Abstract
Troponin C (TnC), present in all striated muscle, is the Ca2+-activated trigger that initiates myocyte contraction. The binding of Ca2+ to TnC initiates a cascade of conformational changes involving the constituent proteins of the thin filament. The functional properties of TnC and its ability to bind Ca2+ have significant regulatory influence on the contractile reaction of muscle. Changes in TnC may also correlate with cardiac and various other muscle-related diseases. We report here the implementation of the PLIMSTEX strategy (Protein Ligand Interaction by Mass Spectrometry, Titration and H/D Exchange) to elucidate the binding affinity of TnC with Ca2+ and, more importantly, to determine the order of Ca2+ binding of the four EF hands of the protein. The four equilibrium constants, K1 = (5 ± 5) × 10 M-1, K2 = (1.8 ± 0.8) × 107 M-1, K3 = (4.2 ± 0.9) × 106 M-1, and K4 = (1.6 ± 0.6) × 106 M-1, agree well with determinations by other methods and serve to increase our confidence in the PLIMSTEX approach. We determined the order of binding to the four EF hands to be III, IV, II, and I by extracting from the H/DX results the deuterium patterns for each EF hand for each state of the protein (apo through fully Ca2+ bound). This approach, demonstrated for the first time, may be general for determining binding orders of metal ions and other ligands to proteins.
The basis of muscle contraction has been well studied over four decades. There are primarily two types of proteins involved in this process: troponin and tropomyosin (1-11). Muscle contraction is initiated by an increase in intracellular free Ca2+ concentration and is controlled by the binding of Ca2+ to troponin (1-3, 8-10, 12-17). Troponin, which is present in skeletal and cardiac muscle of various species of mammals, birds, and some invertebrates, has three subunits: troponin C (TnC), troponin T (TnT), and troponin I (TnI). Each subunit plays a special rule in modulating the movement of muscle filament.
Troponin C, which is the subject of this study, is an 18 kDa protein that is structurally homologous in skeletal and cardiac muscle (1, 3) but has different Ca2+ stoichiometry in these two tissues. Skeletal troponin C has four helix-loop-helix motifs (EF hands) that bind to Ca2+. The EF-hand motif, which is a conserved structure first identified in parvalbumins (18), allows Ca2+ to interact with carboxyl and hydroxyl groups of amino-acid sidechains located at positions 1, 3, 5, 7, 9, and 12 of a 12-membered loop (1, 19). One of the EF hands (EF-I) in cardiac troponin C is defunct so the protein binds only three Ca2+ (1, 2, 7, 20-23). In skeletal troponin C, two of four EF hands are located in the C-terminal (EF-III and EF-IV) and have higher affinities for Ca2+ (Ka ~ 107 M-1) than the other two EF hands; they are usually saturated with Ca2+ or Mg2+ in tissue. The binding of Ca2+ at these two sites helps maintain the interaction between the C-terminus of troponin C, the N-terminus of troponin I, and the C-terminus of troponin T (1, 24-27). The latter subunit binds to tropomyosin on the muscle thin filament, serving as a key in complex formation. When the two N-terminal EF hands (EF-I and EF-II), which have lower affinities for Ca2+ (Ka ~ 105-106 M-1), bind to Ca2+, troponin C undergoes a conformational change to form a hydrophobic core (15, 23, 26, 28-30). The inhibitor region on the C-terminus of troponin I then interacts with this core and releases tropomyosin and actin. The free actins are able to interact with the myosin heads on the thick filament and produce muscle contraction (1-3).
The Ca2+ binding to troponin C and its relation to muscle-contraction disorders have implications in cardiac-related diseases. The amino-acid sequence of troponin C and the phosphorylation of troponin I induced by over-activation of protein kinase A and C (31, 32) affect the binding of Ca2+ to troponin C. Newly designed drugs interact with troponin C and adjust its affinities to Ca2+ (33, 34).
The Ca2+-binding and protein-target binding properties of troponin C have been the subjects of numerous studies using NMR (21, 25, 35-37), calorimetry (38) and fluorescence (26). Hydrogen/deuterium exchange (H/DX) coupled with mass spectrometry is another powerful method to study protein conformation, protein-protein or protein-ligand interactions (39-46). With H/DX, one can measure the relative rates of exchange of backbone amide hydrogen with deuterium and thereby follow changes in H-bonding, which often is parallel to relative solvent accessibilities. Recently, H/DX was applied to study the regulatory function of human cardiac troponin complex and the conformational changes of troponin complex induced by Ca2 binding (47, 48).
We describe here the use of PLIMSTEX (Protein–ligand interactions by mass spectrometry, titration, and H/D exchange) (49-51) to determine again the affinities of Ca2+ to rabbit skeletal troponin C. The purpose of the determination is to test the ability of PLIMSTEX to extract these values in a complex 4:1 interaction. From the determined affinities (equilibrium constants), we calculated the fractional species for the variousTnC-Ca2 states as a function of [Ca2+]. The deuterium exchange patterns of four peptic peptides representing the four EF hands of rabbit skeletal troponin C (EF-I peptide 28-44: DADGGGDISVKELGTVM; EF-II peptide 63-73: EVDEDGSGTID; EF-III peptide 100-109: FRIFDRNADG; EF-IV peptide 136-149: MKDGDKNNDGRIDF) were then measured. Guided by the fractional species calculation, the deuterium patterns for each EF-hand were measured as a function of the various Ca2+ binding states (i.e., 0, 1, 2, 3, and 4) to determine how D distributions change as a function of the binding states. Previous efforts to elucidate the binding properties of Ca2+ to troponin C used NMR, fluorescence measurement, Ca2+ ion-selective electrode measurements, and mutagenesis (36, 37, 52, 53). Although some insights were achieved, a full understanding on the Ca2+ binding order is still lacking. Our strategy provided data that, for the first time, gives the binding order of Ca2+ to full length troponin C in a single and relatively simple experiment. Our ultimate goal is to extend the approach to study the effect of troponin I and troponin T on the affinities and orders of binding of Ca2+ to troponin C.
EXPERIMENTAL PROCEDURE
a. Materials
Rabbit skeletal Troponin C (TnC) was purchased from Ocean Biologics Inc (Seattle, WA). Potassium chloride, calcium chloride, EGTA tetra sodium salt [ethylene-bis(oxyethylenenitrilo)tetraacetic acid tetrasodium], and HEPES hemisodium salt [N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) hemisodium salt] were purchased from Sigma-Aldrich (St.Louis, MO). Deuterium oxide was purchased from Cambridge Isotope Laboratories Inc (Andover, MA). Immobilized pepsin on agarose was purchased from Pierce (Rockford, IL).
b. Apo TnC preparation
TnC was dialyzed against EGTA to obtain apo-TnC. A 1 mL protein solution containing 180 μg of TnC was prepared in 10 mM HEPES and 150 mM KCl. The solution was dialyzed by using Slide-A-Lyzer Dialysis Cassettes 7000 MWCO (Pierce, CA). The dialysis membrane was first boiled two times in 10 mM EGTA for 10 min to remove heavy metals from the membrane and then soaked in deionized H2O for 10 min. The protein solution was then injected into the cassette and dialyzed against 10 mM HEPES, 150 mM KCl and 1 mM EGTA for 2 h. The buffer was exchanged for fresh buffer, and the solution was dialyzed overnight at 4 °C. The protein solution was then dialyzed against 10 mM HEPES, 150 mM KCl for 3 h, fresh buffer was added, and the protein was dialyzed again for 3 h at 4 °C. The concentration of the final protein solution was measured by using a UV spectrophotometer at 280 nm absorption. The protein solution was stored at -80 °C.
c. H/D exchange (H/DX) protocol
H/DX was initiated by diluting the protein solution 1:40 into D2O buffer (10 mM HEPES, 150 mM KCl) at 25 °C. At various times, H/DX was quenched by adding sufficient 1 M HCl at 0 °C to give a final pH of 2.5.
For Global protein H/DX experiments, a quenched protein solution was loaded onto a C8 guard column (1 mm × 15 mm, Optimize Technologies, Oregon, City, OR). The column was pre-equilibrated with 200 μL 0.2% formic acid in water (0 °C). After loading the protein solution, the column was washed with 250 μL 0.2% formic acid in ice cold normal water (0 °C) to back-exchange the labile sites on side chains. The protein was eluted by using a gradient from 5% to 40% solvent B in 3 min, 40% to 100% solvent B in 3 min and back to 5% solvent B in 2 min. (Waters CapLC, Manchester, U.K; solvent A, water containing 0.1% formic acid; solvent B, acetonitrile containing 0.1% formic acid).
For peptide-level H/DX experiments, 5 μL of immobilized pepsin on agarose was added to the solution. Protein digestion took place at 0 °C for 3 min. The final solution was centrifuged for 2-3 s to bring down the agarose beads. The peptide-containing supernatant was then loaded onto a C18 guard column (1 mm × 15 mm, Optimize Technologies, Oregon, City, OR). The column was pre-equilibrated with 200 μL of 0.2% formic acid in water (0 °C). After loading, the column was washed with 250 μL of aqueous 0.2% formic acid (H2O) to back-exchange the labile sites. The protein was eluted by using a gradient from 5% to 35% solvent B in 3 min, 35% to 80% solvent B in 4 min, 80% to 100% solvent B in 1 min and back to 5% solvent B in 2 min. All LC connection lines were immersed in a water-ice (0 °C) bath. (Waters nanoACQUITY UPLC, Manchester, U.K; solvent A, water containing 0.1% formic acid; solvent B, acetonitrile containing 0.1% formic acid).
d. LC-ESI/MS analysis with a Q-TOF mass spectrometer
Global protein H/DX results were acquired on a Waters (Micromass) (Manchester, U.K.) Q-TOF Ultima spectrometer equipped with a Z-spray ESI source. The instrument settings were: capillary voltage, 3.0 kV; cone voltage, 80 V; source and desolvation temperatures, 80 °C and 180 °C, respectively. The cone and desolvation gas flow rates were 40 and 400 L/h, respectively. The MS profile used for quadrupole transmission was: scan from m/z 500, dwell for 5% of the scan time, ramp to m/z 1000 for 45% of the scan time, and then dwell at m/z 1000 for 50% of the scan time.
Peptide-level H/DX results were acquired on a Maxis (Bruker) (Bremen, Germany) Q-TOF spectrometer. The instrument settings were: capillary voltage, 3.8 kV; nebulizer gas, 0.4 Bar; drying gas flow rate and temperature, 4.0 L/min and 180 °C; funnel RF, 400 V(pp).
e. LC-ESI/MS/MS analysis of protein digests
To establish the peptide profile from the pepsin digestion of TnC, 100 pmol TnC was digested with pepsin for 3 min, and the peptides were identified by accurate mass and sequencing by product-ion analysis on a Thermo LTQ XL Orbitrap (Thermo Fisher, San Jose, CA). Samples were loaded and eluted by using an Ultra 1D+ UPLC and autosampler (Eksigent, Dublin, CA). A 75 μm diameter column was pulled with a laser-based column puller (Sutter Instruments, Novato, CA) and packed with 12 cm of Magic C18AQ reverse phase media (Michrom Bioresources, Auburn, CA). The column was interfaced via a nanospray source (New Objective, Woburn, MA) and eluted with a 60 min gradient from 2-98% solvent B (acetonitrile with 0.1% formic acid). The spray voltage was 2.0 kV, and the capillary temperature was 200 °C. One full mass spectral acquisition triggered six scans of MS/MS whereby the most abundant precursor ions were activated for sequencing. The product-ion spectra (MS/MS) data were centroided during the acquisition.
f. Mascot database search
Thermo RAW files were processed using extract_msn (2007 version 4.0, Thermo Fisher, San Jose, CA) with a grouping tolerance of 0.8 Da, an intermediate scan setting of 1, and a minimum of 1 scan per group. The NCBI nonredundant database (version 20080718, restricted to mammals) was searched by using MASCOT 2.2.06 (Matrix Science, Oxford, U.K.) with the following settings: enzyme, none; MS tolerance, 10 ppm; MS/MS tolerance, 0.8 Da; maximum number of missed cleavages, 3; peptide charge of 1+, 2+ and 3+; oxidation of methionine was set as variable modification.
g. Data analysis
The uptake of deuterium by the global protein was the average mass differences between the masses of the deuterated protein and the undeuterated protein. The back-exchange rate was measured to be one deuterium loss per minute (45). No correction for back exchange was applied because the time between sample quench and the measurement with the mass spectrometer was less than 3 min, and all data were treated consistently. For global protein analysis, deconvoluted spectra were generated by using the MaxEnt1 algorithm (MassLynX version 4.0). The parameter settings were: resolution, 1.00 Da/channel; uniform Gaussian width at half height, 1.00 Da; minimum intensity ratios, 33% for left peaks and right peaks.
h. Kinetic modeling
The global protein kinetic data were fit with a fixed-rate-constant binning model by MathCAD (Math-Soft. Inc. Cambridge, MA) in which the root mean square (RMS) was minimized (50, 51). All exchangeable H's were separated into four fixed rate-constant bins (k = 10, 1, 0.1, 0.01 min-1). These rate constants were selected because the rates of exchange change measurably in the time frame 0.17 min to 540 min, and the H/DX became relatively constant after 60 min; hence the brackets of 10 min-1 (fast exchangers, half-life ~0.07 min) and 0.01 min-1 (slow exchangers, half-life ~69 min) were chosen. Although the largest rate constant for H/DX is >100 min-1 for unstructured peptides (54), we are yet unable to obtain data at the short times that correspond to this rate constant. Three trials were fit separately, and the results were averaged and reported with standard deviations.
i. Titration and species fraction calculation
The method for fitting the titration curve was described previously (51). Briefly, there are nine parameters involved in the 1:4 protein-ligand binding systems: Ka1, Ka2, Ka3, Ka4, D0, ΔD1, ΔD2, ΔD3, ΔD4; Ka is the binding constant; D0 is the deuterium uptake of protein in the absence of ligands (Apo form); ΔDx is the difference between the average of deuterium uptake of the complex in the presence of x ligands and that of the apo form. The non-linear least squares fitting utilized the “Minimize” function of MathCAD (Math-Soft. Inc. Cambridge, MA) to minimize the root mean square (RMS) of all inputs by optimizing the parameters being searched. To obtain the nine parameters in single fitting cycle with acceptable precision, a significant number of titration points would be needed (>500) by applying a resampling statistical method (51). Because it was difficult to fit the titration curve by varying all nine parameters in one fitting cycle, a repeat fitting cycle strategy was used to obtain the K's and ΔD's values reported here.
The fitting process was started by making a guess of the D's (D0, ΔD1, ΔD2, ΔD3, ΔD4) values. The initial values for D0 was set as experimental data, and the values for ΔD1, ΔD2, ΔD3, ΔD4 were obtained through a PLIMSTEX experiment at high protein concentration (100 times the 1/Ka or Kd as reported previously) (Supplemental Figure 1). We fixed these D's values and searched for K's in the initial fitting process. After the four K's (Ka1, Ka2, Ka3, Ka4) were obtained, the repeat-fitting processes were then started. In first half cycle, we fixed the K's obtained in the initial process and searched for the D's. We then fixed the ΔD1, ΔD2, ΔD3, and searched for Ka1, Ka2, Ka3, Ka4, D0, and ΔD4 to finish the cycle. This fitting cycle was repeated until the relative differences between K's values before and after each cycle of fitting were less than 10%. Three trials of experiments were applied with this strategy separately, and the results were averaged and reported with standard deviations.
Once the binding affinities of Ca2+ were determined, the information for fractionally bound TnC species as a function of Ca2+ was obtained and presented as a deuterium pattern (d0, d1, . . .) in which other isotopes (e.g.,13C, 15N, 18O) had been removed by using an isotopic pattern calculation. To obtain these patterns, each mass list from three experimental trials was extracted from their individual spectra as an x,y file (x: mass-to-charge ratio; y: intensity). A program in MathCAD was applied to find the isotopic peak envelope among the list by providing the chemical formula and charge state of the target peptide. The peak area in each envelope was applied for fractional species calculation, and the isotopes (13C, 15N, 18O) were removed from the output by using the information provided via the Isotope Simulation function in Qual Browser 2.0.7 (Xcalibur, Thermo Fisher, San Jose, CA).
RESULTS
a. H/DX kinetics of apo- and holo-TnC
Binding of four Ca2+ induces a conformational change in troponin C. Previous studies showed that the structure of the holo (Ca2+ saturated) state of skeletal troponin C is more structurally open than the semi-apo state (two Ca2+ bound). The center partial-loop structure between the N and C-terminal EF hands forms a less flexible helix whereas the Ca2+ is bound to the two EF hands near the N-terminus (EF-I and EF-II) (1, 4, 12, 55). A detailed structure of apo-troponin C has eluded determination owing to the unstructured C-terminus in the absence of Ca2+ (37, 56).
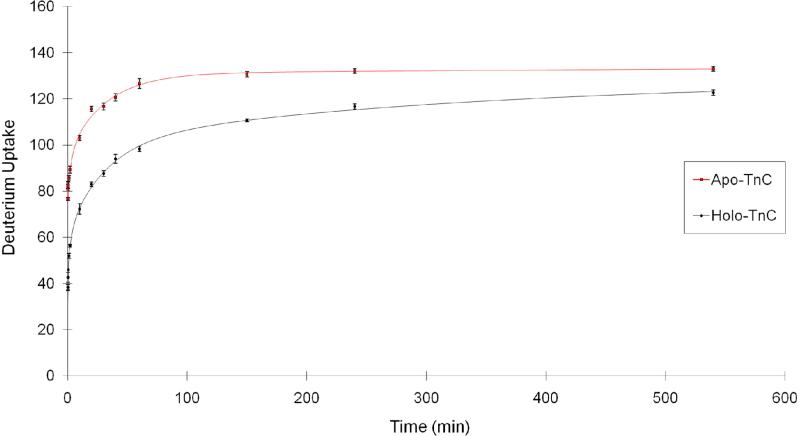
We began our study by measuring the kinetics of H/DX of rabbit skeletal troponin C in the Ca2+ saturated (holo) and Ca2+ absent (apo) states (Figure 1); the output is a plot of deuterium uptake as a function of exchange times (10 s to 9 h). To generate the holo state of troponin C, we made the protein stock solution (40 μM) containing 10 mM HEPES, 150 mM KCl, and 1 mM CaCl2, pH 7.4. Using KCl mimics physiological conditions, and excess Ca2+ (the ratio of Ca2+ concentration to protein concentration was 25) insures that the four EF hands of troponin C are saturated with Ca2+. To generate the apo state of troponin C, we replaced CaCl2 with 3 mM EGTA to insure that no Ca2+ was present in the protein.
Figure 1.
Kinetics of H/D exchange for rabbit skeletal troponin C. Apo-troponin C in 3 mM EGTA (squares, red) shows more extensive D uptake than holo troponin C in 1 mM CaCl2 (diamonds, black). The fitted curves are shown as solid lines.
Rabbit skeletal troponin C has 158 exchangeable amide protons (excluding one proline). Because the percentage of D2O in the experiment was 97%, the maximum number of observable exchange events is 153. After 20 min of H/DX, the mass of apo troponin C shifted by 116 ± 1 Da, indicating that ~75% of the amide sites are exchangeable at this time. This high level of exchange is in accord with the general view that troponin C in solution is highly flexible, dynamic, and solvent-exposed in the absence of Ca2+. In the presence of Ca2+, the number of amides undergoing H/DX decreased to 83 ± 1 Da after 20 min of exchange, indicating that ~32 amide sites no longer exchange as a consequence of Ca2+ binding. A more stabilized secondary structure, an increase in hydrogen bonding, a decrease in solvent accessibility of some amide sites, and/or a combination of these factors decreased the H/DX rates.
To understand quantitatively how Ca2+ binding affects the exchange rate, we fit the kinetic curve with four exchange rate bins (k = 10, 1, 0.1, 0.01 min-1) (Table 1). The results show that the apo state has 79 ± 1 sites with an exchange rate constant of 10 min-1, whereas the holo state has only 26 ± 2 sites with the same rate constant, indicating that ~53 fast-exchanging sites are affected by Ca2+ binding. In the holo state, these sites shift to the lower exchange rates including those with an exchange rate constant of 1 min-1 where the apo state has 1 ± 1 sites whereas the holo state has 24 ± 2 sites. There are also significant differences for those amides undergoing exchange with a rate constant of 0.01 min-1; whereas the holo state has 61 ± 3 sites, 37 ± 4 amides in the apo state exchange at this corresponding low rate. The overall shift to states having low exchange rate constants is consistent with troponin C possessing a more rigid secondary structure upon Ca2+ binding.
Table 1.
Numbers of amide hydrogen undergoing exchange for D for apo and holo troponin C. The kinetic modeling used four fixed exchange rate constants and “binned” the number of amides with respect to the rate constants.
| Kinetic Fit | # of H's per fixed-rate bin | |||
|---|---|---|---|---|
| k (min-1 ) | 10 | 1 | 0.1 | 0.01 |
| Apo | 79 ± 1 | 1 ± 1 | 16 ± 3 | 37 ± 4 |
| Holo | 26 ± 2 | 24 ± 2 | 3 ± 2 | 61 ± 3 |
b. Affinities for Ca2+ binding of troponin C
Several methods can be used to measure the binding affinities for protein-protein interactions and protein-small molecule interactions; these methods include FRET (fluorescence resonance energy transfer) (57), NMR (58), CD (circular dichorism) (59), calorimetry (60) and SPR (surface plasmon resonance) (61, 62). Because there is a significant difference in deuterium uptake between apo-TnC and holo-TnC, we can apply PLIMSTEX (Protein–ligand interactions by mass spectrometry, titration, and H/DX) to measure the equilibrium constants for Ca2+ binding to TnC (45, 49,51, 63) in a relatively simple experiment.
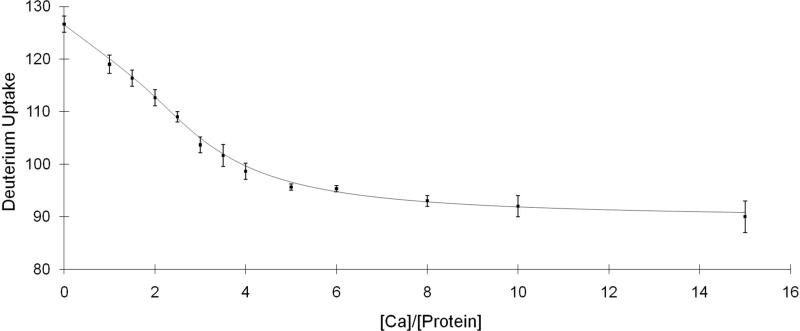
Our titration format varies the relative concentration of Ca2+ and monitors by MS the extent of H/DX as a function of time. Because EF-III and EF-IV are known from previous studies to be high-affinity sites, they are often saturated with Ca2+. To insure the protein was in the full apo state (no bound Ca2+), we carried out a two-step dialysis procedure (see Experimental). The deuterium uptake of the dialyzed TnC was comparable to that of TnC in the presence of EGTA (data not shown), indicating the dialysis was successful in removing Ca2+ from the protein. This purified protein sample was used as the starting material for the titration (Figure 2); the troponin C concentration for the titration was 0.3 μM, which falls into the range of Kd's determined previously (64). The time for H/DX (97% D2O buffer, 10 mM HEPES, 150 mM KCl, pH 7.4) was necessarily constant at 1 h, a time for which H/DX had become relatively constant, and for which small errors in sampling time would have minimal impact on the titration. We also performed the titration at high protein concentration, 15 μM, which is ~100 times the Kd values (Supplemental figure 1). A titration at this protein concentration will usually give a “sharp break” curve that reveals the stoichiometry of the ligand binding (49); in this case, rabbit skeletal troponin C binds four Ca2+, in agreement with previous findings. The other purpose for conducting this “sharp break” titration is to obtain an estimate of the ΔD's values to aid the PLIMSTEX curve fitting process (see Experimental). At high protein concentration, the equilibrium shifts toward the protein-ligand complex, and the resulting ΔD's values will be similar to the actual ΔD's, which report the change in the protein secondary structure owing to each ligand binding.
Figure 2.
PLIMSTEX curve of Troponin C titrated with Ca2+ and followed by MS-based H/DX. The concentration of the protein for the titration was 0.3 μM.
In this manner, we obtained ΔD1, ΔD2, ΔD3, and ΔD4 as 6, 15, 31, and 37, respectively. These values serve as the initial guesses for the repeat-fitting cycle described in the experimental section. The relative variations of K2, K3 and K4 all decreased to ~10% after we performed three cycles of the fitting (Supplemental Figure 2). Although the relative variation of K1 was not constrained over each fitting cycle, probably because the number of experimental points is insufficient, its value still is in a reasonable range. Given that the affinities are known and that the main purpose of the study is to investigate the order of binding, we did not pursue the titration further because additional improvements would not affect significantly the determination of the binding order.
Previous studies show that skeletal troponin C has two Ca2+ binding sites, EF-III and EF-IV, located near the C-terminus; their binding affinities are comparable at (2.1 ± 0.7) × 107 M-1 (64). Two additional sites are located near the N-terminus of skeletal troponin C, EF-I and EF-II, and their binding affinities for Ca2+ are in the range of 3 × 105 to 5 × 106 M-1 (20, 64). Although the equilibrium constants show variability from study to study (possibly because the affinities are relatively small), the consensus is that the equilibrium constants for the N-terminal EF hands are in the range of 105-106 M-1 and smaller than those for the C terminus (37).
We fit the titration curve to a non-linear least squares model that was previously described (51). The output of the fitting produced four Ka's (Table 2), two with high values of (5 ± 5) × 107 M-1 and (1.8 ± 0.8) × 107 M-1, which are within a factor of 3 of previous results. The other two Ka's have smaller values of (4.2 ± 0.9) × 106 M-1 and (1.6 ± 0.6) × 106 M-1, which are also within the range of values from previous studies. The fitting parameter D0 (126.5 ± 0.1), which represents the deuterium uptake in the apo state is in agreement with the experimental results (127 ± 2).
Table 2.
Four Ca2+ binding constants of troponin C obtained by fitting PLIMSTEX titration curves.
| K | Experimental Value | Literature Values |
|---|---|---|
| K1 | (5.3 ± 4.7 ) × 107 M-1 | 2 × 107 M-1 (1);(2.1 ± 0.7 ) × 107 M-1(64) |
| K2 | (1.8 ± 0.8 ) × 107 M-1 | 2 × 107 M-1 (1);(2.1 ± 0.7 ) × 107 M-1(64) |
| K3 | (4.2 ± 0.9) × 106 M-1 | 5 × 106 M-1 (20);(3.2 ± 1.3 ) × 105 M-1(64) |
| K4 | (1.6 ± 0.6) × 106 M-1 | 5 × 106 M-1 (20) ;(3.2 ±1.3 ) × 105 M-1(64); |
| D4 | 38 ± 1 | N/A |
| D0 | 127 ± 1 | N/A |
| RMS | 0.62 |
c. Fractional species and binding order
The titration study provides us the four equilibrium constants (Ka) for Ca2+ binding to troponin C. These values are not new, and they can be determined by other approaches. Remarkable, however, is that three of the four values (K2, K3 and K4) can be extracted with reasonable precision from a single titration, probably because each addition of Ca2+ to the protein induces a change in conformation that responds to H/DX. Because the binding affinities are different, it is likely that that Ca2+ binding is cooperative and occurs, at least in part, in a stepwise manner.
The next level of information is the order of binding, which we sought to determine by following the H/DX extent at the EF-hand level. One prospect is to examine the deuterium distribution for the various EF hands as a function of Ca2+ concentration. These EF-hand regions may be removed from the protein following H/DX as peptides by pepsin digestion and submitted to LC-MS analysis. When an EF hand binds Ca2+, we expect the extent of H/DX will decrease. By identifying the state of Ca2+ binding (e.g., 0, 1, 2, 3, 4) for which the change occurs, we should be able to determine in a straightforward way the order for Ca2+ binding. The interaction between Ca2+ and troponin C at any titration point, however, results in a mixture of Ca2+-binding states, and the deuterium distribution of any given EF hand at each titration point is of a mixture of different Ca2+ bound states. The challenge is to extract from this mixture of states the deuterium distribution of an EF hand for a single state (Figure 3).
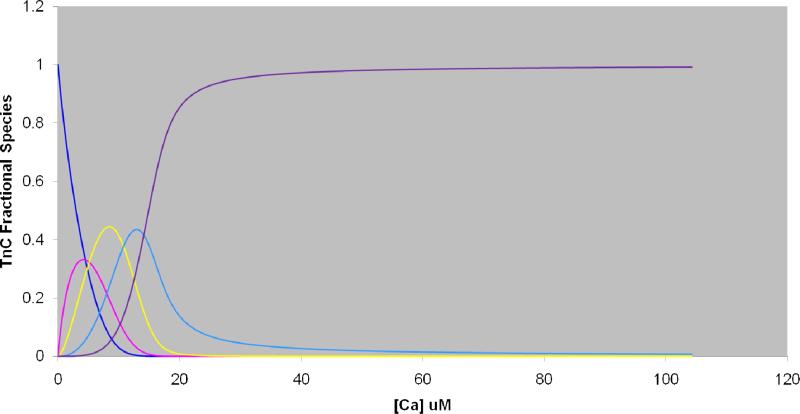
Figure 3.
Fractional species curves showing the distribution of each TnC:xCa2+ as a function of Ca2+ concentration (μM). (Blue: zero Ca2+-bound; Pink: 1 Ca2+-bound;, Yellow: 2 Ca2+-bound; Light blue: 3 Ca2+-bound; Purple: 4 Ca2+- bound).
The four equilibrium constants, determined by PLIMSTEX or any other method, are a source of fractional species information that allow the determination of the population of the various Ca2+ states as a function of [Ca2+]. We hypothesize that knowing these fractional species will lead us to the required deuterium distributions. To test this hypothesis, we used the previously reported and likely more reliable K1 value (2.1 × 107 M-1) and the K2, K3, and K4 values (1.8 × 107 M-1, 4.2 × 106 M-1, 1.6 × 106 M-1, respectively) determined by PLIMSTEX to calculate the fractional species as a function of total [Ca2+] and determine the population of the five troponin C species, TnC:xCa2+ (x = 0-4) when the protein concentration was 4.2 μM (Figure 3). The reason for choosing a literature K1 is the precision for our K1 value is not high because the titration is underdetermined, and we don't want that to affect the precision for the fractional species determination. We chose seven Ca2+ concentrations from the fractional-species plot, including [Ca2+] = 0 (100% apo TnC) and Ca2+ = 2 mM (100% holo TnC) (Table 3) for our search for the required deuterium distributions. To obtain the needed experimental data, we conducted H/DX for 1 h in 90% D2O buffer (10 mM HEPES, 150 mM KCl, pH 7.4), quenched the exchanging solution, digested the protein with pepsin for 3 min on ice-water, and analyzed the peptide mixture by LC/MS.
Table 3.
Various solution concentrations chosen for H/DX to determine the order of binding of Ca2+ with troponin C. The values are calculated fractional species of each Ca2+-bound TnC species and are taken from the fractional species curves in Figure 3.
| [Ca] μM | TnC-0Ca | TnC-1Ca | TnC-2Ca | TnC-3Ca | TnC-4Ca |
|---|---|---|---|---|---|
| 0 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| 8.4 | 7.4% | 19.7% | 44.5% | 23.7% | 4.7% |
| 10.4 | 2.3% | 10.4% | 39.8% | 35.6% | 11.9% |
| 12.1 | 0.0% | 4.9% | 29.7% | 42.6% | 22.8% |
| 13.8 | 0.0% | 0.0% | 18.2% | 43.1% | 38.7% |
| 16.7 | 0.0% | 0.0% | 0.0% | 29.1% | 70.9% |
| 2000 | 0.0% | 0.0% | 0.0% | 0.0% | 100.0% |
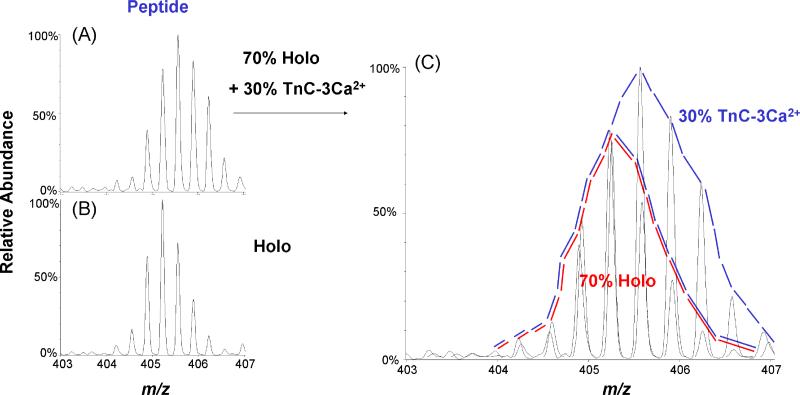
MS analysis directly gave us the complete isotopic distribution for specific deuterated peptides, but this distribution represents various states of the protein, not the single state we seek. Nevertheless, from these distributions, we can calculate the representative isotopic distribution for each Ca2+-bound troponin C species (see Figure 4 for an example of this idea). The upper left spectrum shows the deuterium-containing isotopic distribution when [Ca2+] = 16.7 μM for the triply charged peptic peptide FRIFDRNADG, which is from the EF-III region. From Table 3, we know that there is 30% TnC-3Ca2+ species and 70% of TnC-4Ca2+ species in the solution when the total [Ca2+] is 16.7 μM. Because we know the deuterium distribution of this peptide when [Ca2+] = 2 mM (representing 100% TnC-4Ca2+ species, lower left in Figure 4), we can remove that pattern from the experimental distribution to give the deuterium distribution for the peptide representing this region of the protein when it exists as TnC-3Ca2+ (right pattern in Figure 4).
Figure 4.
(A) Deuterium-containing isotopic distribution of the triply charged peptic peptide 100-109 (FRIFDRNADG). The distribution can be represented as a mixture of 70% holo (TnC-4Ca2+, red area in C) and 30% TnC-3Ca2+ states as shown in (C), which is an enlarged view of A. (B) Deuterium-containing isotopic distribution of triply charged peptic peptide 100-109 (FRIFDRNADG). The distribution represents 100% Holo state (TnC-4Ca2+) in 1 mM CaCl2. (C) Enlarged view of (A).
To sort out the required isotopic distributions, we implemented an algorithm with Mathcad 2001 Professional (Math-Soft, Inc., Cambridge, MA). We extracted mass lists containing all peptide information from each spectrum and input them into MathCAD as x,y files for peak-finding and fractional-species calculation. The basis for this algorithm is in matrix algebra as given in equation 1.
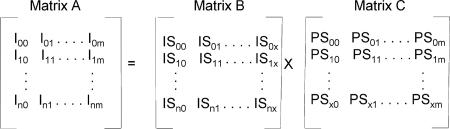 |
(1) |
In this equation, matrix A is the input of the experimental results; that is, the isotopic abundances for a single peptide I, from peak 0 to peak n as a function of the various ligand concentrations. Matrix C provides the fractional species information; that is, the population of each species, PS, from species 0 to x as a function of the ligand concentrations. For troponin C, binding to Ca2+, x = 4, and there exist five species: TnC-0Ca2+, TnC-1Ca2+, TnC-2Ca2+, TnC-3Ca2+, and TnC-4Ca2+. Matrix B is the output of isotopic abundances of species IS from peak 0 to peak n as a function of various Ca2+ binding states.
In the implementation, we normalized each row of each matrix so that their sum is equal to 1, and we required that the values in matrix B be positive. We fit the output in matrix B to the best values; this procedure requires minimizing the differences between matrix A and the product of matrix B and C. The intensity input can be the intensity of the peak centroid or its area in a peak-profile mode; the latter was used in this work so that each part in the isotopic distribution pattern was considered, in principle increasing the accuracy of the calculation. By comparing the distribution of the peaks and their area, we obtained a deuterium isotopic pattern of each peptic peptide as a function of the Ca2+ binding state. Changes in H-bonding or solvent accessibility for various regions of the protein induced by Ca2+ binding can be determined by simply analyzing the shift of the isotopic distribution.
d. Order of Ca2+ binding
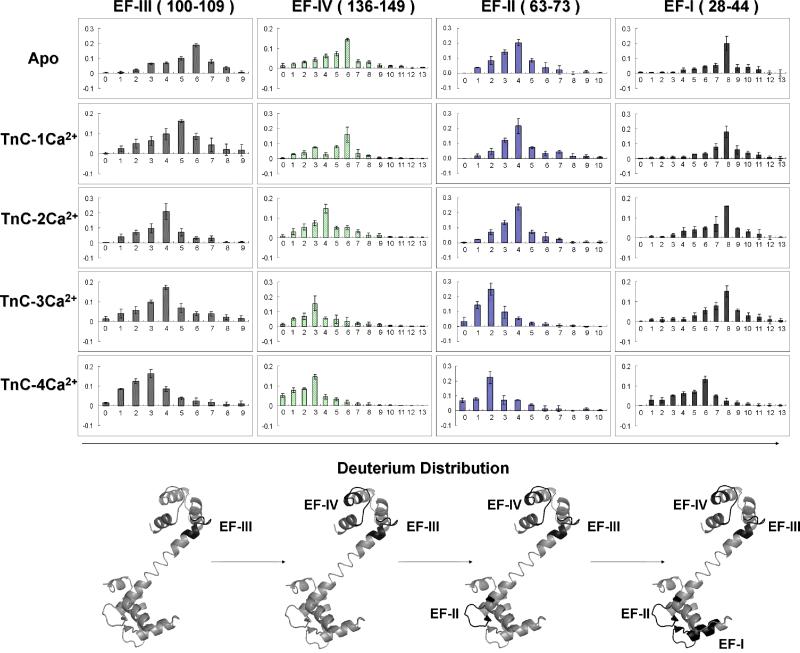
Our hypothesis is that changes in the deuterium distributions for peptides from regions involved in Ca2+ binding as a function of the states of the protein (TnC-0Ca2+ to TnC-4Ca2+) will report the order of Ca2+ binding. To test this hypothesis, we needed four abundant peptic peptides each representing one of the four EF hands to follow as a function of the fractional species calculation, and we were able to obtain these reporter peptides by pepsin digestion. The four peptides are doubly charged DADGGGDISVKELGTVM (28-44) containing EF-I; doubly charged, EVDEDGSGTID (63-73) containing EF-II; doubly charged FRIFDRNADG (100-109) containing EF-III; and triply charged, MKDGDKNNDGRIDF (136-149) containing EF-IV. (One set of mass spectra of these four peptic peptides from three trials are presented in Supplemental Figure 3-6). The outcome of the matrix-algebra calculation gave us the deuterium distributions convolved with the native distribution of C-13, N-15 and O-18 of each EF hand for the five states of Ca2+ binding to troponin C. We then removed all isotope components except D for these four unique peptic peptides by using the theoretical isotope ratio provided via the Isotope Simulation function in Qual Browser 2.0.7 (Xcalibur, Thermo Fisher, San Jose, CA). The isotope deconvolution process is illustrated in supplemental Figure 7. Briefly, each deuterium-containing isotopic pattern is a mixture of various numbers of deuterium-containing species (d0, d1, d2...) with their isotopic species. By knowing the chemical formula for a particular peptide, we can calculate the isotope distribution (except that of D from H/DX) and remove it from the distribution, leaving a pure deuterium distribution (Figure 5). We submit that any slight change of the deuterium uptake can be readily revealed by this strategy.
Figure 5.
Top: Each column represents the deuterium distributions of the peptic peptide of EF hands: III, IV, II, I (left to right). Each row represents the various Ca2+ bound TnC states: Apo (Absent Ca2+) to 4 Ca2+ bound TnC (top to bottom). Bottom: X-ray crystal structure of rabbit skeletal troponin C (PDB: 1TCF) in gray, and the four EF hands are in black.
Comparing the variation of the deuterium-distribution centroid for the four peptic peptides as a function of different Ca2+ bound troponin C states (Figure 5), we see that the first shift from higher to lower deuterium content (indicating onset of protection) occurs for peptic peptide 100-109 containing EF-III for the transition of TnC-0Ca2+ to TnC-1Ca2+ whereas the deuterium distributions of the peptides representing the other three EF hands remain largely unaffected. This indicates that EF-III in skeletal troponin C is first to bind Ca2+, although the binding may not be complete as the D distribution continues to shift to more protection as more calcium binds. Peptic peptide 136-149 from EF-IV showed a similar but more conclusive shift from a centroid of six to four deuteriums for TnC-2Ca2+, indicating that EF-IV is the second Ca2+ binding site. Similar to EF-III, the D distribution shows additional minor shifts as the protein continues to take up calcium. Although the shifts for EF-III and IV do not occur sharply as various states of Ca2+ binding are achieved, the results clearly show the higher propensity for binding Ca2+ by EF-III and EF-IV (~107 M-1) than by EF-II and EF-I. Sykes and coworkers (53) studied the binding of Ca2+ to synthetic EF-III and EF-IV peptides of troponin C by 1H-NMR. Their results show that Ca2+ binds to synthetic EF-III peptide; the resulting Ca2+-EF-III then associates with the apo-EF-IV peptide to form a heterodimer Ca2+-EF-III/EF-IV. The heterodimer then binds the second calcium to give 2Ca2+-EFIII/EFIV. Our results on the intact protein agree with this general picture of stepwise binding.
Because the N-terminal EF-I and EF-II have lower binding affinities to Ca2+ (~106 M-1), they should bind after EF-III and IV. Indeed, the deuterium content (most abundant ion) of the peptic peptide containing EF-II shifts from four to two deuteriums at the transition to TnC-3Ca2+, whereas the peptic peptide representing EF-I shifts from eight to six deuteriums at the transition to TnC-4Ca2+. Thus, the third Ca2+ binds at EF-II followed by the last Ca2+ binding at EF-I. Sykes and coworkers (37) also studied Ca2+ binding to EF hands in N-terminus of chicken skeletal troponin C by using NMR. Although the overall binding picture for full length TnC remained unclear, they could conclude that EF-II is the first site in the N-terminus of troponin C that binds to Ca2+ and EF-I is the last Ca2+ binding site, a conclusion that is consistent with the results of H/DX presented here.
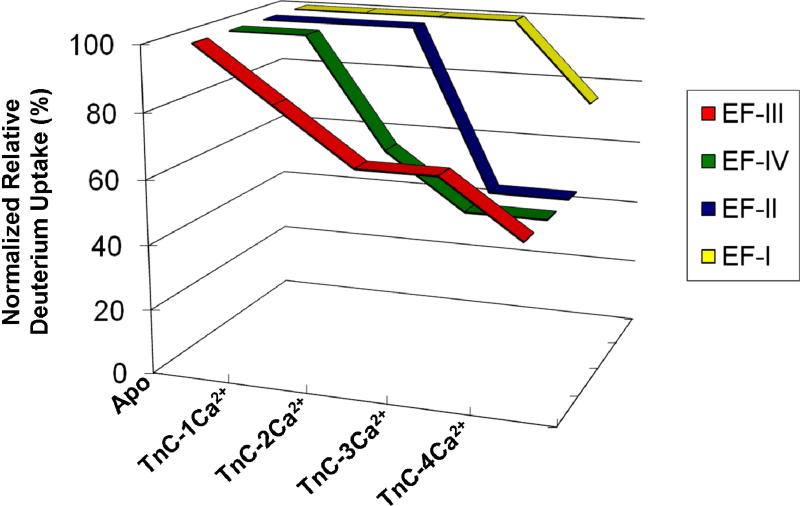
To illustrate more clearly the order of Ca2+ binding, we normalized the deuterium- uptake values for the four peptides to their maximum deuterium uptake. This allows us to view the relative deuterium uptake as a function of different TnC-Ca2+ states (Figure 6). The outcome clearly shows that the order of Ca2+ binding to the four Ca2+ binding sites in intact skeletal troponin C is EF-III > EF-IV > EF-II >EF-I, although there is likely to be some simultaneous binding of Ca2+ by EF-III and IV.
Figure 6.
A plot of the normalized relative deuterium uptake based on the centroids of the deuterium distributions for four peptic peptides containing the four EF hands as a function of various Ca2+-bound TnC states.
DISCUSSION
The advantage of H/DX coupled with MS is that changes in the extent of H/DX, whether caused by increased H bonding or decreased solvent accessibility or both, can be used to compare two or more states. The conclusions do not depend highly on the abundances of the various isotopomers but principally on their m/z. The PLIMSTEX strategy further extends the advantage of H/DX by affording, via ligand titrations at a fixed concentration of protein, the protein-ligand binding affinities. The equilibrium constants are essential for the design of an experimental approach to extract deuterium distributions for regions of a protein as a function of its binding state.
We illustrate the approach to determine the binding order of Ca2+ to troponin C. Although troponin C is a well-studied protein, establishing the order of Ca2+ binding has proven difficult. The kinetics of HDX at the protein or global level demonstrates that the protein is considerably stabilized as a consequence of Ca2+ binding. The differences in the apo and holo states allow the binding affinities of troponin C with Ca2+ to be measured in a single-titration format. The values of the equilibrium constants allow calculation of fractional species for troponin C. From H/DX experiments at known fractional species, we can extract the deuterium distribution of four regions of the protein that bind Ca2+. Those distributions shift to lower mass when Ca2+ binds at the site, providing a basis for determining the binding order of the four Ca2+ ions.
Previous studies on Ca2+ binding to troponin C by Ca2+ titration (36, 37, 52, 53, 64) indicated the binding is cooperative and affected by pH. Those studies, however, did not give a clear outcome of the binding order of Ca2+ to troponin C probably because the similar binding affinities of Ca2+ in each terminal region of troponin C cause the states to coexist at various Ca2+ concentrations. Nevertheless, our results indicate the Ca2+ binding to skeletal troponin C occurs in the order EF-III > EF-IV > EF-II > EF-I. This determination is attributed to the high sensitivity of MS in the H/DX platform and the ability to extract from the data specific D distributions for the five binding states of the protein.
The reasons for this trend of binding are not completely understood. Preferential binding at the C terminus is well-established. Binding details may be an outcome of the various charge states of the protein in different pH environments or the difference of the secondary and tertiary structure of the Ca2+ binding sites with respect to individual Ca2+ binding (52). It will be of interest to see whether this approach can be applied to determine binding orders of other proteins and ligands.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Henry Rohrs for assistance with the LTQ-Orbitrap analysis and database searching.
Funding: This research was supported by the NCRR of the NIH (Grant No. 21 2P41RR000954) to MLG.
Abbreviations
- TnC
Troponin C
- PLIMSTEX
Protein Ligand Interaction by Mass Spectrometry, Titration and H/D Exchange
- MS
mass spectrometry
- ESI
electrospray ionization
- Da
Dalton
- H/DX
hydrogen/deuterium exchange
- NMR
nuclear magnetic resonance
- D2O
deuterium oxide
- HCl
hydrochloric acid
- UPLC
ultra performance liquid chromatography
- Q-TOF
quadrupole time-of-flight
- MWCO
molecular weight cut off
Footnotes
SUPPORTING INFORMATION AVAILABLE
Supplemental figures are included in the Supporting Information section. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Filatov AGK, Bulargina TV, Gusev NB. Troponin: structure, properties, and mechanism of functioning. Biochemistry (Moscow) 1999;64:1155–1164. [PubMed] [Google Scholar]
- 2.Grabarek Z, Tao T, Gergely J. Molecular mechanism of troponin-C function. Journal of Muscle Research and Cell Motility. 1992;13:383–393. doi: 10.1007/BF01738034. [DOI] [PubMed] [Google Scholar]
- 3.Zot AS, Potter JD. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annual Review of Biophysics and Biophysical Chemistry. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]
- 4.Herzberg O, James MNG. Refined crystal structure of troponin C from turkey skeletal muscle at 2.0 A resolution. Journal of Molecular Biology. 1988;203:761–779. doi: 10.1016/0022-2836(88)90208-2. [DOI] [PubMed] [Google Scholar]
- 5.Gulati J, Babu A, Su H, Zhang YF. Identification of the regions conferring calmodulin-like properties to troponin C. Journal of Biological Chemistry. 1993;268:11685–11690. [PubMed] [Google Scholar]
- 6.Li Y, Mui S, Brown JH, Strand J, Reshetnikova L, Tobacman LS, Cohen C. The crystal structure of the C-terminal fragment of striated-muscle - tropomyosin reveals a key troponin T recognition site. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7378–7383. doi: 10.1073/pnas.102179999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardia contractility. Annual Review of Physiology. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S. Crystal structure of troponin and the molecular mechanism of muscle regulation. J Electron Microsc (Tokyo) 2005;54:i35–41. doi: 10.1093/jmicro/54.suppl_1.i35. [DOI] [PubMed] [Google Scholar]
- 9.Davis JP, Tikunova SB. Ca2+ exchange with troponin C and cardiac muscle dynamics. Cardiovasc Res. 2008;77:619–626. doi: 10.1093/cvr/cvm098. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y-B, Lou F, Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J Physiol. 2009;587:155–163. doi: 10.1113/jphysiol.2008.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul DM, Morris EP, Kensler RW, Squire JM. Structure and orientation of troponin in the thin filament. J. Biol. Chem. 2009;284:15007–15015. doi: 10.1074/jbc.M808615200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satyshur KA, Rao ST, Pyzalska D, Drendel W, Greaser M, Sundaralingam M. Refined structure of chicken skeletal muscle troponin C in the two- calcium state at 2.0A resolution. J. Biol. Chem. 1988;263:1628–1647. [PubMed] [Google Scholar]
- 13.Jayashree S, Terence T, George NP., Jr. Conformational variation of calcium-bound troponin C. Proteins: Structure, Function, and Genetics. 1999;37:510–511. doi: 10.1002/(sici)1097-0134(19991201)37:4<510::aid-prot2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Sykes BD. Pulling the calcium trigger. Nat Struct Mol Biol. 2003;10:588–589. doi: 10.1038/nsb0803-588. [DOI] [PubMed] [Google Scholar]
- 15.Vinogradova MV, Stone DB, Malanina GG, Karatzaferi C, Cooke R, Mendelson RA, Fletterick RJ. Ca2+-regulated structural changes in troponin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5038–5043. doi: 10.1073/pnas.0408882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y-B, Brandmeier B, Irving M. Structural changes in troponin in response to Ca2+ and myosin binding to thin filaments during activation of skeletal muscle. Proceedings of the National Academy of Sciences. 2006;103:17771–17776. doi: 10.1073/pnas.0605430103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reece KL, Moss RL. Intramolecular interactions in the N-domain of cardiac troponin C are important determinants of calcium sensitivity of force development. Biochemistry. 2008;47:5139–5146. doi: 10.1021/bi800164c. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki H KR. Calcium-binding proteins 1: EF-hands. Protein Profile. 1994;4:297–490. [PubMed] [Google Scholar]
- 19.Gillis TE, Marshall CR, Tibbits GF. Functional and evolutionary relationships of troponin C. Physiol. Genomics. 2007;32:16–27. doi: 10.1152/physiolgenomics.00197.2007. [DOI] [PubMed] [Google Scholar]
- 20.Calvert MJ, Ward DG, Trayer HR, Trayer IP. The importance of the carboxyl-terminal domain of cardiac troponin C in Ca2+-sensitive muscle regulation. J. Biol. Chem. 2000;275:32508–32515. doi: 10.1074/jbc.M005764200. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Saude E, Wang X, Pearlstone J, Smillie L, Sykes B. Kinetic studies of calcium and cardiac troponin I peptide binding to human cardiac troponin C using NMR spectroscopy. European Biophysics Journal. 2002;31:245–256. doi: 10.1007/s00249-002-0227-1. [DOI] [PubMed] [Google Scholar]
- 22.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Wang X, Sykes B. Structural based insights into the role of troponin in cardiac muscle pathophysiology. Journal of Muscle Research and Cell Motility. 2004;25:559–579. doi: 10.1007/s10974-004-5879-2. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Gergely J, Tao T. Proximity relationships between residue 117 of rabbit skeletal troponin-I and residues in troponin-C and actin. Biophys. J. 2001;81:321–333. doi: 10.1016/S0006-3495(01)75702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MX, Sykes BD. Role of the Structural Domain of Troponin C in Muscle Regulation: NMR Studies of Ca2+ binding and subsequent interactions with regions 1-40 and 96-115 of troponin I. Biochemistry. 2000;39:2902–2911. doi: 10.1021/bi992579n. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson RE, Irving M, Corrie JET, Trentham DR, Sykes BD. NMR Structure of a bifunctional rhodamine labeled N-domain of troponin C complexed with the regulatory “witch” peptide from troponin I: implications for in situ fluorescence studies in muscle fibers. Biochemistry. 2003;42:4333–4348. doi: 10.1021/bi027041n. [DOI] [PubMed] [Google Scholar]
- 27.Blumenschein TMA, Stone DB, Fletterick RJ, Mendelson RA, Sykes BD. Dynamics of the C-terminal region of TnI in the troponin complex in solution. Biophys. J. 2006;90:2436–2444. doi: 10.1529/biophysj.105.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang ZY, Sarkar S, Gergely J, Tao T. Ca2(+)-dependent interactions between the C-helix of troponin-C and troponin-I. Photocross-linking and fluorescence studies using a recombinant troponin-C. J. Biol. Chem. 1990;265:4953–4957. [PubMed] [Google Scholar]
- 29.Wang Z, Gergely J, Tao T. Characterization of the Ca(2+)-triggered conformational transition in troponin C. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:11814–11817. doi: 10.1073/pnas.89.24.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foguel D, Suarez MC, Barbosa C, Rodrigues JJ, Sorenson MM, Smillie LB, Silva JL. Mimicry of the calcium-induced conformational state of troponin C by low temperature under pressure. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10642–10646. doi: 10.1073/pnas.93.20.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandra M, Dong W-J, Pan B-S, Cheung HC, Solaro RJ. Effects of protein kinase A phosphorylation on signaling between cardiac troponin I and the N-terminal domain of cardiac troponin C. Biochemistry. 1997;36:13305–13311. doi: 10.1021/bi9710129. [DOI] [PubMed] [Google Scholar]
- 32.Finley NL, Rosevear PR. Introduction of negative charge mimicking protein kinase C phosphorylation of cardiac troponin I: Effects on cardiac troponin C. J. Biol. Chem. 2004;279:54833–54840. doi: 10.1074/jbc.M408304200. [DOI] [PubMed] [Google Scholar]
- 33.Baryshnikova OK, Li MX, Sykes BD. Defining the binding site of Levosimendan and its analogues in a regulatory cardiac troponin C-troponin I complex. Biochemistry. 2008;47:7485–7495. doi: 10.1021/bi800438k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li MX, Robertson IM, Sykes BD. Interaction of cardiac troponin with cardiotonic drugs: A structural perspective. Biochemical and Biophysical Research Communications. 2008;369:88–99. doi: 10.1016/j.bbrc.2007.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spyracopoulos L, Sykes BD. Structure, Dynamics, and Thermodynamics of the Structural Domain of Troponin C in Complex with the Regulatory Peptide 1-40 of Troponin I. Biochemistry. 2001;40:10063–10077. doi: 10.1021/bi010748+. [DOI] [PubMed] [Google Scholar]
- 36.Olle T, Torbjorn D, Sture F, Eva T. Calcium and Cadmium Binding to Troponin C. European Journal of Biochemistry. 1983;134:453–457. doi: 10.1111/j.1432-1033.1983.tb07588.x. [DOI] [PubMed] [Google Scholar]
- 37.Li MX, Gagne SM, Tsuda S, Kay CM, Smillie LB, Sykes BD. Calcium binding to the regulatory N-domain of skeletal muscle troponin C occurs in a stepwise manner. Biochemistry. 1995;34:8330–8340. doi: 10.1021/bi00026a014. [DOI] [PubMed] [Google Scholar]
- 38.Yamada K. Thermodynamic analyses of calcium binding to troponin C, calmodulin and parvalbumins by using microcalorimetry. Molecular and Cellular Biochemistry. 1999;190:39–45. [PubMed] [Google Scholar]
- 39.Bobst CE, Abzalimov RR, Houde D, Kloczewiak M, Mhatre R, Berkowitz SA, Kaltashov IA. Detection and characterization of altered conformations of protein pharmaceuticals using complementary mass spectrometry-based approaches. Analytical Chemistry. 2008;80:7473–7481. doi: 10.1021/ac801214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Englander S. Hydrogen exchange and mass spectrometry: A historical perspective. Journal of The American Society for Mass Spectrometry. 2006;17:1481–1489. doi: 10.1016/j.jasms.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konermann L, Pan J, Liu Y-H. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chemical Society Reviews. 2011;40:1224–1234. doi: 10.1039/c0cs00113a. [DOI] [PubMed] [Google Scholar]
- 42.Bou-Assaf GM, Chamoun JE, Emmett MR, Fajer PG, Marshall AG. Advantages of isotopic depletion of proteins for hydrogen/deuterium exchange experiments monitored by mass spectrometry. Analytical Chemistry. 2010;82:3293–3299. doi: 10.1021/ac100079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalmers MJ, Busby SA, Pascal BD, West GM, Griffin PR. Differential hydrogen/deuterium exchange mass spectrometry analysis of protein-ligand interactions. Expert Review of Proteomics. 2011;8:43–59. doi: 10.1586/epr.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engen JR. Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS. Analytical Chemistry. 2009;81:7870–7875. doi: 10.1021/ac901154s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sperry JB, Shi X, Rempel DL, Nishimura Y, Akashi S, Gross ML. A Mass Spectrometric Approach to the Study of DNA-Binding Proteins: Interaction of Human TRF2 with Telomeric DNA. Biochemistry. 2008;47:1797–1807. doi: 10.1021/bi702037p. [DOI] [PubMed] [Google Scholar]
- 46.Hopper ED, Roulhac PL, Campa MJ, Patz Jr EF, Fitzgerald MC. Throughput and Efficiency of a Mass Spectrometry-Based Screening Assay for Protein-Ligand Binding Detection. Journal of the American Society for Mass Spectrometry. 2008;19:1303–1311. doi: 10.1016/j.jasms.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowlessur D, Tobacman LS. Troponin Regulatory Function and Dynamics Revealed by H/D Exchange-Mass Spectrometry. Journal of Biological Chemistry. 2010;285:2686–2694. doi: 10.1074/jbc.M109.062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bou-Assaf GM, Chamoun JE, Emmett MR, Fajer PG, Marshall AG. Complexation and calcium-induced conformational changes in the cardiac troponin complex monitored by hydrogen/deuterium exchange and FT-ICR mass spectrometry. International Journal of Mass Spectrometry In Press, Corrected Proof. doi: 10.1016/j.ijms.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu MM, Rempel DL, Du Z, Gross ML. Quantification of Protein-Ligand Interactions by Mass Spectrometry, Titration, and H/D Exchange: PLIMSTEX. Journal of the American Chemical Society. 2003;125:5252–5253. doi: 10.1021/ja029460d. [DOI] [PubMed] [Google Scholar]
- 50.Zhu MM, Chitta R, Gross ML. PLIMSTEX: a novel mass spectrometric method for the quantification of protein-ligand interactions in solution. International Journal of Mass Spectrometry. 2005;240:213–220. [Google Scholar]
- 51.Zhu MM, Rempel DL, Gross ML. Modeling data from titration, amide H/D exchange, and mass spectrometry to obtain protein-ligand binding constants. Journal of the American Society for Mass Spectrometry. 2004;15:388–397. doi: 10.1016/j.jasms.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Iida S. Calcium Binding to Troponin C. II. A Ca2+ Ion Titration Study with a Ca2+ Ion Sensitive Electrode. J Biochem. 1988;103:482–486. doi: 10.1093/oxfordjournals.jbchem.a122296. [DOI] [PubMed] [Google Scholar]
- 53.Gary SS, Robert SH, Brian DS. Stoichiometry of calcium binding to a synthetic heterodimeric troponin-C domain. Biopolymers. 1992;32:391–397. doi: 10.1002/bip.360320415. [DOI] [PubMed] [Google Scholar]
- 54.Bai Y, Milne JS, Mayne L, Englander SW. Primary structure effects on peptide group hydrogen exchange. PROTEINS: Structure, Function, and Genetics. 1993;17:75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houdusse A, Love ML, Dominguez R, Grabarek Z, Cohen C. Structures of four Ca2+-bound troponin C at 2.0 A resolution: further insights into the Ca2+-switch in the calmodulin superfamily. Structure. 1997;5:1695–1711. doi: 10.1016/s0969-2126(97)00315-8. [DOI] [PubMed] [Google Scholar]
- 56.Shaw GS, Golden LF, Hodges RS, Sykes BD. Interactions between paired calcium-binding sites in proteins: NMR determination of the stoichiometry of calcium binding to a synthetic troponin-C peptide. Journal of the American Chemical Society. 1991;113:5557–5563. [Google Scholar]
- 57.Khamir M, Adam DH, Raghunandan K, Peter JW, Jennifer JL. A computational approach to inferring cellular protein-binding affinities from quantitative fluorescence resonance energy transfer imaging. PROTEOMICS. 2009;9:5371–5383. doi: 10.1002/pmic.200800494. [DOI] [PubMed] [Google Scholar]
- 58.Lee F, Samantha R, Dan F. Determination of protein-ligand binding affinity by NMR: observations from serum albumin model systems. Magnetic Resonance in Chemistry. 2005;43:463–470. doi: 10.1002/mrc.1574. [DOI] [PubMed] [Google Scholar]
- 59.Mayhood TW, Windsor WT. Ligand binding affinity determined by temperature-dependent circular dichroism: Cyclin-dependent kinase 2 inhibitors. Analytical Biochemistry. 2005;345:187–197. doi: 10.1016/j.ab.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 60.Bains G, Freire E. Calorimetric determination of cooperative interactions in high affinity binding processes. Analytical Biochemistry. 1991;192:203–206. doi: 10.1016/0003-2697(91)90207-a. [DOI] [PubMed] [Google Scholar]
- 61.Monfregola L, Vitale RM, Amodeo P, Luca SD. A SPR strategy for high-throughput ligand screenings based on synthetic peptides mimicking a selected subdomain of the target protein: A proof of concept on HER2 receptor. Bioorganic & Medicinal Chemistry. 2009;17:7015–7020. doi: 10.1016/j.bmc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Blow N. Proteins and proteomics: life on the surface. Nat Meth. 2009;6:389–393. [Google Scholar]
- 63.Rempel DL, Zhao J, Giblin DE, Gross ML. Probing Ca2+-Induced Conformational Changes in Porcine Calmodulin by H/D Exchange and ESI-MS: Effect of Cations and Ionic Strength. Biochemistry. 2003;42:15388–15397. doi: 10.1021/bi035188o. [DOI] [PubMed] [Google Scholar]
- 64.Potter JD, Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J. Biol. Chem. 1975;250:4628–4633. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.