Abstract
Eukaryotic genomes exist as an elaborate three-dimensional structure in the nucleus. Recent studies have shown that this higher-order organization of the chromatin fiber is coupled to various nuclear processes including transcription. In fission yeast, we demonstrated that RNA polymerase III (Pol III)-transcribed genes such as tRNA and 5S rRNA genes, dispersed throughout chromosomal arm regions, localize to centromeres in interphase. This centromeric association of Pol III genes, mediated by the condensin complex, becomes prominent during mitosis. Here, we discuss potential roles of the Pol III gene-mediated genome organization during interphase and mitosis, and hypothesize that the interphase genome structure serves as a scaffold for the efficient assembly of condensed mitotic chromosomes and that tethering of chromosomal arm regions to centromeres allows chromosomes to properly segregate along the spindle microtubules during anaphase.
Keywords: Genome organization, RNA polymerase III, chromosome condensation
1. Introduction
Large-scale sequencing for various eukaryotes has provided complete genomic sequences accompanied by lists of genes and their regulatory elements. Chromatin fibers that carry those genomic sequences are in fact present in the nucleus as a complex three-dimensional (3D) structure. At the chromosomal level, it has been shown that DNA fibers are non-randomly arranged into chromosomal territories (Lanctot et al., 2007; Fraser and Bickmore, 2007). It is becoming clear that the 3D structure of the genome is linked to numerous nuclear processes such as transcription (Cook, 1999; Misteli, 2007). For instance, it is well known that the tandemly repeated ribosomal RNA genes are transcribed by RNA polymerase I (Pol I) in a subnuclear compartment, the nucleolus, where Pol I and rRNA genes are concentrated (Long and Dawid, 1980; Shaw and Jordan, 1995). Classes of Pol II-transcribed genes are enriched at transcriptional factories or nuclear speckles, likely contributing to efficient transcription and processing of the transcripts (Cook, 1999; Lamond and Spector, 2003; Chakalova et al., 2005; Sutherland and Bickmore, 2009). This is also true for Pol III-transcribed genes such as tRNA and 5S rRNA genes. 5S rRNA genes arranged as contiguous repeats in many eukaryotes often localize to the nucleolar periphery (Matera et al., 1995; Haeusler and Engelke, 2006). While 5S rRNA genes are dispersed in fission yeast and a few other organisms, they still co-localize at the nucleolar periphery (Iwasaki et al., 2010). Moreover, tRNA genes cluster in the nucleolus of budding yeast (Thompson et al., 2003). DNA replication and repair also involve higher-order genome organization through the establishment of replication factories and repair centers, respectively (Cook, 1999; Lisby et al., 2003; Kitamura et al., 2006; Misteli, 2007). Although the observed higher-order genome structures in the nucleus have been shown to be linked to various nuclear processes, the mechanisms that organize the functional genome structures have remained unknown.
We have used fission yeast as a model system to investigate the relationships between the global genome organization and genomic functions, and have modeled the 3D structure of the fission yeast genome using the latest genomics approach that combines the molecular biology procedure called chromosome conformation capture (3C) and massively parallel DNA sequencing (Tanizawa et al., 2010). This study suggests the existence of chromosomal territories and transcription factories in this model organism. Moreover, we have shown that Pol III-transcribed genes can localize to centromeres, thereby contributing to the global genome organization (Iwasaki et al., 2010). In this article, we focus on the molecular mechanism that drives the Pol III gene-mediated genome organization in fission yeast, and the roles of this organization during interphase and during mitosis.
2. Overview of the Pol III gene-mediated global genome organization
The molecular process by which the Pol III machineries transcribe Pol III genes such as tRNA and 5S rRNA genes has been intensely studied (Willis, 1993; Roeder, 1996; Paule and White, 2000; Huang and Maraia, 2001). The Pol III transcription involves several transcription factor complexes that direct the accurate positioning of Pol III on tRNA and 5S rRNA genes (Paule and White, 2000; Geiduschek and Kassavetis, 2001). The TFIIIB and TFIIIC transcription factor complexes are sufficient to recruit Pol III to tRNA genes, although an additional transcription factor, TFIIIA, is required for loading Pol III onto 5S rRNA genes. These Pol III transcription machineries are conserved from yeast to human (Huang and Maraia, 2001; Geiduschek and Kassavetis, 2001).
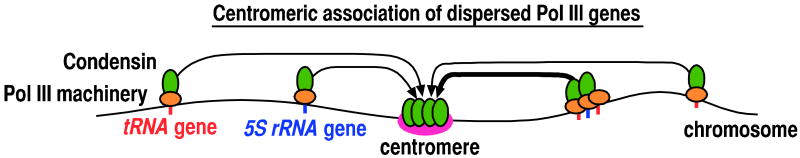
It has recently been shown that Pol III transcription machineries are not evenly distributed throughout the nucleus of fission yeast (Iwasaki et al., 2010). While TFIIIA is enriched in the nucleolus, TFIIIC is preferentially localized to 5-10 discrete spots associated with the nuclear periphery. Moreover, TFIIIB and Pol III are concentrated at centromeres. Besides the localization of these Pol III transcription machineries, Pol III genes themselves, in dispersed locations throughout the fission yeast genome, frequently localize to centromeres. This centromeric association of Pol III genes is mediated by the condensin complex (Figure 1).
Figure 1. The centromeric association of Pol III genes.
A number of Pol III genes such as tRNA and 5S rRNA genes dispersed throughout the fission yeast genome associate with centromeres. Red and blue vertical bars indicate tRNA and 5S rRNA genes, respectively. The thick arrow indicates more frequent localization of Pol III genes at centromeres. The current hypothesis is that Pol III transcription machinery binding to Pol III genes interacts with condensin, which in turn mediates tethering of Pol III genes to centromeres through interaction between condensin complexes.
Condensin has been shown to function in chromosome condensation during mitosis (Laemmli et al., 1992; Koshland and Strunnikov, 1996; Yanagida, 1998; Hirano, 2000; Hagstrom and Meyer, 2003; Nasmyth and Haering, 2005; Hirano, 2006). The condensin complex consists of five subunits, two structural maintenance of chromosomes (SMC) proteins and three non-SMC proteins, which are conserved from yeast to human (Koshland and Strunnikov, 1996; Hagstrom and Meyer, 2003; Losada and Hirano, 2005). In addition to its known role in chromosome compaction, there is accumulating evidence that the condensin complex participates in other nuclear processes including transcriptional regulation (Hagstrom and Meyer, 2003; Wood et al., 2010). Recent observations in fission yeast have demonstrated an additional role for condensin in the interphase global genome organization that associates Pol III genes with centromeres. Moreover, the nucleolar clustering of tRNA genes in budding yeast is disrupted in the condensin mutants, indicating that this role of condensin in the Pol III gene-mediated genome organization is not unique to fission yeast (Haeusler et al., 2008). Considering that Pol III gene-mediated genome organization in yeast involves evolutionarily conserved protein complexes such as condensin and Pol III transcription machineries, a similar genome organization might be conserved in higher eukaryotes.
3. Mechanism of the Pol III gene-mediated global genome organization
A series of results argues that condensin is recruited to Pol III genes through its binding to a component of the Pol III transcription machineries, likely TFIIIC (D'Ambrosio et al., 2008; Haeusler et al., 2008; Iwasaki et al., 2010). Condensin can also be targeted to the kinetochore portions of the centromeres through a mechanism involving Mis6 kinetochore protein (Nakazawa et al., 2008). In fission yeast, while 50 tRNA genes are encoded at centromeres, more than 100 non-centromeric tRNA genes are dispersed along the chromosomal arms and approximately 30 5S rRNA genes are also scattered across the genome. Condensin likely binds to both the centromeric tRNA genes as well as the non-centromeric Pol III genes. Condensin mediates associations between two DNA duplexes through interactions with other condensin complexes (Hirano, 2006). Therefore, it is likely that the centromeric association of Pol III genes is mediated by interaction between the condensin complexes present at centromeres and those distributed through the chromosomal arms, although it is not yet clear whether the non-centromeric Pol III genes are just targeted to the kinetochore portions of centromeres, or just the centromeric tRNA genes, or both. In any case, relatively more condensin molecules localize at centromeres than individual non-centromeric Pol III gene loci, which might explain why Pol III genes are frequently tethered to centromeres rather than other Pol III gene regions. It is still possible that association among non-centromeric Pol III genes might occur infrequently and also be mediated by the same mechanism.
Unexpectedly, Pol III transcription was shown to have an inhibitory effect on the centromeric localization of Pol III genes. For instance, inhibition of Pol III transcription promotes the centromeric association of Pol III genes. This was further confirmed by the observation that the sfc3-1 TFIIIC mutation which reduces Pol III transcription enhances the centromeric association of Pol III genes. Binding of TFIIIC and condensin to Pol III genes is also increased in the sfc3-1 mutant. It has been shown that TFIIIC dissociates from tRNA genes during Pol III transcription (Kassavetis et al., 1990; Roberts et al., 2003). We speculate that a reduction in the transcription frequency of Pol III genes might stabilize the association of the TFIIIC complex carrying the mutant Sfc3 protein with the Pol III genes, and that this in turn might result in the recruitment of condensin molecules and enhance the centromeric association of Pol III genes. In other words, TFIIIC together with the condensin complex might be released from Pol III genes during their transcription, leading to the dissociation of the Pol III genes from centromeres. Thus, increased Pol III transcription can inhibit the centromeric localization of Pol III genes.
4. Roles of the Pol III gene-mediated global genome organization during interphase
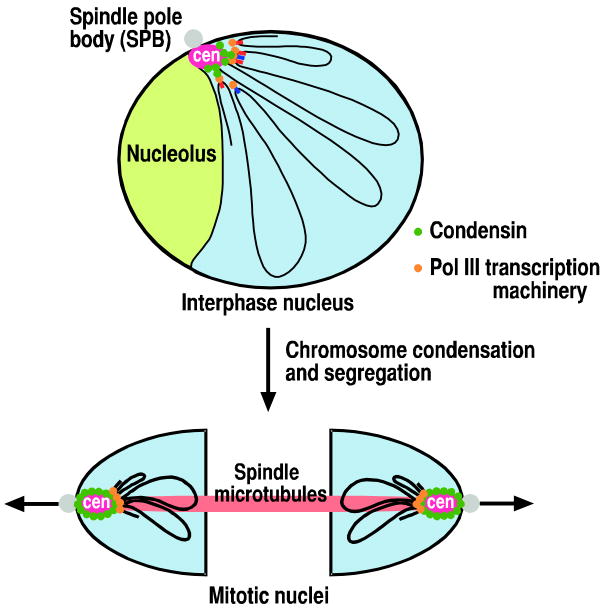
Although Pol III genes are dispersed throughout the fission yeast genome, many of them dynamically associate with centromeres, which are often positioned at the nuclear periphery near the surface boundary between the nucleoplasm and the nucleolus (Iwasaki et al., 2010). Therefore, the linear DNA fiber is folded into various chromatin loops emanating from centromeres in the fission yeast nucleus (Figure 2). The biological functions of this global genome organization involving Pol III genes and condensin complexes during interphase are not well understood. There are several possibilities that we can propose based on recent observations from our lab and others. The first possibility is that the interphase genome structure might be used as a scaffold for the efficient assembly of condensed mitotic chromosomes. This possibility is supported by observations that the centromeric association of Pol III genes becomes prominent during mitosis, indicating that global genome organizations during interphase and mitosis are at least not completely independent.
Figure 2. A schematic model for global chromosome organization by the centromeric association of Pol III genes.
In interphase, the centromeric association of Pol III genes at the nuclear periphery near the surface boundary between the nucleoplasm and the nucleolus is mediated by condensin interacting with Pol III transcription machinery (top panel). The centromeric association of Pol III genes likely influences global higher-order chromosome structure, possibly helping mediate formation of numerous chromatin loops derived from centromeres. Pol III transcription is negatively correlated with the centromeric association of Pol III genes. In mitosis, the centromeric association of Pol III genes contributes to chromosome condensation essential for faithful chromosome segregation (bottom panel).
Tethering of Pol III genes to centromeres might be required for boundary activity which prevents heterochromatin spreading into neighboring euchromatin domains. tRNA gene situated between heterochromatin and euchromatin domains functions as a chromatin boundary in budding yeast and fission yeast (Donze and Kamakaka, 2001; Oki and Kamakaka, 2005; Scott et al., 2006; Noma et al., 2006). Alu and another SINE element, B2, which are transcribed by Pol III, are also involved in forming chromatin boundaries in mammals (Willoughby et al., 2000; Lunyak et al., 2007). It is possible that tethering of Pol III genes to centromeres might create a physical barrier for heterochromatin proteins to encroach beyond the boundaries, resulting in separation of heterochromatin and euchromatin domains present in distinct chromatin loops.
The Pol III gene-mediated genome organization might be linked to a global transcriptional regulation of Pol III genes. This hypothesis is supported by the observation that the decrease and increase in Pol III transcription are connected to the more and less frequent associations of Pol III genes with centromeres, respectively. Concentration of Pol III genes and their transcription machineries at a specific subnuclear domain, such as centromeres in fission yeast, may facilitate Pol III transcription (Iwasaki et al., 2010). This is also observed in human cells where Pol III transcription occurs in about 2000 discrete sites accumulating numerous Pol III molecules (Pombo et al., 1999). Enrichment of ongoing Pol III transcription at a subnuclear domain, often near or in the nucleolus, might also be beneficial for efficient processing of the Pol III transcripts. It has been shown that several components of RNaseP, the splicing complex for tRNA molecules, are nucleolar in yeast and in mammals (Bertrand et al., 1998; Jarrous et al., 1999).
In fission yeast, centromeres are often positioned at the nucleolar periphery where Pol III genes are frequently located. Enrichment of Pol III genes near the nucleolus may facilitate the coordination of Pol III transcription with cellular processes in the nucleolus, especially Pol I transcription which synthesizes rRNA molecules. It has been shown that Pol I and Pol III transcription processes are co-regulated by the extracellular signal-regulated kinase (ERK) and the retinoblastoma protein (RB) (White, 2005). Considering that 5S rRNA, synthesized by Pol III, as well as other rRNA molecules synthesized by Pol I, are finally assembled into the ribosome within the nucleolus, it is probably important for efficient ribosome assembly that these two types of transcription are located close to each other. In human cells, the 5S rRNA gene array is also localized to the nucleolar periphery (Matera et al., 1995).
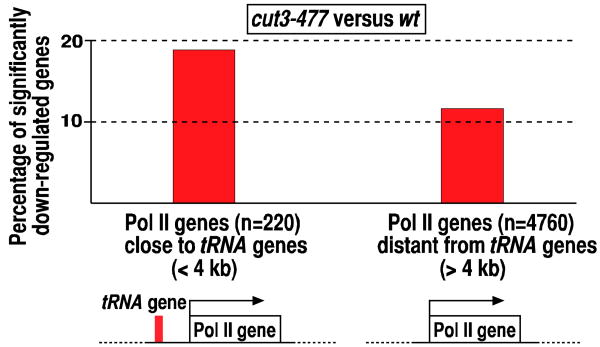
In budding yeast, the nucleolar clustering of active tRNA genes represses nearby Pol II genes, a process referred to as tRNA gene-mediated (tgm) silencing (Hull et al., 1994; Wang et al., 2005a). Similarly, in fission yeast, Pol II genes present near Pol III genes are often down-regulated in the cut3-477 condensin mutant where Pol III transcription is enhanced (Figure 3). We speculate that Pol III transcription or its favored chromatin environment might inhibit efficient Pol II transcription occurring in the vicinity of the Pol III gene.
Figure 3. Reduction of transcript levels of Pol II genes located near tRNA genes in the condensin mutant (cut3-477).
The graph indicates proportions of significantly down-regulated Pol II genes comparing the cut3-477 condensin mutant to wild-type (wt) in two groups, those positioned less than or more than 4 kb away from a tRNA gene. Significance of the difference is p<0.01.
Another interesting aspect of the Pol III gene-mediated global genome organization is that centromeres associating with Pol III genes are connected to the spindle pole body (SPB) which in turn is attached to cytoplasmic microtubules (Funabiki et al., 1993; Ding et al., 1997; King et al., 2008). Therefore, cytoplasmic contexts can influence the global genome organization in the nucleus through the microtubule-dependent positioning of SPB and centromeres.
5. Role of the centromeric association of Pol III genes in the assembly of condensed mitotic chromosomes
Recent findings in fission yeast demonstrate that the centromeric association of Pol III genes becomes prominent during mitosis (Figure 2; Iwasaki et al., 2010). This Pol III gene-mediated genome organization is mediated by the interaction between Pol III transcription machinery and the condensin complex, which is essential for chromosome condensation during mitosis and meiosis. It is thought that condensin mediates numerous interactions between DNA duplexes residing within a chromosome, and functions in some of the steps of chromosome compaction (Hirano, 2006). However, the exact molecular processes underlying chromosome compaction remain elusive (Gassmann et al., 2004; Belmont, 2006). We propose that the condensin-mediated global genome organization through Pol III genes accounts for a part of the assembly process for the condensed mitotic chromosomes.
In anaphase cells of fission yeast, Pol III genes distributed through the chromosomal arms are tethered to centromeres, and chromosomes are segregated along the spindle microtubules (Figure 4). The centromere is the chromosomal domain where kinetochore microtubules attach and where the pulling force is generated. Therefore, tethering chromosomal arm regions to the centromere can facilitate chromosome movement along spindle microtubules during anaphase. In condensin mutant cells, centromeres are pulled by spindle microtubules, but chromosomal arms are left behind with the Pol III genes, called φ- shaped chromosomes, further suggesting that condensin-mediated tethering of chromosomal arms to centromeres is an architectural requirement for mitotic chromosome segregation (Figure 4; Saka et al., 1994). If an association occurs between Pol III genes and centromeres present on different chromosomes or sister chromatids, mitotic chromosomes may not segregate properly, as mitotic chromosome fibers may become entangled during anaphase. Therefore, a molecular mechanism, which has remained elusive, likely directs Pol III genes present at chromosomal arms to interact with a centromere on the same chromosome and the same sister chromatid.
Figure 4. Role of the centromeric association of Pol III genes in the assembly of condensed mitotic chromosomes.
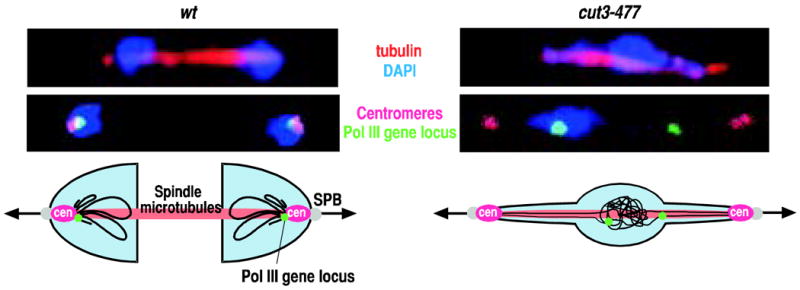
Immunofluorescent images of cells stained for tubulin (red) were merged with DAPI signals (blue; top panels). The c417 Pol III gene locus (green) and centromeres (magenta) visualized by FISH were merged with DAPI signals (blue; middle panels). Anaphase cells were subjected to the microscopic analysis. Intra-nuclear positioning of centromeres and the Pol III gene locus is depicted in the schematic diagrams (bottom panels). In wild-type cells (wt), centromeres associate with Pol III genes dispersed through the chromosomal arm regions and are pulled by spindle microtubules, and as a result chromosomes are properly segregated along the spindle microtubules during anaphase (left). In the cut3-477 condensin mutant, the centromeric association of Pol III genes is diminished and centromeres are segregated without holding the chromosomal arms (right). We hypothesize that tethering of Pol III genes to centromeres is a part of the proper assembly process for the condensed mitotic chromosomes, which is essential for faithful chromosome segregation.
Accumulation of the condensin complex at the centromere was shown in many organisms including budding yeast, fission yeast, fly, nematode, and human (Steffensen et al., 2001; Hagstrom et al., 2002; Ono at al., 2004; Wang et al., 2005b; Nakazawa et al., 2008). In addition, loss of condensin function results in similar chromosome segregation defects such as φ-shaped chromosomes and lagging chromosomes in various organisms (Saka et al., 1994; Freeman et al., 2000; Lavoie et al., 2000; Steffensen et al., 2001; Hagstrom et al., 2002; Hudson et al., 2003; Ono et al., 2004). It has recently been reported in budding yeast that condensin associates with tRNA genes and that condensin mutations affect clustering of tRNA genes in the nucleolus (D'Ambrosio et al., 2008; Haeusler et al., 2008). Furthermore, molecular mechanisms of chromosome segregation are generally well-conserved from fission yeast to human (Yanagida, 2005). Therefore, it is conceivable that chromosome compaction mediated through the associations among Pol III genes and centromeres are likely to be conserved among eukaryotes.
6. Conclusion
Pol III genes associate with centromeres during interphase and those associations become prominent during mitosis. The Pol III gene-dependent genome organization mediated by condensin is likely to be a part of the assembly process for the condensed chromosomes during mitosis. The exact mechanism of this global genome organization remains unclear, but it certainly appears to involve interactions between condensin and the Pol III transcription machinery, likely TFIIIC. We previously showed that TFIIIC participates in organizing higher-order genome structure in fission yeast (Noma et al., 2006). More than 60 loci dispersed across the fission yeast genome contain bound TFIIIC without Pol III association. These loci are referred to as COC (Chromosome-Organizing Clamps), based on the observation that these sites are occupied by high concentrations of TFIIIC that are tethered to the nuclear periphery. In budding yeast, there are 8 loci called extra TFIIIC (ETC) that are bound by TFIIIC without Pol III binding (Moqtaderi and Struhl, 2004). TFIIIC binding to specific DNA sequences is critical for boundary function demarcating chromosomal domains in both fission yeast and budding yeast (Noma et al., 2006; Simms et al., 2008; Valenzuela et al., 2009). Pol III transcription machineries have been mapped throughout the human genome and COC/ETC loci are identified in human cells (Raha et al., 2010; Barski et al., 2010; Moqtaderi et al., 2010; Oler et al., 2010; Canella et al., 2010). Human ETC sites are often positioned near CTCF binding sites (Moqtaderi et al., 2010). CTCF is known to function in insulator activity and in global genome organization in higher eukaryotes (Wallace and Felsenfeld, 2007; Phillips and Corces, 2009). Thus, TFIIIC that can bind to both Pol III genes as well as COC/ETC loci might have a general role in chromatin domain formation through higher-order genome organization in eukaryotes.
Genomes of single cell eukaryotes such as fission yeast display a specific functional architecture. For instance, active genes, functionally-related genes, LTR retrotransposons, telomeres, and centromeres tend to associate (Funabiki et al., 1993; Cam et al., 2008; Tanizawa et al., 2010). As described above, Pol III genes and COC/ETC loci also participate in organizing the higher-order genome structure (Noma et al., 2006; Iwasaki et al., 2010), suggesting that the 3D genome structure in the nucleus is generated by the coexistence of various genome-organizing mechanisms. Recent observations in fission yeast have shed light on an additional complexity in the global genome-organizing mechanism, linking the interphase genome structure to the mitotic chromosome architecture. To understand the fundamental importance of global genome organizations in various nuclear processes, further elucidation of the molecular mechanisms that direct the relevant genome organizations is essential. The existence of genetic, genomic, cell biology, and biochemical tools in the model organism fission yeast, the simplicity of its genome, and similarities of global genome organizations with mammals, make this system ideal for exploring the genome-organizing mechanisms conserved among eukaryotes.
Acknowledgments
We apologize to all the colleagues whose work could not be cited for space constraints. We thank Louise Showe for critically reading the manuscripts and Marion Sacks for editorial assistance. Our work was supported by National Institutes of Health grant CA010815 and funded by the NIH Director's New Innovator Award Program DP2-OD004348, the V foundation, the Edward Mallinckrodt, Jr. foundation, and the Wistar Pilot Project Support Fund.
Abbreviations
- Pol III
RNA polymerase III
- TFIIIA
Transcription factor IIIA
- TFIIIB
Transcription factor IIIB
- TFIIIC
Transcription factor IIIC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barski A, Chepelev I, Liko D, Cuddapah S, Fleming AB, Birch J, Cui K, White RJ, Zhao K. Pol II and its associated epigenetic marks are present at Pol III-transcribed noncoding RNA genes. Nat Struct Mol Biol. 2010;17:629–634. doi: 10.1038/nsmb.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont AS. Mitotic chromosome structure and condensation. Curr Opin Cell Biol. 2006;18:632–638. doi: 10.1016/j.ceb.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Houser-Scott F, Kendall A, Singer RH, Engelke DR. Nucleolar localization of early tRNA processing. Genes Dev. 1998;12:2463–2468. doi: 10.1101/gad.12.16.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam HP, Noma K, Ebina H, Levin HL, Grewal SI. Host genome surveillance for retrotransposons by transposon-derived proteins. Nature. 2008;451:431–436. doi: 10.1038/nature06499. [DOI] [PubMed] [Google Scholar]
- Canella D, Praz V, Reina JH, Cousin P, Hernandez N. Defining the RNA polymerase III transcriptome: Genome-wide localization of the RNA polymerase III transcription machinery in human cells. Genome Res. 2010;20:710–721. doi: 10.1101/gr.101337.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakalova L, Debrand E, Mitchell JA, Osborne CS, Fraser P. Replication and transcription: shaping the landscape of the genome. Nat Rev Genet. 2005;6:669–677. doi: 10.1038/nrg1673. [DOI] [PubMed] [Google Scholar]
- Cook PR. The organization of replication and transcription. Science. 1999;284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio C, Schmidt CK, Katou Y, Kelly G, Itoh T, Shirahige K, Uhlmann F. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 2008;22:2215–2227. doi: 10.1101/gad.1675708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Kamakaka RT. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. Embo J. 2001;20:520–531. doi: 10.1093/emboj/20.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–417. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- Freeman L, Aragon-Alcaide L, Strunnikov A. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J Cell Biol. 2000;149:811–824. doi: 10.1083/jcb.149.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Vagnarelli P, Hudson D, Earnshaw WC. Mitotic chromosome formation and the condensin paradox. Exp Cell Res. 2004;296:35–42. doi: 10.1016/j.yexcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Geiduschek EP, Kassavetis GA. The RNA polymerase III transcription apparatus. J Mol Biol. 2001;310:1–26. doi: 10.1006/jmbi.2001.4732. [DOI] [PubMed] [Google Scholar]
- Haeusler RA, Engelke DR. Spatial organization of transcription by RNA polymerase III. Nucleic Acids Res. 2006;34:4826–4836. doi: 10.1093/nar/gkl656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Pratt-Hyatt M, Good PD, Gipson TA, Engelke DR. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev. 2008;22:2204–2214. doi: 10.1101/gad.1675908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom KA, Holmes VF, Cozzarelli NR, Meyer BJ. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 2002;16:729–742. doi: 10.1101/gad.968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom KA, Meyer BJ. Condensin and cohesin: more than chromosome compactor and glue. Nat Rev Genet. 2003;4:520–534. doi: 10.1038/nrg1110. [DOI] [PubMed] [Google Scholar]
- Hirano T. Chromosome cohesion, condensation, and separation. Annu Rev Biochem. 2000;69:115–144. doi: 10.1146/annurev.biochem.69.1.115. [DOI] [PubMed] [Google Scholar]
- Hirano T. At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- Huang Y, Maraia RJ. Comparison of the RNA polymerase III transcription machinery in Schizosaccharomyces pombe, Saccharomyces cerevisiae and human. Nucleic Acids Res. 2001;29:2675–2690. doi: 10.1093/nar/29.13.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev Cell. 2003;5:323–336. doi: 10.1016/s1534-5807(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Hull MW, Erickson J, Johnston M, Engelke DR. tRNA genes as transcriptional repressor elements. Mol Cell Biol. 1994;14:1266–1277. doi: 10.1128/mcb.14.2.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki O, Tanaka A, Tanizawa H, Grewal SI, Noma K. Centromeric localization of dispersed Pol III genes in fission yeast. Mol Biol Cell. 2010;21:254–265. doi: 10.1091/mbc.E09-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrous N, Wolenski JS, Wesolowski D, Lee C, Altman S. Localization in the nucleolus and coiled bodies of protein subunits of the ribonucleoprotein ribonuclease P. J Cell Biol. 1999;146:559–572. doi: 10.1083/jcb.146.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis GA, Braun BR, Nguyen LH, Geiduschek EP. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990;60:235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–438. doi: 10.1016/j.cell.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura E, Blow JJ, Tanaka TU. Live-cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell. 2006;125:1297–1308. doi: 10.1016/j.cell.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D, Strunnikov A. Mitotic chromosome condensation. Annu Rev Cell Dev Biol. 1996;12:305–333. doi: 10.1146/annurev.cellbio.12.1.305. [DOI] [PubMed] [Google Scholar]
- Laemmli UK, Kas E, Poljak L, Adachi Y. Scaffold-associated regions: cis-acting determinants of chromatin structural loops and functional domains. Curr Opin Genet Dev. 1992;2:275–285. doi: 10.1016/s0959-437x(05)80285-0. [DOI] [PubMed] [Google Scholar]
- Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet. 2007;8:104–115. doi: 10.1038/nrg2041. [DOI] [PubMed] [Google Scholar]
- Lavoie BD, Tuffo KM, Oh S, Koshland D, Holm C. Mitotic chromosome condensation requires Brn1p, the yeast homologue of Barren. Mol Biol Cell. 2000;11:1293–1304. doi: 10.1091/mbc.11.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M, Mortensen UH, Rothstein R. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol. 2003;5:572–577. doi: 10.1038/ncb997. [DOI] [PubMed] [Google Scholar]
- Long EO, Dawid IB. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–1287. doi: 10.1101/gad.1320505. [DOI] [PubMed] [Google Scholar]
- Lunyak VV, Prefontaine GG, Nunez E, Cramer T, Ju BG, Ohgi KA, Hutt K, Roy R, Garcia-Diaz A, Zhu X, Yung Y, Montoliu L, Glass CK, Rosenfeld MG. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science. 2007;317:248–251. doi: 10.1126/science.1140871. [DOI] [PubMed] [Google Scholar]
- Matera AG, Frey MR, Margelot K, Wolin SL. A perinucleolar compartment contains several RNA polymerase III transcripts as well as the polypyrimidine tract-binding protein, hnRNP I. J Cell Biol. 1995;129:1181–1193. doi: 10.1083/jcb.129.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Moqtaderi Z, Struhl K. Genome-wide occupancy profile of the RNA polymerase III machinery in Saccharomyces cerevisiae reveals loci with incomplete transcription complexes. Mol Cell Biol. 2004;24:4118–4127. doi: 10.1128/MCB.24.10.4118-4127.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqtaderi Z, Wang J, Raha D, White RJ, Snyder M, Weng Z, Struhl K. Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nat Struct Mol Biol. 2010;17:635–640. doi: 10.1038/nsmb.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa N, Nakamura T, Kokubu A, Ebe M, Nagao K, Yanagida M. Dissection of the essential steps for condensin accumulation at kinetochores and rDNAs during fission yeast mitosis. J Cell Biol. 2008;180:1115–1131. doi: 10.1083/jcb.200708170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- Noma K, Cam HP, Maraia RJ, Grewal SI. A role for TFIIIC transcription factor complex in genome organization. Cell. 2006;125:859–872. doi: 10.1016/j.cell.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Oki M, Kamakaka RT. Barrier function at HMR. Mol Cell. 2005;19:707–716. doi: 10.1016/j.molcel.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Oler AJ, Alla RK, Roberts DN, Wong A, Hollenhorst PC, Chandler KJ, Cassiday PA, Nelson CA, Hagedorn CH, Graves BJ, Cairns BR. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. Nat Struct Mol Biol. 2010;17:620–628. doi: 10.1038/nsmb.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Fang Y, Spector DL, Hirano T. Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol Biol Cell. 2004;15:3296–3308. doi: 10.1091/mbc.E04-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MR, White RJ. Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res. 2000;28:1283–1298. doi: 10.1093/nar/28.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo A, Jackson DA, Hollinshead M, Wang Z, Roeder RG, Cook PR. Regional specialization in human nuclei: visualization of discrete sites of transcription by RNA polymerase III. Embo J. 1999;18:2241–2253. doi: 10.1093/emboj/18.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha D, Wang Z, Moqtaderi Z, Wu L, Zhong G, Gerstein M, Struhl K, Snyder M. Close association of RNA polymerase II and many transcription factors with Pol III genes. Proc Natl Acad Sci U S A. 2010;107:3639–3644. doi: 10.1073/pnas.0911315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DN, Stewart AJ, Huff JT, Cairns BR. The RNA polymerase III transcriptome revealed by genome-wide localization and activity-occupancy relationships. Proc Natl Acad Sci U S A. 2003;100:14695–14700. doi: 10.1073/pnas.2435566100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder RG. Nuclear RNA polymerases: role of general initiation factors and cofactors in eukaryotic transcription. Methods Enzymol. 1996;273:165–171. doi: 10.1016/s0076-6879(96)73016-1. [DOI] [PubMed] [Google Scholar]
- Saka Y, Sutani T, Yamashita Y, Saitoh S, Takeuchi M, Nakaseko Y, Yanagida M. Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. Embo J. 1994;13:4938–4952. doi: 10.1002/j.1460-2075.1994.tb06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KC, Merrett SL, Willard HF. A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains. Curr Biol. 2006;16:119–129. doi: 10.1016/j.cub.2005.11.065. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Jordan EG. The nucleolus. Annu Rev Cell Dev Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Simms TA, Dugas SL, Gremillion JC, Ibos ME, Dandurand MN, Toliver TT, Edwards DJ, Donze D. TFIIIC binding sites function as both heterochromatin barriers and chromatin insulators in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:2078–2086. doi: 10.1128/EC.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen S, Coelho PA, Cobbe N, Vass S, Costa M, Hassan B, Prokopenko SN, Bellen H, Heck MM, Sunkel CE. A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr Biol. 2001;11:295–307. doi: 10.1016/s0960-9822(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Sutherland H, Bickmore WA. Transcription factories: gene expression in unions? Nat Rev Genet. 2009;10:457–466. doi: 10.1038/nrg2592. [DOI] [PubMed] [Google Scholar]
- Tanizawa H, Iwasaki O, Tanaka A, Capizzi JR, Wickramasinghe P, Lee M, Fu Z, Noma KI. Mapping of long-range associations throughout the fission yeast genome reveals global genome organization linked to transcriptional regulation. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Haeusler RA, Good PD, Engelke DR. Nucleolar clustering of dispersed tRNA genes. Science. 2003;302:1399–1401. doi: 10.1126/science.1089814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela L, Dhillon N, Kamakaka RT. Transcription independent insulation at TFIIIC-dependent insulators. Genetics. 2009;183:131–148. doi: 10.1534/genetics.109.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev. 2007;17:400–407. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Haeusler RA, Good PD, Thompson M, Nagar S, Engelke DR. Silencing near tRNA genes requires nucleolar localization. J Biol Chem. 2005a;280:8637–8639. doi: 10.1074/jbc.C500017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BD, Eyre D, Basrai M, Lichten M, Strunnikov A. Condensin binding at distinct and specific chromosomal sites in the Saccharomyces cerevisiae genome. Mol Cell Biol. 2005b;25:7216–7225. doi: 10.1128/MCB.25.16.7216-7225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ. RNA polymerases I and III, growth control and cancer. Nat Rev Mol Cell Biol. 2005;6:69–78. doi: 10.1038/nrm1551. [DOI] [PubMed] [Google Scholar]
- Willis IM. RNA polymerase III. Genes, factors and transcriptional specificity. Eur J Biochem. 1993;212:1–11. doi: 10.1111/j.1432-1033.1993.tb17626.x. [DOI] [PubMed] [Google Scholar]
- Willoughby DA, Vilalta A, Oshima RG. An Alu element from the K18 gene confers position-independent expression in transgenic mice. J Biol Chem. 2000;275:759–768. doi: 10.1074/jbc.275.2.759. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Severson AF, Meyer BJ. Condensin and cohesin complexity: the expanding repertoire of functions. Nat Rev Genet. 2010;11:391–404. doi: 10.1038/nrg2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida M. Fission yeast cut mutations revisited: control of anaphase. Trends Cell Biol. 1998;8:144–149. doi: 10.1016/s0962-8924(98)01236-7. [DOI] [PubMed] [Google Scholar]
- Yanagida M. Basic mechanism of eukaryotic chromosome segregation. Philos Trans R Soc Lond B Biol Sci. 2005;360:609–621. doi: 10.1098/rstb.2004.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]