10.1152/ajpheart.1999.277.6.H2145. [DOI] [PubMed] [Google Scholar]
- Pittman RN, Golub AS, Carvalho H. Measurement of oxygen in the microcirculation using phosphorescence quenching microscopy. Adv Exp Med Biol. 2010;662:157–162. doi: 10.1007/978-1-4419-1241-1_22. [DOI] [PubMed] [Google Scholar]
- Poole DC. Influence of exercise training on skeletal muscle oxygen delivery and utilization. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung: Scientific Foundations. New York: Raven Press; 1997. [Google Scholar]
- Poole DC, Barstow TJ, McDonough P, Jones AM. Control of oxygen uptake during exercise. Med Sci Sports Exerc. 2008a;40:462–474. doi: 10.1249/MSS.0b013e31815ef29b. [DOI] [PubMed] [Google Scholar]
- Poole DC, Behnke BJ, McDonough P, Wilson DF. Measurement of muscle microvascular oxygen pressures: compartmentalization of phosphorescent probe. Microcirc. 2004;11:317–326. doi: 10.1080/10739680490437487. [DOI] [PubMed] [Google Scholar]
- Poole DC, Behnke BJ, Padilla DJ. Dynamics of muscle microcirculatory oxygen exchange. Med Sci Sports Exerc. 2005a;37:1559–1566. doi: 10.1249/01.mss.0000177471.65789.ce. [DOI] [PubMed] [Google Scholar]
- Poole DC, Brown MD, Hudlicka O. Counterpoint: There is not capillary recruitment in active skeletal muscle during exercise. J Appl Physiol. 2008b;104:891–893. doi: 10.1152/japplphysiol.00779.2007a. discussion 893–4. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ferreira LF. Oxygen exchange in muscle of young and old rats: muscle-vascular-pulmonary coupling. Exp Physiol. 2007;92:341–346. doi: 10.1113/expphysiol.2006.036764. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ferreira LF, Behnke BJ, Barstow TJ, Jones AM. The final frontier: oxygen flux into muscle at exercise onset. Exerc Sport Sci Rev. 2007;35:166–173. doi: 10.1097/jes.0b013e318156e4ac. [DOI] [PubMed] [Google Scholar]
- Poole DC, Kindig CA, Behnke BJ. V̇O2 kinetics in different disease states. In: Jones AM, Poole DC, editors. Oxygen uptake kinetics in sport, exercise and medicine. London: Routledge; 2005b. [Google Scholar]
- Poole DC, Mathieu-Costello O. Analysis of capillary geometry in rat subepicardium and subendocardium. Am J Physiol. 1990;259:H204–H210. doi: 10.1152/ajpheart.1990.259.1.H204. [DOI] [PubMed] [Google Scholar]
- Poole DC, Mathieu-Costello O. Capillary and fiber geometry in rat diaphragm perfusion fixed in situ at different sarcomere lengths. J Appl Physiol. 1992;73:151–159. doi: 10.1152/jappl.1992.73.1.151. [DOI] [PubMed] [Google Scholar]
- Poole DC, Musch TI. Muscle microcirculatory O2 exchange in health and disease. Adv Exp Med Biol. 2010;662:301–307. doi: 10.1007/978-1-4419-1241-1_43. [DOI] [PubMed] [Google Scholar]
- Poole DC, Musch TI, Kindig CA. In vivo microvascular structural and functional consequences of muscle length changes. Am J Physiol. 1997;272:H2107–H2114. doi: 10.1152/ajpheart.1997.272.5.H2107. [DOI] [PubMed] [Google Scholar]
- Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol. 2000;88:186–194. doi: 10.1152/jappl.2000.88.1.186. [DOI] [PubMed] [Google Scholar]
- Poole DC, Wagner PD, Wilson DF. Diaphragm microvascular plasma PO2 measured in vivo. J Appl Physiol. 1995;79:2050–2057. doi: 10.1152/jappl.1995.79.6.2050. [DOI] [PubMed] [Google Scholar]
- Rattigan S, Appleby GJ, Miller KA, Steen JT, Dora KA, Colquhoun EQ, Clark MG. Serotonin inhibition of 1-methylxanthine metabolism parallels its vasoconstrictor activity and inhibition of oxygen uptake in perfused rat hindlimb. Acta Physiol Scand. 1997a;161:161–169. doi: 10.1046/j.1365-201X.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- Rattigan S, Bradley EA, Richards SM, Clark MG. Muscle metabolism and control of capillary blood flow: insulin and exercise. Essays Biochem. 2006;42:133–144. doi: 10.1042/bse0420133. [DOI] [PubMed] [Google Scholar]
- Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997b;46:1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- Rattigan S, Wheatley C, Richards SM, Barrett EJ, Clark MG. Exercise and insulin-mediated capillary recruitment in muscle. Exerc Sport Sci Rev. 2005;33:43–48. [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol. 1998;85:310–317. doi: 10.1152/jappl.1998.85.1.310. [DOI] [PubMed] [Google Scholar]
- Renkin EM, Gray SD, Dodd LR. Filling of microcirculation in skeletal muscles during timed India ink perfusion. Am J Physiol. 1981;241:H174–H186. doi: 10.1152/ajpheart.1981.241.2.H174. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B, Wagner PD. Determinants of maximal exercise V̇O2 during single leg knee-extensor exercise in humans. Am J Physiol. 1995a;268:H1453–H1461. doi: 10.1152/ajpheart.1995.268.4.H1453. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995b;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol. 1998;85:627–634. doi: 10.1152/jappl.1998.85.2.627. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson ER, Kendrick K, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Wagner PD. Red blood cell transit time in man: theoretical effects of capillary density. Adv Exp Med Biol. 1994;361:521–532. doi: 10.1007/978-1-4615-1875-4_91. [DOI] [PubMed] [Google Scholar]
- Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol. 2003;95:1055–1062. doi: 10.1152/japplphysiol.00308.2003. [DOI] [PubMed] [Google Scholar]
- Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at V̇O2 max. J Appl Physiol. 1992;73:1067–1076. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. Oxford: Oxford University Press; 1993. [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988;241:1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI. Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 2003;285:H251–H258. doi: 10.1152/ajpheart.01086.2002. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gollnick PD. Handbook of Physiology. Section 10: Skeletal muscle. Bethesda, MD: American Physiological Society; 1983. Skeletal muscle adaptability: significance for metabolism and performance; pp. 555–631. 1983. [Google Scholar]
- Sarelius IH, Duling BR. Direct measurement of microvessel hematocrit, red cell flux, velocity, and transit time. Am J Physiol. 1982;243:H1018–H1026. doi: 10.1152/ajpheart.1982.243.6.H1018. [DOI] [PubMed] [Google Scholar]
- Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol. 1988;64:649–657. doi: 10.1152/jappl.1988.64.2.649. [DOI] [PubMed] [Google Scholar]
- Schaffartzik W, Barton ED, Poole DC, Tsukimoto K, Hogan MC, Bebout DE, Wagner PD. Effect of reduced hemoglobin concentration on leg oxygen uptake during maximal exercise in humans. J Appl Physiol. 1993;75:491–498. doi: 10.1152/jappl.1993.75.2.491. [DOI] [PubMed] [Google Scholar]
- Sietsema KE, Ben-Dov I, Zhang YY, Sullivan C, Wasserman K. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest. 1994;105:1693–1700. doi: 10.1378/chest.105.6.1693. [DOI] [PubMed] [Google Scholar]
- Sietsema KE, Cooper DM, Perloff JK, Rosove MH, Child JS, Canobbio MM, Whipp BJ, Wasserman K. Dynamics of oxygen uptake during exercise in adults with cyanotic congenital heart disease. Circulation. 1986;73:1137–1144. doi: 10.1161/01.cir.73.6.1137. [DOI] [PubMed] [Google Scholar]
- Smaje L, Zweifach BW, Intaglietta M. Micropressures and capillary filtration coefficients in single vessels of the cremaster muscle of the rat. Microvasc Res. 1970;2:96–110. doi: 10.1016/0026-2862(70)90055-5. [DOI] [PubMed] [Google Scholar]
- Vetterlein F, dal Ri H, Schmidt G. Capillary density in rat myocardium during timed plasma staining. Am J Physiol. 1982;242:H133–H141. doi: 10.1152/ajpheart.1982.242.2.H133. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290:E1191–E1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Krustrup P, Dawson E, Secher NH. Arm blood flow and oxygenation on the transition from arm to combined arm and leg exercise in humans. J Physiol. 2003;547:641–648. doi: 10.1113/jphysiol.2002.034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voter WA, Gayeski TE. Determination of myoglobin saturation of frozen specimens using a reflecting cryospectrophotometer. Am J Physiol. 1995;269:H1328–H1341. doi: 10.1152/ajpheart.1995.269.4.H1328. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Hoppeler H, Saltin B. Determinants of Maximal Oxygen Uptake. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung: Scientific Foundations. New York: Raven Press; 1997. [Google Scholar]
- Wagner WW. Recruitment of Gas Exchange Vessels. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung: Scientific Foundations. New York: Raven Press; 1997. [Google Scholar]
- Wearn JT, Ernstene AC, Bromer AW, Barr JS, German WJ, Zschiesche LJ. The normal behavior of the pulmonary blood vessels with observations on the intermittence of the flow of blood in the arterioles and capillaries. Am J Physiol. 1934;109:236–256. [Google Scholar]
- Welsh DG, Segal SS. Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circ Res. 1996;79:551–559. doi: 10.1161/01.res.79.3.551. [DOI] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology: People and Ideas. New york: Oxford University Press; 1996. [Google Scholar]
- Whalen WJ, Buerk D, Thuning C, Kanoy BE, Jr, Duran WN. Tissue PO2, V̇O2, venous PO2 and perfusion pressure in resting dog gracilis muscle perfused at constant flow. Adv Exp Med Biol. 1976;75:639–655. doi: 10.1007/978-1-4684-3273-2_75. [DOI] [PubMed] [Google Scholar]
- Whalen WJ, Nair P, Buerk D, Thuning CA. Tissue PO2 in normal and denervated cat skeletal muscle. Am J Physiol. 1974;227:1221–1225. doi: 10.1152/ajplegacy.1974.227.6.1221. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Raven PB, Whipp BJ. Unaltered oxygen uptake kinetics at exercise onset with lower-body positive pressure in humans. Exp Physiol. 1996;81:695–705. doi: 10.1113/expphysiol.1996.sp003970. [DOI] [PubMed] [Google Scholar]
- Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53:2175–2183. doi: 10.1016/j.jacc.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youd JM, Newman JM, Clark MG, Appleby GJ, Rattigan S, Tong AC, Vincent. MA. Increased metabolism of infused 1-methylxanthine by working muscle. Acta Physiol Scand. 1999;166:301–308. doi: 10.1046/j.1365-201x.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005;99:1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]


