Summary
Recent genome-wide single nucleotide polymorphism (SNP) association studies (GWAS) have identified a number of SNPs that were significantly associated with coronary artery disease (CAD) and myocardial infarction (MI). However, many independent replication studies in other populations are needed to unequivocally confirm the GWAS association. To assess GWAS association, we have established a case-control cohort consisting of 1,231 well-characterized MI patients and 560 controls without detectable coronary stenosis, all selected from the Cleveland Genebank population. The Genebank cohort has a sufficient power to detect the association between MI and four GWAS SNPs, including rs17465637 within the MIA3 gene, rs2943634 (intergenic), rs6922269 in MTHFD1L, and rs599839 near SORT1. SNPs were genotyped by TaqMan assays and follow-up multivariate logistic regression analysis with incorporation of significant covariates showed significant association with MI for MIA3 SNP rs17465637 (P-adj=0.0034) and SORT1 SNP rs599839 (P-adj=0.009). The minor allele G of rs599839 was also associated with a decreased LDL-C level of 5–9 mg/dL per allele, but not with HDL-C or triglyceride levels. No association for MI or lipid levels was found for SNPs rs2943634 and rs6922269 (P-adj>0.05). Our results establish two SNPs, rs17465637 in MIA3 and rs599839 near SORT1 as significant risk factors for MI in the American Genebank Caucasian population.
Keywords: genome-wide association study (GWAS), single nucleotide polymorphism (SNP), myocardial infarction (MI), coronary artery disease, genetics, LDL, SNP rs17465637, SNP rs599839
Introduction
Coronary artery disease (CAD), along with its leading complication of myocardial infarction (MI), is the leading cause of mortality and disability worldwide (Lloyd-Jones et al., 2009). CAD is considered to be a complex disease that is caused by multiple genetic factors, environmental factors, and their interactions (Topol et al., 2006; Wang, 2005b; Wang, 2005a). As genetic factors play a substantial role in inherited risk for CAD/MI (Nora et al., 1980), it is important to identify these specific molecular and genetic determinants. Large scale genome-wide association studies (GWAS) with 500,000 to 1,000,000 single nucleotide polymorphisms (SNPs) have been developed as a popular strategy for identifying genetic factors for common, complex disease traits such as CAD and MI.
Recent GWAS have identified a number of candidate genetic loci for CAD and MI (Erdmann et al., 2009; Helgadottir et al., 2007; Kathiresan et al., 2009; McPherson et al., 2007; Samani et al., 2007; Samani et al., 2009; Tregouet et al., 2009). One limitation of GWAS is a high rate of false positives; thus, rigorous replication studies in many independent populations from different independent research groups are needed to replicate the findings. To date, the very first chromosome 9p21 locus for CAD and MI identified by GWAS has been replicated in more than 30 independent studies. GWAS also reported a number of other candidate loci that confer risk of or protection from CAD and MI, including SNPs rs17465637 on chromosome 1q41, rs2943634 on 2q36.3, rs6922269 on 6q25.1, rs599839 on 1p13.3, rs9818870 on 3q22.3 and rs501120 on 10q11.21 (Erdmann et al., 2009; Kathiresan et al., 2009; Samani et al., 2007; Samani et al., 2009). However, further replication studies are needed to establish the unequivocal association between these SNPs and CAD/MI using other independent populations. We have established a well-characterized case-control population of 1,231 MI patients and 560 non-CAD controls (Shen et al., 2007), which has a sufficient power for assessing the association between MI and four GWAS SNPs: rs17465637 within the MIA3 gene, rs2943634 (intergenic), rs6922269 in MTHFD1L, and rs599839 near SORT1. The aim of this study was to determine whether any of these four SNPs was associated with risk of MI in our U.S. Caucasian population from the Northeast Ohio area (Cleveland Clinic Genebank population).
Materials and Methods
Study population
We conducted a case-control association study involving a total population of 1,231 unrelated MI patients and 560 normal controls as previously reported by us (Shen et al., 2007). The study subjects were selected from the Cleveland Clinic Genebank program, which ascertained patients who were evaluated at the Cardiac Catheterization Laboratories. Clinical diagnosis of CAD and MI was carried out by a panel of cardiologists. Individuals with a stenosis of more than 70% or with a history of revascularization procedures (percutaneous coronary angioplasty – PTCA, coronary artery bypass graft - CABG) were classified as CAD patients. CAD patients with a previous diagnosis of MI were classified as MI patients (Shen et al., 2007; Wang et al., 2004). A myocardial infarction was diagnosed on the basis of chest pain of ≥ 30-minute duration, electrocardiogram patterns consistent with patterns of acute MI, and significant elevation of cardiac enzymes (Shen et al., 2007; Wang et al., 2004). All Caucasian patients who were enrolled in the first year of the program (a total of 1,231 patients) were selected for this study. The 560 controls were chosen from Cleveland Genebank, and included individuals who underwent coronary angiography. Only Caucasian individuals without atherosclerotic lesions detected by angiography were included. Every participant had fasted blood drawn, a lipid profile completed, glucose levels measured, and each subject completed a health questionnaire as well. This study was approved by the Cleveland Clinic Institutional Review Board on Human Subject Research. Informed consent was obtained from all participants, and the investigation conformed to the guidelines of the Declaration of Helsinki.
Isolation of genomic DNA and SNP genotyping
Human genomic DNA was isolated from whole blood with the Puregene Kits (Gentra). SNP genotyping was performed using the 5’ nuclease discrimination assay (Taqman Assays, Applied Biosystems) on an ABI PRISM 7900HT Sequence Detection System (as previously described by us) (Abdullah et al., 2008; Hu et al., 2008). The quality of SNP genotyping was verified by direct DNA sequence analysis of 36 samples. The results from the Taqman Assay were 100% in agreement with those from the sequencing analysis. In addition, all of the DNA samples for case and control subjects were run in the same batches.
Statistical analysis
Allelic association of a SNP with a disease trait was assessed with Pearson’s 2x2 contingency table Chi-square test, implemented within SAS Ver 9.00 (SAS Institute Inc) as described previously (Shen et al., 2007; Shen et al., 2008; Xu et al., 2010). Odds ratios and 95% confidence intervals (CI) were estimated through SAS Ver 9.00. Multivariate logistic regression analysis was also performed using SAS Ver 9.00 to test relationships between SNPs and to account for significant covariates as described (gender, age, smoking, body mass index, hypertension, diabetes, total cholesterol, and triglyceride level) (Shen et al., 2007; Shen et al., 2008; Xu et al., 2010). All SNPs were tested for Hardy-Weinberg equilibrium among the normal controls using a chi-square test with one degree of freedom from an online software program (http://www.genes.org.uk/software/hardy-weinberg.shtml). Point-wise statistical significance was adjusted for multiple tests with the Bonferroni method.
Power analysis was estimated using two group Chi-square tests of two unequal proportions and performed using the nQuery Advisor 7.0 using reported ORs for individual SNPs (Samani et al., 2007) and their minor allele frequencies for the U.S. residents with northern and western European ancestry (HapMap Public Release #28 http://hapmap.ncbi.nlm.nih.gov/). Our Genebank case-control cohort has a power of 73%, 74%, 73%, and 77% for detecting MI association for SNPs rs17465637, rs2943634, rs6922269, and rs599839, respectively.
Results
Significant association of SNPs rs17465637 in MIA3 and rs599839 near SORT1 with MI in an American Caucasian population
We performed a case-control study with 1,231 MI patients and 560 controls from the Cleveland Genebank population. The same study population was used in a previous study to evaluate the association between an LRP8 SNP and platelet aggregation/MI (Shen et al., 2007). All study subjects were of American Caucasian descent. The average age at onset for case subjects was 60.6±12.1 years, and the average age at examination for control subjects was 53.5±12.1 years. As expected, the male/female ratio and the rates of smoking, hypertension, diabetes, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were higher in the case population than in the control population.
Four SNPs (rs17465637 within the MIA3 gene, rs2943634 (intergenic), rs6922269 in MTHFD1L, and rs599839 near SORT1) were genotyped in the study population. None of the SNPs demonstrated deviation from Hardy-Weinberg equilibrium (Table 1) (PHW > 0.01). After adjusting for the significant clinical covariates of age, gender, smoking, hypertension, diabetes, BMI, total cholesterol, and triglyceride levels, a significant association was identified for SNPs rs17465637 in MIA3 and rs599839 near SORT1 with MI; P-adj=0.0034 and P-adj=0.009, respectively (Table 1). In both cases, the minor allele conferred a protective effect on MI with an adjusted OR of 0.75.
Table 1.
Allelic association of 4 GWAS SNPs with MI in an American Genebank Caucasian population.
| SNP | Chromosomal location |
Minor Allele |
Frequency | OR (95% CI)a |
P value* | Adjusted OR (95% CI)a |
|||
|---|---|---|---|---|---|---|---|---|---|
| (gene) | Control | Case | HWb | Observedc | Adjustedd | ||||
| rs17465637 | 1q41 (MIA3) |
A | 29.37 | 26.68 | 0.88 (0.75–1.02) |
0.577 | 0.097 | 0.0034 | 0.75 (0.62–0.91) |
| rs2943634 | 2q36.3 (intergenic) |
A | 32.66 | 33.50 | 1.04 (0.89–1.21) |
0.032 | 0.626 | 0.596 | 1.05 (0.87–1.26) |
| rs6922269 | 6q25.1 (MTHFD1L) |
A | 26.14 | 25.84 | 0.98 (0.84–1.16) |
0.718 | 0.852 | 0.718 | 0.96 (0.78–1.18) |
| rs599839 | 1p13.1 (near SORT1) |
G | 22.89 | 19.76 | 0.83 (0.70–0.99) |
0.980 | 0.041 | 0.009 | 0.75 (0.61–0.93) |
OR, odds ratio, CI, confidence interval.
HW, P value for Hardy-Weinberg disequilibrium test.
Observed, nominal P-value.
Adjusted, P value obtained after adjustment for gender, age, smoking, body mass index, hypertension, diabetes, total cholesterol, and triglyceride level.
A P value of ≤0.0125 was considered to be statistically significant after adjusting for multiple tests with the Bonferroni method.
No significant allelic association was detected for intergenic SNP rs2943634 and MTHFD1L SNP rs6922269 with MI (Table 1).
Significant association of SNP rs599839 near SORT1 with plasma LDL-C levels
Further statistical analysis was performed for the association between the four SNPs and plasma LDL-C, HDL-C, and triglyceride levels in the study population. General linear model analysis showed no significant Bonferroni-corrected P-values for HDL-C and triglyceride concentrations under an additive, dominant, or recessive model (Tables 2–4). Interestingly, for LDL-C levels, one of the SNPs, rs599839 near SORT1, showed significant association in an additive model in 1,231 MI cases and the combined population of 1,791 subjects with P-adj values of 0.0015 and 0.0003, respectively (Table 3). In the population of 560 controls, the association was significant before adjustment for significant clinical covariates (P-obs=0.0009). However, this significance was reduced to P-adj=0.017 after adjustment, which is higher than the accepted threshold for Bonferroni-corrected significance (0.05/4=0.013). The association remained significant under a dominant model (P-adj=0.000038 and 0.000034 in 1,231 MI cases and the combined population, respectively) but not under a recessive model (best P-adj=0.012). Each minor allele G decreases LDL-C levels by 4.87–9.19 mg/dL (AA=103.71 +/− 35.88; GA=98.84 +/− 34.35; GG=89.65 +/− 32.43).
Table 2.
Assessment of association between the 4 SNPs and HDL-C levels in the Genebank population assuming an additive model.
| HDL-C |
P value* |
|||||
|---|---|---|---|---|---|---|
| 1231 MI cases | 560 controls | Combined | ||||
| SNP | P-ob | P-adj | P-ob | P-adj | P-ob | P-adj |
| rs17465637 | 0.7225 | 0.7137 | 0.2325 | 0.7508 | 0.9371 | 0.9854 |
| rs2943634 | 0.3281 | 0.5749 | 0.6522 | 0.9120 | 0.7648 | 0.8643 |
| rs6922269 | 0.7018 | 0.9339 | 0.9287 | 0.9076 | 0.8768 | 0.8928 |
| rs599839 | 0.3653 | 0.1434 | 0.8596 | 0.0166 | 0.1923 | 0.0175 |
P-ob was obtained from general linear modeling where the predictor is coded with the number of risk alleles, but without any other covariates.
P-adj was obtained from general linear modeling after adjustment for gender, age, smoking, diabetes, hypertension, total cholesterol, triglyceride level and MI.
A P value of ≤0.0125 was considered to be statistically significant after adjusting for multiple tests with the Bonferroni method.
Table 4.
Assessment of association between 4 SNPs and triglyceride levels (TG) in the Genebank population assuming an additive model.
| TG |
P value* |
|||||
|---|---|---|---|---|---|---|
| 1231 MI cases | 560 controls | Combined | ||||
| SNP | P-ob | P-adj | P-ob | P-adj | P-ob | P-adj |
| rs17465637 | 0.5234 | 0.3656 | 0.8580 | 0.6907 | 0.5581 | 0.6900 |
| rs2943634 | 0.3439 | 0.1672 | 0.2097 | 0.2671 | 0.1567 | 0.1141 |
| rs6922269 | 0.8617 | 0.3953 | 0.7195 | 0.6214 | 0.7649 | 0.2806 |
| rs599839 | 0.9752 | 0.2294 | 0.4206 | 0.0343 | 0.7801 | 0.0283 |
P-adj was obtained from general linear modeling after adjustment for gender, age, smoking, diabetes, hypertension, total cholesterol and MI.
A P value of ≤0.0125 was considered to be statistically significant after adjusting for multiple tests with the Bonferroni method.
Table 3.
Assessment of association between 4 SNPs and LDL-C levels in the Genebank population assuming an additive model.
| LDL-C |
P value* |
|||||
|---|---|---|---|---|---|---|
| 1231 MI cases | 560 controls | Combined | ||||
| SNP | P-ob | P-adj | P-ob | P-adj | P-ob | P-adj |
| rs17465637 | 0.6063 | 0.8613 | 0.1815 | 0.6724 | 0.9477 | 0.7817 |
| rs2943634 | 0.3283 | 0.8985 | 0.2793 | 0.8799 | 0.8835 | 0.7508 |
| rs6922269 | 0.0476 | 0.6028 | 0.8742 | 0.2456 | 0.1019 | 0.2371 |
| rs599839 | 0.0002 | 0.0015 | 0.0009 | 0.0173 | 0.000009 | 0.0003 |
P-adj was obtained from general linear modeling after adjustment for gender, age, smoking, diabetes, hypertension, total cholesterol, triglyceride level and MI.
A P value of ≤0.0125 was considered to be statistically significant after adjusting for multiple tests with the Bonferroni method.
DISCUSSION
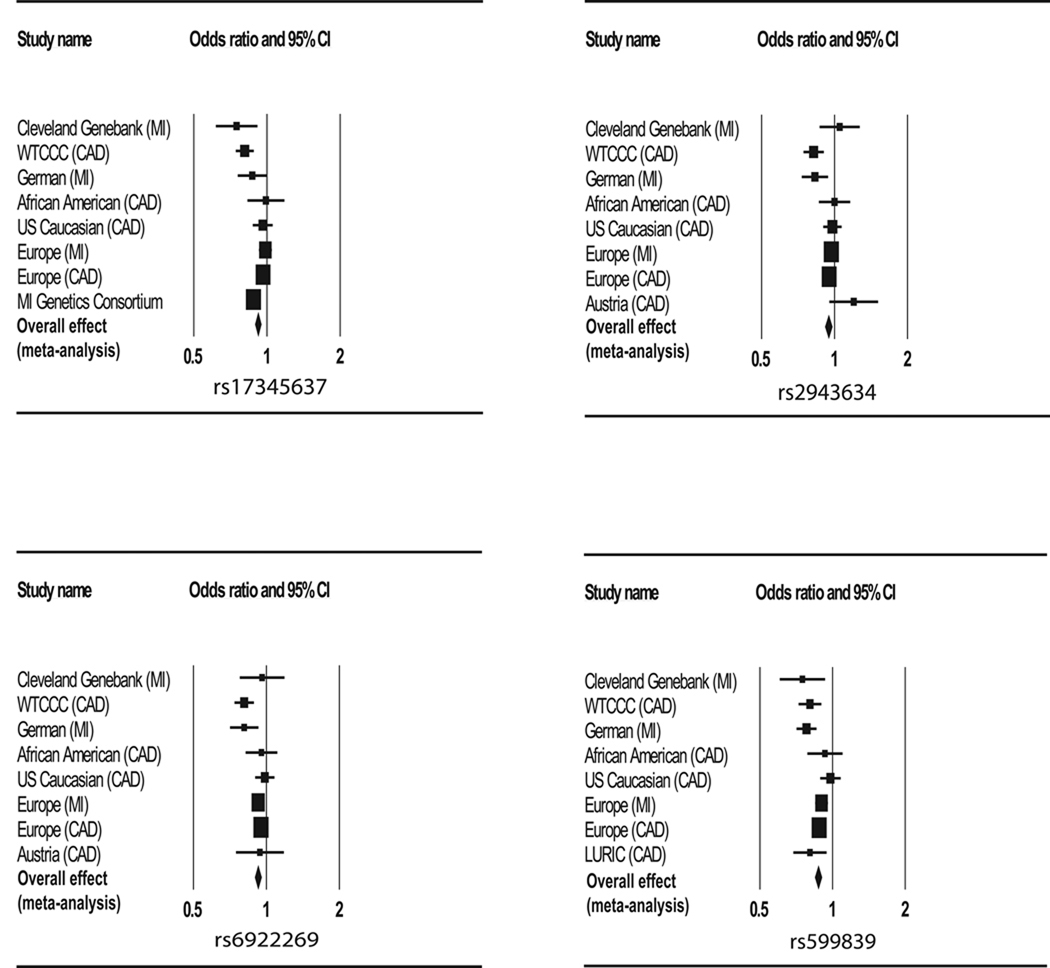
In this study, we assessed four SNPs (rs17465637 within the MIA3 gene, rs2943634 (intergenic), rs6922269 in MTHFD1L, and rs599839 near SORT1) for their association with MI in an American Caucasian population. These four SNPs have previously been identified as being associated with CAD and/or MI by GWAS (WTCCC CAD and German MI studies, Figure 1) (Samani et al., 2007). The recruited MI cases and control subjects used in this study were carefully ascertained and very strict criteria were used to define both the MI phenotype and the normal phenotype. All the control subjects showed no detectable stenosis, as verified by angiography. Two of the SNPs, rs17465637 in MIA3 and rs599839 near SORT1, demonstrated significant allelic association with MI after adjustment by multivariate logistic regression analysis incorporating the significant covariates of age, gender, smoking, hypertension, diabetes, BMI, total cholesterol, and triglyceride levels (Table 1). SNP rs599839 was also associated with plasma LDL-C levels. The minor allele G of rs599839 confers a protective effect on MI and is associated with decreased LDL-C levels.
Figure 1.
Forest plot of SNP effect estimates for CAD/MI. The data from original GWAS and follow-up replication studies are summarized fro 4 SNPs, including rs17465637 within the MIA3 gene, rs2943634 (intergenic), rs6922269 in MTHFD1L, and rs599839 near SORT1. The X-axis represents the odds ratios (ORs) and 95% confidence intervals (CI) as well as the weight (marked as a filled box) from different studies, including Cleveland Genebank (this study), WTCCC (CAD) and German (MI) (Samani et al., 2007), Europe (MI) and Europe (CAD) (Samani et al., 2009), African-American (CAD) and USA (CAD) (Bressleret al., 2010), Austria (CAD) (Muendlein et al., 2009), MI Genetics Consortium (Kathiresan et al., 2009), and LURIC (CAD) (Kleber et al., 2010). The overall effect of each SNP from meta-analysis of the combined data is shown as a filled diamond. The effect of each SNP was based on the minor allele, thus conversion was made for some studies. The plot was created using the CMA program (http://www.meta-analysis.com/pages/features/forest_plots.html).
SNP rs17465637 on chromosome 1q41 is located in the MIA3 gene, which encodes melanoma inhibitor protein 3 required for export of collagen VII (COL7A1) from the endoplasmic reticulum (Saito et al., 2009), and appears to be a tumor suppressor of malignant melanoma (Arndt & Bosserhoff, 2007). MIA3 may be involved in facilitating the migration of monocytic cells through fibrinogene or human microvascular endothelial cells (Arndt et al., 2007), which may increase the risk of plaque formation. However, it remains to be further established whether MIA3 is directly related to CAD and MI.
SNP rs599839 on chromosome 1p13.1 is located in the 3’ untranslated region of the PSRC1 gene and is near the SORT1 gene. SNP rs599839 has been reported to be associated with LDL-C levels (Sandhu et al., 2008; Willer et al., 2008; Kleber et al., 2010). The minor allele G was associated with increased expression levels of SORT1 mRNA and that overexpression of SORT1 led to a significant increase in LDL uptake into cells (Linsel-Nitschke et al., 2010). A very recent study further showed that overexpression of SORT1 resulted in a decrease in total plasma cholesterol and LDL-C levels (Musunuru et al., 2010). The study also demonstrated that knockdown of SORT1 expression by siRNA caused a 46 percent increase in total cholesterol and a more than twofold increase in LDL-C levels (Musunuru et al., 2010). Thus, SORT1 appears to be the causal gene for reduced LDL-C levels at the 1p13.3 locus, and may lower the risk of MI by decreasing the LDL-C levels.
SNP rs17465637 in MIA3 was identified as a probable genetic locus for CAD only after combining the Wellcome Trust Case Consortium study and the German MI family study (more than 80% probability of a true association) (Samani et al., 2007). SNP rs599839 near SORT1 was also identified as a probable genetic locus after combining the two studies (Samani et al., 2007). A follow-up study by the Myocardial Infarction Genetics Consortium replicated the association between rs17465637 and MI (Kathiresan et al., 2009) (Figure 1). However, a study by the Coronary Artery Disease Consortium (Samani et al., 2009) and another study with an African-American population and a U.S. Caucasian population (Bressleret al., 2010) failed to confirm the association (Figure 1). Our study provides a timely reassessment and provides new evidence that supports the association between SNP rs17465637 and MI. The association between SNP rs599839 near SORT1 and CAD was replicated in the Coronary Artery Disease Consortium study (Samani et al., 2009), but not in another replication study with an African-American population and a U.S. Caucasian population (Bressleret al., 2010). In the Ludwigshafen Risk and Cardiovascular Health replication study, the association between SNP rs599839 and CAD/MI was confirmed (Kleber et al., 2010). Our study further replicated the association of SNP rs599839 near SORT1 with MI in an American Caucasian population. In our Genebank population, the other two SNPs, intergenic SNP rs2943634 and SNP rs6922269 in MTHFD1L, did not exhibit any association with MI although the 95% confidence intervals for ORs for these two SNPs overlap with those in the original GWAS studies (Figure 1). Our results are more consistent with a recent replication study in Austria and another study in the U.S. that did not detect association between rs6922269 and rs2943634 and CAD (Muendlein et al., 2009; Bressleret al., 2010) (Figure 1).
One limitation of this study is that, as in many other human genetics studies, the sample size is fixed. And the phenotypes under study (MI, lipid levels) are complex and involve multiple small-to modest-sized effects of genes and polygenic/environmental backgrounds. As such, the results may be biased. Furthermore, the relatively small sample size with a limited power of 73% to 77% and the small effect of their contribution to MI may explain why SNPs rs2943634 and rs6922269 did not show significant association with MI.
In conclusion, we found significant associations of SNP rs17465637 in the MIA3 gene on chromosome 1q41 and SNP rs599839 near the SORT1 gene on chromosome 1p13.3 with MI in an American Caucasian population. We also found a significant association between SNP rs599839 and LDL-C levels in the same population. However, no significant association was found with MI for intergenic SNP rs2943634 on 1q36.3 and rs6922269 in MTHFD1L on 6q25.1. These results represent an important expansion of GWAS findings into an American Caucasian Genebank population.
Acknowledgments
This work was supported by the National Institutes of Health Grants P50 HL81011, the Cleveland Clinic Summer Internship to Shaker Heights High School student A.Z.W., the American Heart Association grants (09BGIA2460022, 11SDG5510001, and 11IRG5570046), the China National Basic Research Program (973 program 2007CB512002), China Nation 863 Scientific Program (2006AA02Z476), the Hubei Province Natural Science Key Program (2008CDA047), and the Key Academic Program Leader Award of Wuhan City (200951830560).
References
- Abdullah KG, Li L, Shen GQ, Hu Y, Yang Y, MacKinlay KG, Topol EJ, Wang QK. Four SNPS on chromosome 9p21 confer risk to premature, familial CAD and MI in an American Caucasian population (GeneQuest) Ann. Hum. Genet. 2008;72:654–657. doi: 10.1111/j.1469-1809.2008.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt S, Bosserhoff AK. Reduced expression of TANGO in colon and hepatocellular carcinomas. Oncol. Rep. 2007;18:885–891. [PubMed] [Google Scholar]
- Arndt S, Melle C, Mondal K, Klein G, von EF, Bosserhoff AK. Interactions of TANGO and leukocyte integrin CD11c/CD18 regulate the migration of human monocytes. J. Leukoc. Biol. 2007;82:1466–1472. doi: 10.1189/jlb.0407219. [DOI] [PubMed] [Google Scholar]
- Bressler J, Folsom AR, Couper DJ, Volcik KA, Boerwinkle E. Genetic variants identified in a European genome-wide association study that were found to predict incident coronary heart disease in the atherosclerosis risk in communities study. Am. J. Epidemiol. 2010;171:14–23. doi: 10.1093/aje/kwp377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann J, Grosshennig A, Braund PS, Konig IR, Hengstenberg C, Hall AS, Linsel-Nitschke P, Kathiresan S, Wright B, Tregouet DA, Cambien F, Bruse P, Aherrahrou Z, Wagner AK, Stark K, Schwartz SM, Salomaa V, Elosua R, Melander O, Voight BF, O'Donnell CJ, Peltonen L, Siscovick DS, Altshuler D, Merlini PA, Peyvandi F, Bernardinelli L, Ardissino D, Schillert A, Blankenberg S, Zeller T, Wild P, Schwarz DF, Tiret L, Perret C, Schreiber S, El Mokhtari NE, Schafer A, Marz W, Renner W, Bugert P, Kluter H, Schrezenmeir J, Rubin D, Ball SG, Balmforth AJ, Wichmann HE, Meitinger T, Fischer M, Meisinger C, Baumert J, Peters A, Ouwehand WH, Deloukas P, Thompson JR, Ziegler A, Samani NJ, Schunkert H. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat. Genet. 2009;41:280–282. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- Hu Y, Li L, Seidelmann SB, Timur AA, Shen PH, Driscoll DJ, Wang QK. Identification of association of common AGGF1 variants with susceptibility for Klippel-Trenaunay syndrome using the structure association program. Ann. Hum. Genet. 2008;72:636–643. doi: 10.1111/j.1469-1809.2008.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Ardissino D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Mannucci PM, Schwartz SM, Siscovick DS, Yee J, Friedlander Y, Elosua R, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Kathiresan S, Meigs JB, Williams G, Nathan DM, MacRae CA, O'Donnell CJ, Salomaa V, Havulinna AS, Peltonen L, Melander O, Berglund G, Voight BF, Kathiresan S, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Musunuru K, Daly MJ, Purcell S, Voight BF, Purcell S, Nemesh J, Korn JM, McCarroll SA, Schwartz SM, Yee J, Kathiresan S, Lucas G, Subirana I, Elosua R, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Samani NJ, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall AS, Schunkert H, Erdmann J, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Schunkert H, Samani NJ, Erdmann J, Ouwehand W, Hengstenberg C, Deloukas P, Scholz M, Cambien F, Reilly MP, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein SE, Rader DJ, Scheffold T, Berger K, Stoll M, Huge A, Girelli D, Martinelli N, Olivieri O, Corrocher R, Morgan T, Spertus JA, McKeown P, Patterson CC, Schunkert H, Erdmann E, Linsel-Nitschke P, Lieb W, Ziegler A, Konig IR, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Engert JC, Do R, Xie C, Anand S, Kathiresan S, Ardissino D, Mannucci PM, Siscovick D, O'Donnell CJ, Samani NJ, Melander O, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Altshuler D. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber ME, Renner W, Grammer TB, Linsel-Nitschke P, Boehm BO, Winkelmann BR, Bugert P, Hoffmann MM, Marz W. Association of the single nucleotide polymorphism rs599839 in the vicinity of the sortilin 1 gene with LDL and triglyceride metabolism, coronary heart disease and myocardial infarction. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis. 2010;209:492–497. doi: 10.1016/j.atherosclerosis.2009.09.068. [DOI] [PubMed] [Google Scholar]
- Linsel-Nitschke P, Heeren J, Aherrahrou Z, Bruse P, Gieger C, Illig T, Prokisch H, Heim K, Doering A, Peters A, Meitinger T, Wichmann HE, Hinney A, Reinehr T, Roth C, Ortlepp JR, Soufi M, Sattler AM, Schaefer J, Stark K, Hengstenberg C, Schaefer A, Schreiber S, Kronenberg F, Samani NJ, Schunkert H, Erdmann J. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis. 2010;208:183–189. doi: 10.1016/j.atherosclerosis.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boerwinkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muendlein A, Saely CH, Rhomberg S, Sonderegger G, Loacker S, Rein P, Beer S, Vonbank A, Winder T, Drexel H. Evaluation of the association of genetic variants on the chromosomal loci 9p21.3, 6q25.1, and 2q36.3 with angiographically characterized coronary artery disease. Atherosclerosis. 2009;205:174–180. doi: 10.1016/j.atherosclerosis.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora JJ, Lortscher RH, Spangler RD, Nora AH, Kimberling WJ. Genetic--epidemiologic study of early-onset ischemic heart disease. Circulation. 1980;61:503–508. doi: 10.1161/01.cir.61.3.503. [DOI] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Deloukas P, Erdmann J, Hengstenberg C, Kuulasmaa K, McGinnis R, Schunkert H, Soranzo N, Thompson J, Tiret L, Ziegler A. Large scale association analysis of novel genetic loci for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2009;29:774–780. doi: 10.1161/ATVBAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH, Song K, Yuan X, Johnson T, Ashford S, Inouye M, Luben R, Sims M, Hadley D, McArdle W, Barter P, Kesaniemi YA, Mahley RW, McPherson R, Grundy SM, Bingham SA, Khaw KT, Loos RJ, Waeber G, Barroso I, Strachan DP, Deloukas P, Vollenweider P, Wareham NJ, Mooser V. LDL-cholesterol concentrations: a genome-wide association study. Lancet. 2008;371:483–491. doi: 10.1016/S0140-6736(08)60208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen GQ, Li L, Girelli D, Seidelmann SB, Rao S, Fan C, Park JE, Xi Q, Li J, Hu Y, Olivieri O, Marchant K, Barnard J, Corrocher R, Elston R, Cassano J, Henderson S, Hazen SL, Plow EF, Topol EJ, Wang QK. An LRP8 variant is associated with familial and premature coronary artery disease and myocardial infarction. Am. J. Hum. Genet. 2007;81:780–791. doi: 10.1086/521581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen GQ, Li L, Rao S, Abdullah KG, Ban JM, Lee BS, Park JE, Wang QK. Four SNPs on chromosome 9p21 in a South Korean population implicate a genetic locus that confers high cross-race risk for development of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2008;28:360–365. doi: 10.1161/ATVBAHA.107.157248. [DOI] [PubMed] [Google Scholar]
- Topol EJ, Smith JC, Plow EF, Wang Q. Genetic susceptibility to myocardial infarction and coronary artery disease. Hum. Mol. Genet. 2006;15:R117–R123. doi: 10.1093/hmg/ddl183. [DOI] [PubMed] [Google Scholar]
- Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, Grosshennig A, Linsel-Nitschke P, Perret C, DeSuremain M, Meitinger T, Wright BJ, Preuss M, Balmforth AJ, Ball SG, Meisinger C, Germain C, Evans A, Arveiler D, Luc G, Ruidavets JB, Morrison C, van der HP, Schreiber S, Neureuther K, Schafer A, Bugert P, El Mokhtari NE, Schrezenmeir J, Stark K, Rubin D, Wichmann HE, Hengstenberg C, Ouwehand W, Ziegler A, Tiret L, Thompson JR, Cambien F, Schunkert H, Samani NJ. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat. Genet. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- Wang Q. Molecular genetics of coronary artery disease. Curr. Opin. Cardiol. 2005b;20:182–188. doi: 10.1097/01.hco.0000160373.77190.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. Advances in the genetic basis of coronary artery disease. Curr. Atheroscler. Rep. 2005a;7:235–241. doi: 10.1007/s11883-005-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Rao S, Shen GQ, Li L, Moliterno DJ, Newby LK, Rogers WJ, Cannata R, Zirzow E, Elston RC, Topol EJ. Premature Myocardial Infarction Novel Susceptibility Locus on Chromosome 1P34-36 Identified by Genomewide Linkage Analysis. Am. J. Hum. Genet. 2004;74:262–271. doi: 10.1086/381560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, vey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang F, Wang B, Li X, Li C, Wang D, Xiong X, Wang P, Lu Q, Wang X, Yang Q, Yin D, Huang Y, Ji L, Wang N, Chen S, Cheng X, Liao Y, Ma X, Su D, Chen G, Xia H, Shi L, Tu X, Wang QK. Minor allele C of chromosome 1p32 single nucleotide polymorphism rs11206510 confers risk of ischemic stroke in the Chinese Han population. Stroke. 2010;41:1587–1592. doi: 10.1161/STROKEAHA.110.583096. [DOI] [PubMed] [Google Scholar]