Abstract
Recent data suggest that transitions between the relaxed (R) and tense (T) state of hemoglobin control the reduction of nitrite to nitric oxide (NO) by deoxyhemoglobin. This reaction may play a role in physiologic NO homeostasis and be a novel consideration for the development of the next generation of hemoglobin-based blood oxygen carriers (HBOCs, i.e. artificial blood substitutes). Herein we tested the effects of chemical stabilization of bovine hemoglobin in either the T- (THb) or R-state (RHb) on nitrite reduction kinetics, NO-gas formation and ability to stimulate NO-dependent signaling. These studies were performed over a range of fractional saturations that is expected to mimic biological conditions. The initial rate for nitrite-reduction decreased in the following order RHb > bHb > THb, consistent with the hypothesis that the rate constant for nitrite reduction is faster with R-state Hb and slower with T-state Hb. Moreover, RHb produced more NO-gas and inhibited mitochondrial respiration more potently than both bHb and THb. Interestingly, at low oxygen fractional saturations, THb produced more NO and stimulated nitrite-dependent vasodilation more potently than bHb despite both derivatives having similar initial rates for nitrite reduction and a more negative reduction potential in THb versus bHb. These data suggest that cross-linking of bovine hemoglobin in the T-state conformation leads to a more effective coupling of nitrite reduction to NO-formation. Our results support the model of allosteric regulation of nitrite reduction by deoxyhemoglobin and show that cross-linking hemoglobins in distinct quaternary states can generate products with increased NO yields from nitrite reduction that could be harnessed to promote NO-signaling in vivo.
Keywords: Hypoxia, blood flow, oxygen sensing, blood substitute, nitrite reduction
1. Introduction
Hemoglobin is a tetrameric protein whose primary role as a reversible carrier of oxygen and carbon dioxide is regulated by allosteric effectors that modulate structural transitions between relaxed (R-state, high oxygen affinity) and tense (T-state, low-oxygen affinity) conformations. The physiologic importance of the hemoglobin R-T transition is now appreciated to go beyond oxygen delivery with roles in regulating vascular nitric oxide (NO) metabolism and signaling [1; 2]. The concept forwarded is that NO, a key mediator of vascular homeostasis, is produced by deoxyhemoglobin mediated nitrite reduction [3; 4]. Nitrite is an anion that is ubiquitous in mammalian systems being formed by both nitrate reduction and NO-oxidation [5; 6]. This so-called hemoglobin nitrite-reductase activity has been proposed to contribute to hypoxic blood flow by increasing local NO concentrations at sites where hemoglobin becomes deoxygenated [3; 4; 7].
Early studies by Haldane [8], Brooks [9] and Doyle [10], described the reduction of nitrite by deoxyhemoglobin via the following reaction scheme:
However only recently, has this reaction been considered as a viable mechanism for producing bioactive NO in vivo to stimulate vasodilation, with key data supporting this hypothesis stemming from the assessment of the kinetics of this reaction at different oxygen fractional saturations [3; 4; 11; 12]. Interestingly, the rate constant for this reaction (assessed by measuring deoxyhemoglobin consumption) increases with increasing oxygen fractional saturations in parallel with increased R- state character [11; 12; 13]. In contrast, increasing oxygen fractional saturations decreases the concentration of deoxyhemoglobin, i.e. one of the substrates for the nitrite-reduction reaction. This combination results in a bell shaped dependence of initial rate of nitrite reduction versus oxygen fractional saturation, with maximal initial rates centered around hemoglobin's P50 (oxygen affinity, pO2 at which hemoglobin is half-saturated with oxygen molecules) [4; 12; 14]. The increased rate constant with oxygen fractional saturation is explained by either ‘R’-state hemoglobin being a more facile electron donor due to a more negative redox potential and / or having a more accessible heme pocket relative to ‘T’-state hemoglobin [4; 15; 16; 17; 18; 19; 20]. Overall, these data suggest a coordinated regulation of hemoglobin desaturation and NO-formation from nitrite reduction [1].
An important concept that has emerged from these studies is that deoxyhemoglobin and nitrite mediated NO-generation will counter-balance the NO-scavenging reaction by the heme, an effect that will become prominent as hemoglobin is desaturated. This has been demonstrated by measuring the ability of deoxyhemoglobin to modulate nitrite-mediated vasodilatation of isolated rat aortic rings, which provides a sensitive read-out for NO signaling [21; 22]. The physiological role for deoxyhemoglobin and red blood cells as mediators of hypoxic blood flow in vivo via nitrite reduction remains under investigation, and more recently this reactivity has been discussed from a therapeutic perspective as a means to attenuate the hypertensive effects of hemoglobin-based oxygen carriers (HBOCs) [23; 24; 25; 26]. Concerns with transmission of infectious agents, transfusion related toxicities, limited supplies and stability of donated red cells have fuelled interest in the development of acellular modified hemoglobins as blood substitutes.
These are typically cross-linked products with higher molecular weights which have significantly improved circulatory half-life versus non-modified cell-free hemoglobin and a range of oxygen affinities consistent with stabilization in either the R- or T-state conformation. However, NO-scavenging by acellular cross-linked hemoglobins remains a concern, with a recent meta-analysis of data from several clinical trials suggesting that this reaction is central to the observed toxicity of these products [27; 28]. This observation has led us and others to propose the use of nitrite as an adjunct therapy to mitigate HBOC-induced hypertension [23; 26; 29]. In this model, nitrite-derived NO would counter HBOC mediated NO-scavenging. Consistent with this hypothesis, both deoxygenated cross-linked hemoglobins and polyethylene glycol modified hemoglobin reduce nitrite with faster kinetics compared to the corresponding native hemoglobin [16; 24; 25]. Moreover, we have shown that nitrite administration at the onset of resuscitation with HBOC-201 (OPK Biotech, Cambridge, MA), a ‘T’-state stabilized bovine hemoglobin, can attenuate the hypertensive response that is typically observed after infusion of this product in a hemorrhagic shock model in vivo [26]. This has led to the suggestion that the next generation of HBOCs should be designed to have optimal nitrite reductase activities. This is an intriguing possibility since nitrite reduction can be affected independently by altering the oxygen affinity of the hemoglobin (and hence deoxyheme substrate concentration) as well as the reduction potential and accessibility of the heme centers (i.e. rate constant). For instance, although an R-state stabilized hemoglobin may not become deoxygenated to the same extent as a T-state product at a given oxygen tension, a faster rate constant may allow for faster nitrite reduction. On the other hand although a T-state stabilized product may have a slower rate constant for nitrite reduction compared to an R-state stabilized product, by virtue of it being more easily deoxygenated and therefore providing more deoxyheme substrate, it may reduce nitrite faster. In other words, the potential consequences for modulating nitrite reduction by cross-linking hemoglobin are not easily predictable, especially at different fractional saturations. This latter concept is further emphasized by the possibility that intermediates formed as a result of nitrite – oxyhemoglobin reactions may facilitate NO-release from nitrosylheme formed during nitrite-deoxyhemoglobin reactions; a process termed oxidative denitrosylation [30]. As a result, in evaluating the potential for nitrite-HBOC interactions to attenuate NO-scavenging, it is important to evaluate nitrite reduction kinetics together with the ability to stimulate NO-signaling under the different fractional saturations that will be populated in vivo.
Herein, we tested the effects of R- or T- state stabilization of cross-linked hemoglobins on nitrite reduction kinetics, NO-formation and mediation of vasodilation as a function of fractional saturation. We used bovine hemoglobin which was cross-linked with glutaraldehyde under conditions that produce strictly ‘R’ – or ‘T’ state stabilized products [31].
2. Materials and Methods
2.1 Materials
All reagents were purchased from Sigma–Aldrich (St Louis, MO) except MahmaNONOate and L-NMMA, which were obtained from Axxora Platform (San Diego, CA). Male Sprague–Dawley rats (200–250 g) were purchased from Harlan (Indianapolis, IN). All studies were performed following Institutional Animal Care and Use approved procedures
2.2 Purification of Bovine Hemoglobin
Bovine hemoglobin (bHb) was purified from lysed bovine red blood cells by tangential flow filtration (TFF) as described in the literature [32; 33; 34].
2.3 Polymerization of bHb
T-state polymerized bHb (THb) was synthesized according to a previously published procedure [35; 36; 37]. Briefly, 30 grams of purified bHb was diluted with 20 mM phosphate buffer (PB) (pH 8.0) to approximately 1200 mL in an airtight bottle, which was connected to a vacuum manifold and kept cold in an ice-water bath. The bHb solution was then subjected to alternative vacuum and argon (Ar) purging cycles under continuous stirring for 4 hours to remove the majority of O2. Subsequently, 300 mL of ice-cold Na2S2O4 solution (1.5 mg/mL) was then added into the bHb solution slowly with a syringe pump (Razel Scientific, St. Albans, VT) to remove the residual O2. The pO2 of the solution was measured using a RapidLab 248 (Siemens, Malvern, PA) blood gas analyzer until the pO2 of the bHb solution attained a value of 0 mm Hg. At this point, a deoxygenated glutaraldehyde solution preequilibrated with Ar was titrated into the deoxygenated bHb solution at a glutaraldehyde to bHb molar ratio of 30:1. The polymerization reaction was kept under continuous stirring at 37°C in the dark with Ar purging for 2 hours and the polymerization reaction was terminated with the addition of 20 mL of 2 M NaBH4 in PB buffer (20 mM, pH 8.0).
R-state polymerized bHb (RHb) was synthesized using the same vacuum manifold system [35; 36; 37]. Briefly, 30 grams of bHb was diluted to 1500 mL in 20 mM PB buffer (pH 8.0). The bHb solution was then subjected to alternative vacuum and O2 purging to remove the majority of N2, CO2 and other impurity gases, and was subsequently saturated with pure O2 for 2 hours in an ice-water bath. The pO2 of the bHb solution was monitored with a RapidLab 248 blood gas analyzer. When the pO2 of the bHb solution was out of the measurement range of the blood gas analyzer (>749 mm Hg), a glutaraldehyde solution preequilibrated with O2 was titrated into the oxygenated bHb solution at a glutaraldehyde:bHb molar ratio of 30:1. The polymerization reaction was kept under continuous stirring at 37°C in the dark with continuous O2 purging for 2 hours. Subsequently, 5 mL of 8 M NaCNBH3 in PB buffer (20 mM, pH 8.0) was first injected into the PolybHb solution to reduce the resultant Schiff bases and reduce the metHb level of the PolybHb solution. The PolybHb solution was continuously stirred for another 30 min in an ice-water bath. At the end of this period, 20 mL of 2M NaBH4 in PB buffer (20 mM, pH 8.0) was added to quench the polymerization reaction. All reactions were repeated in triplicate.
2.4 Clarification and Diafiltration of THb and RHb Solutions
The resulting THb and RHb solutions were initially clarified by filtering them through a column packed with autoclaved glass wool to remove large particles. The clarified THb and RHb solutions were then diluted from 1500 mL to 2000 mL and then subjected to 4 cycles of diafiltration via TFF with a 500 kDa hollow fiber cartridge (Spectrum Labs, Rancho Dominguez, CA) against an ice-cold modified lactated Ringer's solution (NaCl 115 mmol/L, KCl 4 mmol/L, CaCl2-2H2O 1.4 mmol/L, NaOH 13 mmol/L, sodium lactate 27 mmol/L and N-acetyl-L-cysteine 2 g/L). At the final diafiltration step, the resulting concentrated THb/RHb solution was immediately stored at -80°C for future use.
2.5 Equilibria of O2-bHb/THb/RHb Solutions
O2-bHb/THb/RHb equilibrium curves were measured using a Hemox Analyzer (TCS Scientific Corp., Southampton, PA) at 37°C. Samples were mixed with 5 mL of Hemox buffer (pH 7.4, TCS Scientific Corp.), 20 μL of Additive A, 10 μL of Additive B and 10 μL of antifoaming agent followed by equilibration with compressed air to a pO2 of 145±2 mm Hg at 37°C. Nitrogen was then used to deoxygenate the sample solution while the O2-bHb/THb/RHb saturation was measured as a function of pO2. The P50 (the pO2 at which the bHb/THb/RHb is half-saturated with O2) and cooperativity coefficient (n) were regressed from curve fits of the experimental O2-bHb/THb/RHb equilibrium curves to the Hill equation using IGOR Pro (WaveMetrics Inc, Lake Oswego, OR) [34].
2.6 Absolute Molecular Weight (MW) Distribution of bHb/THb/RHb Solutions
The absolute MW distribution and weight average molar mass (MW) of bHb/THb/RHb solutions was characterized by size exclusion chromatography coupled with multi-angle static light scattering (SEC-MASLS) as described previously in the literature [36].
2.7 Stopped Flow Kinetic Analysis of bHb/THb/RHb Solutions
The rapid kinetics of gaseous ligand reactions with bHb/THb/RHb was measured in an Applied Photophysics SF-17 micro-volume stopped-flow apparatus as previously described in the literature [38]. The kinetics of NO oxidation with oxy-bHb/THb/RHb solutions was measured in the stopped-flow instrument as previously described [39]. NO stock solutions (∼ 2 mM) were prepared by saturating deoxygenated 0.05 M Tris buffer, pH 7.4, in a gas-tight serum bottle with NO gas that was pre-washed with deoxygenated 1 M NaOH and buffer solutions. The NO stock solution was then transferred with a Hamilton syringe to a gastight syringe containing deoxygenated buffer solution to make appropriate concentrations of NO solutions. bHb/THb/RHb solutions (1 μM in heme) were mixed with NO solutions (≤ 50 μM), and the absorbance changes of the reaction were followed at 420 nm. Multiple traces were averaged for each reaction, and fit to exponential equations to obtain reaction rate constants.
2.8 Determination of nitrite reductase activity of native, T-state and R-state bHbs
The reaction of deoxyhemoglobin with nitrite was followed at 37°C, to assess the rate of nitrite reductase activity of native, T-state and R-state bovine hemoglobins (bHb, THb, RHb, respectively). A solution of 30 μM of either hemoglobin (all hemoglobin concentrations refer to heme) in PBS, pH 7.4 supplemented with 100 μM DTPA (diethylenetriaminepenta-acetic acid) was placed in a sealed cuvette and degassed gradually under a stream of argon to obtain a range of fractional saturations. Visible spectra (450 to 700 nm) were collected for 15 minutes every 30 seconds after the addition of either 250 μM or 750 μM sodium nitrite. The change in the concentration of deoxyhemoglobin and other hemoglobin species was determined by spectral deconvolution using reference spectra for nitrosylhemoglobin, methemoglobin, oxyhemoglobin and deoxyhemoglobin as described previously [22; 26]. Typical metHb concentrations in the nitrite reductase assays were 4 ±3%, 13 ±4% and 17±3% for bHb, THb and RHb solutions respectively. Initial rates were calculated considering the initial 10% of the slope of deoxyhemoglobin consumption. Kinetic profiles were always linear during this interval with r2 values greater or equal to 0.95.
2.9 Determination of HbNO stability
Nitrosyl hemoglobin derivatives for bHb, THb and RHb were generated by addition of excess MNO to sodium dithionite-treated hemoglobins in 100mM sodium phosphate buffer supplemented with 100μM DTPA pH 7.4. After 4 minutes of reaction, excess dithionite and MNO were removed by size exclusion chromatography using cooled G-25 columns. Solutions containing 30μM HbNO in 100mM sodium phosphate plus 100μM DTPA were placed in a cuvette at room temperature and atmosphere and concentration changes determined by spectral deconvolution over 20 minutes. Rate constants were obtained by fitting HbNO decomposition profiles to a first order decay model using GraphPad Prism 5.
Nitric oxide formation by native and polymerized bHbs
NO gas formation from nitrite reduction was assessed via ozone-based chemiluminescence using a 280i NO analyzer (GE Sievers). A solution containing 1 mM nitrite in PBS, pH 7.4 with 100 μM DTPA and 50 μL antifoam (GE Sievers) was placed in a sealed chamber connected in line with the chemiluminescence detector and equilibrated at 37°C under anoxic conditions before the addition of hemoglobin. The formation of NO was confirmed by adding cPTIO [2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide] (200 μM).
2.10 Vessel relaxation experiments
Thoracic aortas were isolated from male Sprague-Dawley rats and divided into approximately eight 5-millimeter wide sections. The resulting rings were hung between two hooks connected to a force transducer and placed within a vessel bath chamber containing Krebs-Henseleit buffer as described previously [22; 26]. To study the effect of fractional saturation on nitrite- and NO-mediated vasorelaxation, vessel baths were perfused with gases containing 21 or 0% oxygen plus 5% carbon dioxide and balanced with nitrogen. All vessels were pretreated with indomethacin (5 μM, cyclooxygenase inhibitor) and L-NMMA (NG-monomethyl-L-arginine, 100 μM, inhibitor of nitric oxide synthase) and pre-contracted by the addition of phenylephrine. Vessels were allowed to reach stable tone before the addition of single doses of either the NO donor Mahma-NONOate (MNO, 30 nM) or sodium nitrite (10 μM) in the presence or absence of 20 μM hemoglobin. Vasodilatation by either nitrite or MNO was determined by measuring the change in tension and expressing this difference as a percentage of the initial phenylephrine constriction. Single doses of nitrite and MNO were used in order to limit decomposition of the polymerized hemoglobins in the vessel bath. The precise bath pO2 can vary depending on several factors [40]. Therefore after 5 min of equilibration, aliquots were taken using gas tight syringes, transferred to anaerobic cuvettes and spectra (450-700nm) measured to determine fractional saturation of added hemoglobins. This also allowed determination of the solution concentration of hemoglobin during experiments which was found to vary slightly due to differential settling of bHb versus THb likely due to the higher molecular weight of the latter. It was not possible to deoxygenate vessel bath chambers sufficiently to desaturate R-state Hb and maintain viable vessels (not shown).
2.11 Reduction potential determination
Spectroelectrochemical experiments were performed in an optically transparent thin-layer electrochemical cell similar to that described by Faulkner [41]. The cell consisted of a standard 1-cm path length cuvette fitted with a 1 × 2 cm piece of 52-mesh platinum gauze positioned between an interior wall and a plastic face secured tightly against the mesh using an elastic spacer. A solution containing 0.15–0.25 mM heme plus 0.25–0.5 mM of the electrochemical mediator Ru(NH3)6Cl3 [42] was added to the cuvette and then drawn up across the mesh electrode by capillary action to create a thin layer. A platinum auxiliary electrode and a teflon sleeve for a Ag/AgCl reference electrode were pushed partway through a rubber cap. The cap was fitted across the top of the cell to form an airtight seal. An additional Pt wire was then forced through the cap to make contact with the Pt gauze working electrode. Potentiostat connections could then be made to all three electrodes while simultaneously maintaining an inert atmosphere inside the cell. Potentials were controlled using a Pine Instruments model AFCBP1 bipotentiostat and spectra were obtained using a Shimadzu model 2401 double beam spectrophotometer thermostatted at 25°C. Spectroelectrochemistry was then carried out using an established protocol [43]. Briefly, after the addition of hemoglobin the sealed cuvette was gently flushed with argon for approximately 20 minutes prior to the start of the experiment to remove oxygen and then a low argon flow was continued for the duration of the experiment. The potential of the working electrode was first set to -600 mV for 40 min to reduce the hemoglobins and any residual O2 to ferrous heme and water respectively. A series of potentials were then applied to the platinum minigrid working electrode and an absorbance spectrum was obtained at each potential after 20 minutes equilibration. All potentials were measured against Ag/AgCl and converted to SHE values by considering Ag/AgCl electrode = 0.208 V vs SHE. Reproducible redox potential measurements with RHb were not possible due to an apparent breakdown of this protein indicated by loss of total absorbance over the duration of these experiments (not shown).
2.12 Mitochondrial respiration assays
Purified liver mitochondria suspended in respiration buffer [KCl (120 mmol/L); sucrose (25 mmol/L); HEPES (10 mmol/L); EGTA (1 mmol/L); KH2PO4 (1 mmol/L); MgCl2 (5 mmol/L)] were placed in a closed chamber equipped with an oxygen sensor and treated with substrates to stimulate respiration in the absence or presence of bHb, THb and RHb. When all the oxygen was consumed, an anoxic solution of nitrite was added and then the lid of chamber is removed in order to allow for oxygen to diffuse into the solution. Since the amount of mitochondria utilized in this assay was such that the rate of oxygen consumption by respiration was always greater than the rate of oxygen diffusion into the open chamber, the concentration of oxygen remained close to zero even when the lid had been removed. However, in the presence of bHb, THb and RHb, oxygen consumption by mitochondrial respiration also caused hemoglobin to deoxygenate thereby stimulating nitrite reduction to NO. Nitric oxide inhibits mitochondrial respiration; therefore higher yields of NO production by nitrite reduction were reflected by an earlier increase in oxygen levels in the chamber upon lid removal. Data is expressed as extent of inhibition with respect to treatment with potassium cyanide.
2.14 Statistical analysis
All results are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism 5. The differences between the hemoglobins analyzed were assessed using analysis of variance (ANOVA), followed by a Bonferroni test, using a p value < 0.05.
3. Results
3.1 Characterization of cross-linked hemoglobins
In order to test the contributions of hemoglobin quaternary structure to nitrite reduction kinetics and NO signaling, two polymerized hemoglobins stabilized exclusively under T- and R-state conformations were prepared as previously described in the methods section. Table 1 shows that, consistent with our previous data, THb and RHb had respectively higher and lower P50's compared to bHb. Furthermore, Hill constants are significantly lower (closer to unity) for THb and RHb consistent with these products being locked in a given conformation. Table 1 also shows that cross-linking did not affect the NO-dioxygenation reaction indicated by similar rates for all three hemoglobins, However the reduction potential of THb was significantly lower than bHb, suggesting that the former is thermodynamically a better electron donor. The reduction potential for RHb was not measurable due to excessive heme decomposition during redox potential determination experiments (not shown).
Table 1.
Biophysical properties of bHb, THb and RHb. The errors are standard deviation from triplicate reactions unless otherwise specified.
| Solution | P50 (mm Hg)a | nb | E1/2 (mV vs. SHE)c | k′ox,NO (μM-1s-1)d | MW (kDa)e |
|---|---|---|---|---|---|
| bHb | 27.37±1.57 | 2.84±0.081 | 91.13± 11.51 (n=6) | 18.3 ± 1.8 | 65.3±0.6 |
| THb | 41.16±3.05 | 1.01±0.015 | 65.53± 18.54 (n=12)* | 18.7 ± 2.3 | 1330.3±159 |
| RHb | 1.84±0.78 | 0.69±0.11 | ND | 17.5 ± 5.5 | 6256±886.7 |
O2 affinity (i.e. pO2 at which the bHb/THb/RHb is half-saturated with O2),
cooperativity (Hill) coefficient,
reduction potential,
NO dioxygenation rate constant,
weight-average molar mass.
p<0.01 vs. bHb. ND – not determinable
3.2 Nitrite reduction kinetics by native and polymerized bHbs
Nitrite reduction kinetics were assessed by adding sodium nitrite to bHb, THb and RHb at varying initial fractional saturations and following time-dependent changes in the visible spectrum as shown in figures 1B, 1D and 1F. Since each spectrum represents the summation of the different hemoglobin species present at any given time point, spectra were deconvoluted using reference spectra for oxy-, deoxy-, met, and nitrosyl- standards for each hemoglobin (Figure 1A, 1C, 1E) to generate kinetic profiles for each of these individual species. Previous work has shown that deoxyhemoglobin is the species responsible for the one-electron reduction of nitrite to NO and, furthermore that the rate of this reaction parallels NO formation [4]. Figure 2A shows representative traces of deoxyhemoglobin consumption as a function of time for bHb, THb and RHb at an oxygen fractional saturation of 0.45- 0.5. Initial rates of deoxyhemoglobin consumption were determined and plotted in Fig 2B and 2C. With bHb, the initial rate for loss of deoxyhemoglobin increased with decreased fractional saturation reaching a maximum at ∼ 40% (Fig 2B). The initial rate showed trends towards decreasing at close to zero fractional saturation resulting in an apparent bell-shaped dependence of initial rate with fractional saturation. Fig 2B also shows the initial rate for THb which decreased linearly with decreasing fractional saturations and was significantly slower than bHb. Likewise, the initial rate for RHb mediated nitrite reduction decreased linearly with decreasing fractional saturation but in this case the reaction was significantly faster than bHb (10-30 fold) necessitating the use of lower initial nitrite concentrations (Fig 2C). In summary, initial rates for nitrite reduction assessed by monitoring loss of deoxyhemoglobin decreased in the order RHb > bHb > THb and demonstrated a linear dependence with fractional saturation with the conformationally locked hemoglobins and a bell shaped dependence with bHb that is able to undergo R-T state transitions.
Figure 1.
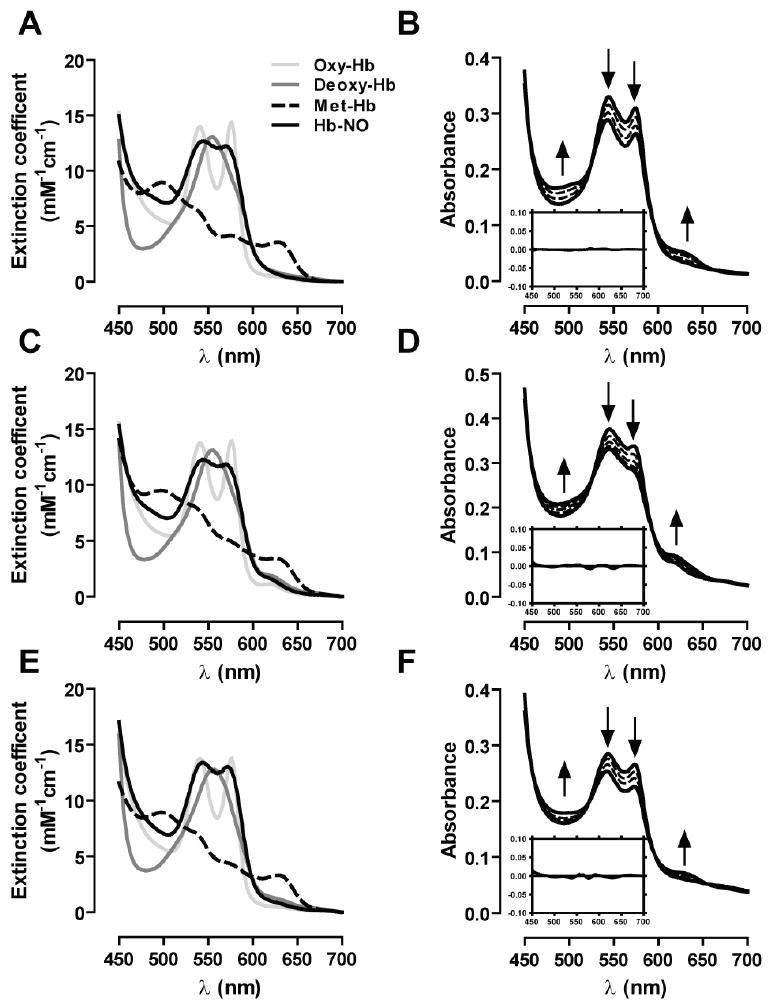
Reference visible spectra for the hemoglobin species expected to be involved in the reaction with nitrite corresponding to A) bHb, C) Thb and E) RHb. Panels B, D and F show representative spectral changes in the reaction of hemoglobin and nitrite for bHb, THb and RHb respectively. Experiments were performed using 30μM hemoglobin and 750μM nitrite with the exception of RHb where 250μM nitrite was used instead. Fractional saturations were bHb: 0.45, THb: 0.4 and RHb: 0.49. Spectra were collected every 30 seconds for 15 minutes. In the interest of clarity, only data collected every 4 minutes is shown in the graph. Inserts show respective residual plots obtained from fitting the experimental data to the reference spectra.
Figure 2.
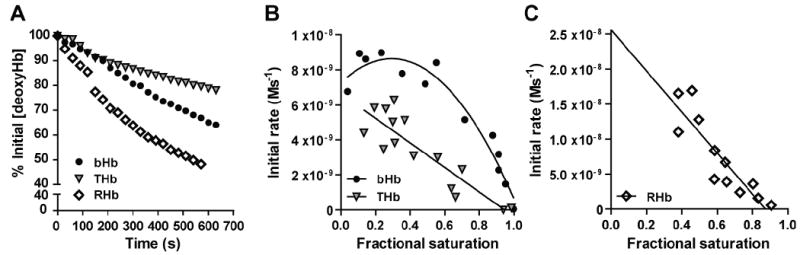
A) Representative kinetic traces showing the consumption of the indicated deoxyhemoglobins (30μM) after the addition of sodium nitrite (750μM for bHb and THb, and 250μM for RHb) in PBS pH 7.4, 37°C and a fractional saturation of 0.45 – 0.5. B) Initial rate for the reaction between nitrite and deoxygenated bHb and THb as function of fractional saturation were calculated using the initial 10% of the deoxyhemoglobin consumption traces shown in panel A. The reaction was performed using 30μM Hb and a nitrite addition of 750μM. C) Initial rate for the reaction between nitrite and deoxygenated RHb as function of fractional saturation were calculated using the initial 10% of the deoxyhemoglobin consumption traces shown in panel A. The reaction was performed using 30μM Hb and a nitrite addition of 250μM.
3.3 Nitric oxide formation from nitrite by native and polymerized bovine hemoglobins
In order to determine whether i) the reaction between nitrite and bHb, RHb and THb leads to NO formation, and ii) whether differential nitrite-reduction kinetics assessed by deoxyhemoglobin consumption translates into concordant NO-production, each hemoglobin (0.5μM) was added to a vessel containing 1 mM sodium nitrite under anoxic conditions and connected in line with a chemiluminescence NO detector. Figure 3A shows representative traces for NO-formation and figure 3B compiled data that demonstrates that all hemoglobins were able to produce NO with the yield of NO being highest with RHb followed by THb and then bHb. NO-formation was confirmed by loss of chemiluminesence signal after addition of the non-heme NO-scavenger C-PTIO (Fig 3A inset). THb and RHb are cross-linked preventing Hb dimerisation at low heme concentrations. However, at 0.5μM heme, bHb will readily dimerize (Kd = 0.2μM) which in turn could affect reactions with nitrite. These experiments were repeated therefore with 5μM heme with NO-formation showing the same relative trend between the 3 different hemoglobins as observed with 0.5μM heme (not shown). Nitrosylhemoglobin (HbNO) is one product of nitrite reduction being formed from the rapid reaction of NO and deoxyferrous heme. In addition to nitrite reduction, differences in NO-formation shown in Fig 3B could also reflect differences in the stability of HbNO and NO-off rates between the 3 hemoglobins studied. To test the latter, the nitrosyl derivatives of each hemoglobin were prepared and their stability determined by following loss of HbNO by visible spectroscopy at 20°C in PBS. Fig 3C show time dependent loss of HbNO for each hemoglobin and Fig 3D the first order rate constants for HbNO loss. The HbNO derivative of RHb was the most stable followed by both bHb and THb suggesting that if significant NO was being generated from HbNO decomposition it would follow the order of THb ∼ bHb ≫ RHb; the opposite to order observed from data presented in Fig 3B.
Figure 3.
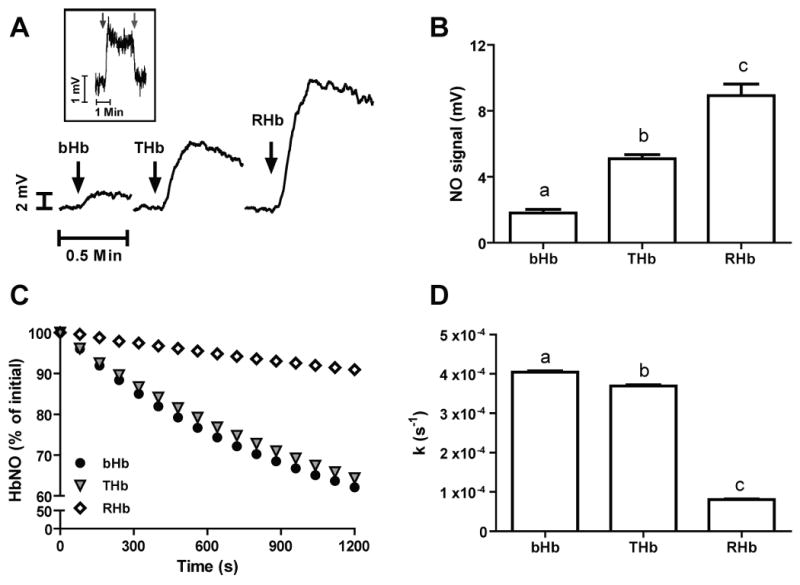
A) Representative traces indicating NO-formation at 0% fractional saturation from the reaction between 0.5μM hemoglobins (as indicated) with 1mM sodium nitrite in PBS pH 7.4 at 37°C. Inset: Representative trace showing the effect of CPTIO addition (25μM, right arrow) to the chemiluminiscence signal generated by the reaction between 0.5μM bHb and 1mM nitrite. Similar results are observed with THb and RHb (not shown). B) Summarized data showing the yield of NO formation by the indicated hemoglobins. Data are means ± SEM, n = 3-5. Different letters denote P < 0.001 versus the other two hemoglobins as determined by one-way ANOVA and Bonferroni's post-test. C) Traces showing the percent decrease of HbNO in 20 minutes for bHb, THb and RHb. Initial concentrations were 22μM, 20μM and 26μM, for bHb, THb, and RHb, respectively. Data are mean ± SEm (n=3) D) First order rate constants for HbNO decomposition. Data are means ± SEM, n = 3. Different letters denote P < 0.001 versus the other two hemoglobins as determined by one-way ANOVA and Bonferroni's post-test.
3.4 Effects of Hb conformation on nitrite dependent modulation of NO-signaling responses
To test if the different kinetics and yield of NO-formation by RHb, THb and bHb shown above, translate into differences in NO-signaling we utilized two biologically relevant assays: NO-dependent inhibition of mitochondrial respiration and stimulation of vessel relaxation. Figure 4A shows representative traces highlighting differential degrees of inhibition of mitochondrial respiration brought about by nitrite-dependent NO formation in the presence of any of the three hemoglobins studied. Nitrite alone (25μM) had no effect on respiration as indicated by no change in the time taken for oxygen to begin to increase after opening the chamber, consistent with the absence of significant NO formation under these conditions. However, in the presence of hemoglobins and nitrite, inhibition of respiration is observed as indicated by increases in oxygen content at earlier times relative to the nitrite-only control. Figure 4B shows that RHb had the highest inhibitory potency followed by THb and bHb. No statistical difference between THb and bHb was observed, although there was a trend (P = 0.065) towards THb leading to greater inhibition.
Figure 4.
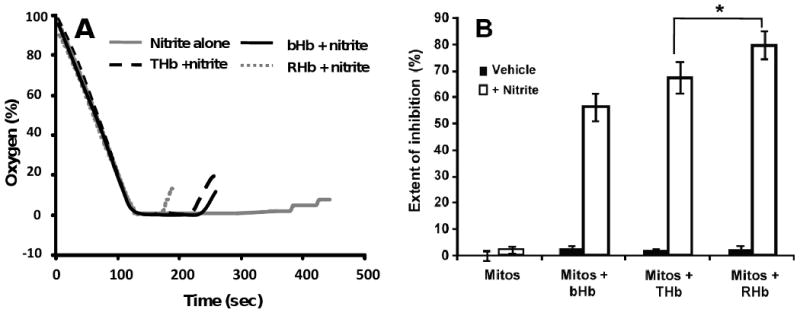
A) Typical oxygen tension traces showing inhibition of mitochondrial respiration by the combination of 25 μM nitrite and 20 μM of the indicated hemoglobins. A shorter time between lid removal (t = 120s) and increases in oxygen levels above zero imply higher degree of inhibition and hence NO formation. B) Summarized data showing the extent of mitochondrial respiration inhibition attained with the indicated hemoglobins. Data are means ± SEM, n = 5, * p <0.05.
In the next series of experiments we used isolated rat aortic rings as a biological readout to assess whether NO derived from nitrite reduction by the different hemoglobins could lead to vasorelaxation. We were not able to include the R-state stabilized hemoglobin in these studies due to the incompatibility of vessel preparations to the low oxygen tensions required to elicit significant RHb deoxygenation (not shown). Cell free hemoglobins have been shown to scavenge NO with high rate constants (k ∼2-9 × 107 M-1s-1 under both oxygenated and deoxygenated conditions [44; 45; 46]). Furthermore, this interaction has been proposed to mediate the hypertensive effects observed upon infusion of HBOC in the circulation of both human patients and experimental animals [27; 28; 47]. Figures 5A and 5B show typical vessel responses to the addition of a single dose of the NO donor, MNO under low and high oxygen tensions respectively. Consistent with previous results, the addition of either bHb or THb inhibited NO mediated vasodilatation. Figure 5C shows a plot of percent inhibition of NO dependent vasodilatation by bHb and THb as a function of oxygen fractional saturation, normalized to the total amount of ferrous hemoglobin added. Both hemoglobins were equally capable of inhibiting vasodilatation by NO, and furthermore inhibition becomes more effective (by ∼2-3 fold) at low versus high fractional saturations. These data demonstrate that both bHb and THb are equally capable of scavenging NO across the entire fractional saturation range.
Figure 5.
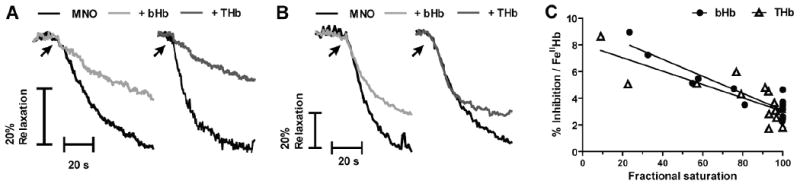
Representative vessel tension traces showing the inhibitory effects of bHb and THb on MNO-dependent vasodilation at A) fractional saturations of 0.54 and 0.57 (bHb and THb, respectively) and B) 0.99 fractional saturations for each hemoglobin. C) bHb and THb mediated inhibition of MNO dependent dilation as a function of fractional saturation. Percent inhibition was calculated by ratio of MNO-induced vasodilation in the presence and absence of hemoglobins and then normalized to the amount of ferrous heme in the vessel bath. Each point corresponds to a single measurement using an individual vessel segment. Lines show linear regression fits with r2 equal to 0.85 and 0.63 for bHb and THb respectively. Both slopes are significantly different from zero with p = < 0.001.
It has been shown that even in the absence of added heme, nitrite induces vasorelaxation at high and low oxygen tensions via NO formation [21; 22; 48; 49]. As a result, the addition of either bHb or THb would be expected to lead to inhibition of nitrite-dependent responses. Nevertheless, data presented in this work also shows that the reaction between nitrite and both deoxygenated bHb and THb leads to the formation of NO (see figure 3). These results suggest the existence of an oxygen-dependent interaction between NO scavenging and NO generating activities by both bHb and THb in the presence of nitrite. Figures 6A and 6B show representative vasodilatory responses induced by a single addition of sodium nitrite to rat aortic rings equilibrated under different oxygen tensions in the absence or presence of bHb and THb. When nitrite is added to aortic rings equilibrated under oxygen tensions giving rise to high fractional saturations, both bHb and THb inhibit nitrite-mediated vasodilatation (Fig 6B and 6C), consistent with their ability to efficiently scavenge NO under these conditions. However, when vessels are equilibrated under oxygen tensions that lead to lower hemoglobin fractional saturations, the addition of both bHb and THb led to significant potentiation of nitrite induced vasodilatation (Fig 6A and 6C). Since both bHb and THb are equally proficient at scavenging NO under low fractional saturations, we propose that the ability of these hemoglobins to reduce nitrite to NO under hypoxic conditions is able to compensate for the NO scavenging activity and cause an effective potentiation of nitrite-mediated vasodilatation. Figure 6C shows the percent inhibition of nitrite-mediated vasodilatation by bHb and THb as a function of oxygen fractional saturation and normalized to the amount of ferrous heme measured in each bioassay chamber. Consistent with the data shown in panels 6A and 6B, lowering the fractional saturation is associated first with a loss of inhibition and then with potentiation of nitrite-mediated responses, as indicated by negative percent inhibition values. Moreover, figure 6C shows that THb is more potent than bHb in stimulating nitrite-dependent vasodilatation.
Figure 6.
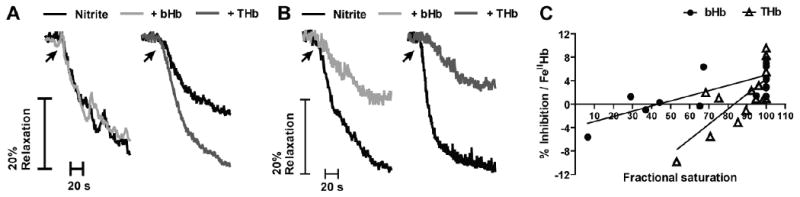
Representative vessel bioassay traces showing the effects of bHb and THb on nitrite-dependent vasodilation at A) 0.45 and 0.53 fractional saturation (bHb and THb, respectively), and B) 0.99 fractional saturation for each hemoglobin. C) bHb and THb mediated inhibition of nitrite-dependent dilation as a function of fractional saturation. Percent inhibition was calculated by ratio of nitrite-induced vasodilation in the presence and absence of hemoglobins and then normalized to the amount of ferrous heme in the vessel bath. Each point corresponds to a single measurement using an individual vessel segment. Lines show linear regression fits with r2 equal to 0.58 and 0.57 for bHb and THb respectively. Both slopes are significantly different from zero with p = 0.0003 and p = 0.0018 for bHb and THb respectively.
4. Discussion
The reactions between nitrite and hemoglobin have been studied for over a century [13]. The recent interest in this reaction stems from its potential to be a source of NO in vivo in vascular beds where hemoglobin deoxygenation occurs. This paradigm may be important in physiologic mechanisms for regulation of hypoxic blood flow [3; 4; 50; 51; 52], in the therapeutic mechanisms for nitrite mediated protection against ischemia induced injury [53] and more recently, dysfunction in this reaction has been implicated in the pathogenesis of human sepsis [54]. Data presented herein further support the concept that deoxyhemoglobin-nitrite reactions are a source of NO that activates NO-dependent signaling pathways.
Nitrite reduction by deoxyhemoglobin is now appreciated to be regulated by protein conformation with the reaction of R-state being faster than T-state [11; 12; 14]. Additionally, vacant ferrous hemes are required for nitrite reduction by hemoglobin. These properties result in a bell-shaped dependence for the initial rate (the product of both nitrite and deoxyheme concentration and the rate constant) of nitrite reduction as a function of fractional saturation with maximal rates observed around the P50 [4; 12]. Data presented herein using native bovine Hb that can undergo T to R transitions, and cross-linked polymeric bovine hemoglobins that have been stabilized in either T- or R-state conformation provide further evidence for this model. With native hemoglobin, the initial rate for nitrite-mediated deoxyhemoglobin consumption exhibited a bell-shaped dependence on fractional saturation, albeit not as pronounced as previously reported with human hemoglobin [4; 12] which possibly reflects species-species variation and requires further investigation. Both RHb and THb showed linear dependent increases in initial rate for nitrite reduction as a function of decreasing fractional saturation consistent with these molecules being conformationally locked and unable to undergo allosteric transition. Also consistent with the proposed model, nitrite reduction was fastest with RHb and slowest with THb. In this model however, it would be expected then that the initial rate for nitrite reduction by bHb at higher fractional saturations (predominantly R-state) would be similar to RHb. However three-fold lower concentrations of nitrite were required in order to follow deoxyhemoglobin consumption kinetics for the latter derivative, indicating a faster rate of reaction. Both a more negative redox potential and increased heme accessibility have been proposed to mediate increased nitrite reduction rates by R-deoxy vs. T-deoxy hemoglobin [55]. The lack of data regarding the redox potential of RHb due to technical limitations in our experimental setup precludes us from formulating an hypothesis in this regard but we note that reports exist in the literature suggesting that hemoglobin cross-linking leads to increased heme accessibility [16] thereby potentially resulting in increased reactivity towards nitrite. In addition, we cannot exclude the possibility that even at high fractional saturations, the presence of low levels of T-state hemes in bHb versus exclusively R-state hemes in RHb, result in a lower rate of nitrite reduction.
Unlike RHb, THb showed slower initial rates compared to bHb across most fractional saturations. Moreover at low fractional saturations where native hemoglobin will be in the T-state, initial rates for native Hb and THb are similar. Interestingly, the reduction potential of THb was lower than that of bHb suggesting that increased rates of nitrite reduction should have been observed (see table 1) if electron exchange with the heme was the rate-limiting step. The fact that no differences in nitrite reduction kinetics were observed under lower fractional saturations suggests that polymerization of T-state hemoglobin may decrease heme accessibility and that this plays an important role in regulating nitrite-reduction. As indicated above, at intermediate fractional saturations bHb will be comprised of both R- and T-state hemes compared to only R- or T-heme in RHb and THb respectively which in turn may affect nitrite reduction kinetics. At a fractional saturation of zero however, where all hemes in bHb and THb are in T-state, differences in nitrite reduction kinetics were not observed despite differences in redox potential. Under these conditions, heme accessibility differences are the likeliest explanation. We also note that this behavior underscores the importance of assessing nitrite reduction kinetics across fractional saturations as determination of this parameter under anoxic conditions (fractional saturation of 0) alone would have suggested that THb and bHb were similar.
Recent studies have questioned whether free NO is formed at all during hemoglobin-nitrite reactions [56]. Formation of NO-gas and the ability of nitrite-derived NO to inhibit mitochondrial respiration and stimulate vasodilation were determined therefore to assess first if different hemoglobins could reduce nitrite to NO and then, whether this NO was competent to elicit two independent signaling responses. This also allowed evaluation of how kinetics of deoxyhemoglobin consumption translated into NO-formation and bioactivity. Anoxic NO-gas formation was highest with RHb, being ∼5 fold greater than native Hb and consistent with the differences observed in deoxyhemoglobin consumption kinetics. Unexpectedly however, THb produced more NO compared to native Hb despite similar deoxyhemoglobin consumption kinetics at zero fractional saturation (See Fig 2C). Data from mitochondrial inhibition experiments showed that the degree of inhibition by hemoglobins and nitrite mirrored NO-gas formation profiles suggesting that bioactive NO is formed from all the hemoglobins; and furthermore, that NO-gas formation under anoxia was a better predictor for activation of NO-signaling than deoxyhemoglobin consumption rates. Vessel relaxation studies also suggested more nitrite-derived NO from THb relative to bHb. The discordance between THb consumption kinetics and NO-formation / signaling suggests that THb more effectively couples nitrite reduction to NO-generation. Recent insights into NO-formation from nitrite-hemoglobin reactions suggest roles for oxidative denitrosylation of nitrosylhemoglobin [30] and formation of a dinitrogen trioxide (N2O3) intermediate which can homolyze to NO and nitrogen dioxide (NO2). In this model, nitrite binding to ferric hemoglobin first forms a ferrous-NO2 radical intermediate that in turn reacts with NO to form N2O3 [19; 57]. These possibilities coupled with other observations showing that nitrite binds to heme via an O-binding mode [58; 59] illustrates the complexity, and potential for multiple reactions that occur to produce NO from nitrite-hemoglobin interactions. It is not clear how T-state polymerization affects these reactions but it is possible that differential effects on any one could result in more efficient NO-production and such information could be useful in the development of HBOCs. Clearly, these data further underscore the importance of assessing functional signaling end points in comparing different hemoglobins and their reactions with nitrite to generate NO.
HBOC mediated scavenging of NO has received considerable interest recently, with clinical development of these products being halted due to potential detrimental effects of NO-scavenging [27]. Nitrite represents a potential adjuvant therapy for HBOC with the concept being that as HBOC deoxygenate in respiring tissues, they would reduce nitrite to form NO and both increase blood flow and replete NO-signaling in general. These concepts are illustrated by data from vessel relaxation experiments which show that hemoglobin, irrespective of it being native or T-state stabilized, inhibits NO-dependent vasodilation to similar extents and consistent with similar rate constants for NO dioxygenation by bHb and THb (Table 1). Notably, we observed that both THb and bHb were 2-fold more efficient at inhibiting MNO-mediated vasorelaxation at low versus high fractional saturations (Figure 5C). This result was unexpected since NO-scavenging rates for oxy- and deoxy hemes, are similar being in fact slightly higher for oxyhemoglobin. We observed MNO itself was ∼2.3 times more potent at inducing vasorelaxation at high versus low oxygen tensions (data not shown) suggesting that the efficacy of NO-soluble guanylate cyclase (sGC) activation signaling pathway is decreased at low oxygen tension. If we consider that the inhibition of NO-mediated vasorelaxation by hemoglobin responds to a competition between hemoglobin and sGC, then as the oxygen tension is lowered the decrease in reactivity between sGC and NO will result in a increased hemoglobin mediated inhibition at lower fractional saturations. In the case of nitrite, both THb and bHb inhibit vasodilatation at high fractional saturations. Previous data have shown that in the absence of added hemoglobin, nitrite promotes vasodilation via NO-formation at high and low oxygen tensions [21; 22]. At high oxygen tensions therefore, native- and THb-dependent inhibition of nitrite-mediated vasorelaxation is likely due to the NO-dioxygenation reaction. However, as the fractional saturation of the hemoglobins is lowered, the inhibitory effect on nitrite dependent vasodilation is lost and eventually transitions into a potentiation of the response. Importantly, the overall effect on vascular tone should be considered as a balance between both hemoglobin-mediated vasoconstriction (NO-scavenging) and vasodilation (nitrite-reduction to NO). Since NO-scavenging occurs across the entire fractional saturation range, the data with nitrite support the model where as hemoglobin deoxygenates, more NO is made from nitrite which counters NO-scavenging reactions eventually shifting to net NO-formation. Interestingly, this counter balancing effect of nitrite reduction was more potent with THb vs native Hb as indicated by an ∼2-fold steeper gradient for percent inhibition versus fractional saturation relationship (Figure 6) which also mirrors increased NO-formation from THb.
4.1 Conclusion
In summary, data presented support the model that allosteric regulation of deoxyhemoglobin-mediated nitrite reduction can yield bioactive NO. Moreover, data show that hemoglobin cross-linking and conformational locking can dramatically impact on nitrite-reduction kinetics, NO-formation and bioactivity thereby resulting in hemoglobins that can more effectively couple nitrite-reduction to stimulation of NO-bioactivity. Finally, these data also show that nitrite reduction kinetics measured by deoxyhemoglobin consumption do not necessarily reflect the yield of NO. In this regard, the determination of NO gas production and assessment of NO-dependent signaling provides a more accurate indicator of the ability of different hemoglobins to produce bioactive NO from nitrite reduction. This observation might be of particular relevance when assessing the ability of different HBOCs to function as nitrite reductases under physiological conditions.
Acknowledgments
This study was supported by grants NIH HL92624 to RPP, NIH HL078840 and DK070862 to AFP, and an American Heart Association pre-doctoral fellowship to DAV (AHA 0815248E)
Abbreviations
- NO
Nitric oxide
- bHb
Native bovine hemoglobin
- THb
T-state cross-linked bovine hemoglobin
- RHb
R-state cross-linked bovine hemoglobin
- HBOC
Hemoglobin-based oxygen carrier
- MNO
Mahma-NONOate
- L-NMMA
L-NG-monomethyl Arginine
- HbNO
Nitrosylhemoglobin
- sGC
Soluble guanylate cyclase
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide
- DTPA
Diethylenetriaminepenta-acetic Acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–35. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 2.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 3.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 4.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–74. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 7.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278:46349–56. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 8.Haldane J. The Red Colour of Salted Meat. The Journal of Hygiene. 1901;1:115–122. doi: 10.1017/s0022172400000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks J. The Action of Nitrite on Haemoglobin in the Absence of Oxygen. Proceedings of the Royal Society of London. Series B - Biological Sciences. 1937;123:368–382. [Google Scholar]
- 10.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256:12393–8. [PubMed] [Google Scholar]
- 11.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res. 2009;42:157–67. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 14.Jensen FB. Nitric oxide formation from the reaction of nitrite with carp and rabbit hemoglobin at intermediate oxygen saturations. FEBS J. 2008;275:3375–87. doi: 10.1111/j.1742-4658.2008.06486.x. [DOI] [PubMed] [Google Scholar]
- 15.Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG. Increased nitrite reductase activity of fetal versus adult ovine hemoglobin. Am J Physiol Heart Circ Physiol. 2009;296:H237–46. doi: 10.1152/ajpheart.00601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaventura C, Henkens R, Alayash AI, Crumbliss AL. Allosteric effects on oxidative and nitrosative reactions of cell-free hemoglobins. IUBMB Life. 2007;59:498–505. doi: 10.1080/15216540601188546. [DOI] [PubMed] [Google Scholar]
- 17.Grubina R, Basu S, Tiso M, Kim-Shapiro DB, Gladwin MT. Nitrite reductase activity of hemoglobin S (sickle) provides insight into contributions of heme redox potential versus ligand affinity. J Biol Chem. 2008;283:3628–38. doi: 10.1074/jbc.M705222200. [DOI] [PubMed] [Google Scholar]
- 18.Roche CJ, Dantsker D, Samuni U, Friedman JM. Nitrite reductase activity of sol-gel-encapsulated deoxyhemoglobin. Influence of quaternary and tertiary structure. J Biol Chem. 2006;281:36874–82. doi: 10.1074/jbc.M603914200. [DOI] [PubMed] [Google Scholar]
- 19.Roche CJ, Friedman JM. NO reactions with sol-gel and solution phase samples of the ferric nitrite derivative of HbA. Nitric Oxide. 2010;22:180–90. doi: 10.1016/j.niox.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salhany JM. Reaction of nitrite with human fetal oxyhemoglobin: a model simulation study with implications for blood flow regulation in sickle cell disease(SCD) Blood Cells Mol Dis. 2010;44:111–4. doi: 10.1016/j.bcmd.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007;292:H3072–8. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 22.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293:H2565–72. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kluger R. Red cell substitutes from hemoglobin-Do we start all over again? Curr Opin Chem Biol. 2010 doi: 10.1016/j.cbpa.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Lui FE, Dong P, Kluger R. Polyethylene glycol conjugation enhances the nitrite reductase activity of native and cross-linked hemoglobin. Biochemistry. 2008;47:10773–80. doi: 10.1021/bi801116k. [DOI] [PubMed] [Google Scholar]
- 25.Lui FE, Kluger R. Enhancing nitrite reductase activity of modified hemoglobin: bis-tetramers and their PEGylated derivatives. Biochemistry. 2009;48:11912–9. doi: 10.1021/bi9014105. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez C, Vitturi DA, He J, Vandromme M, Brandon A, Hutchings A, Rue LW, 3rd, Kerby JD, Patel RP. Sodium nitrite therapy attenuates the hypertensive effects of HBOC-201 via nitrite reduction. Biochem J. 2009;422:423–32. doi: 10.1042/BJ20090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. Jama. 2008;299:2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–97. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Minneci PC, Deans KJ, Shiva S, Zhi H, Banks SM, Kern S, Natanson C, Solomon SB, Gladwin MT. Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis. Am J Physiol Heart Circ Physiol. 2008;295:H743–54. doi: 10.1152/ajpheart.00151.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, Ringwood LA, Jiang A, Hogg N, Kim-Shapiro DB, Gladwin MT. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J Biol Chem. 2007;282:12916–27. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 31.Palmer AF, Sun G, Harris DR. The quaternary structure of tetrameric hemoglobin regulates the oxygen affinity of polymerized hemoglobin. Biotechnology Progress. 2009;25:1803–1809. doi: 10.1002/btpr.265. [DOI] [PubMed] [Google Scholar]
- 32.Elmer J, Buehler PW, Jia Y, Wood F, Harris DR, Alayash AI, Palmer AF. Functional comparison of hemoglobin purified by different methods and their biophysical implications. Biotechnol Bioeng. 106:76–85. doi: 10.1002/bit.22659. [DOI] [PubMed] [Google Scholar]
- 33.Elmer J, Harris DR, Sun G, Palmer AF. Purification of hemoglobin by tangential flow filtration with diafiltration. Biotechnol Prog. 2009;25:1402–1410. doi: 10.1002/btpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer AF, Sun G, Harris DR. Tangential flow filtration of hemoglobin. Biotechnol Prog. 2009;25:189–99. doi: 10.1002/btpr.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabrales P, Sun GY, Zhou YP, Harris DR, Tsai AG, Intaglietta M, Palmer AF. Effects of the molecular mass of tense-state polymerized bovine hemoglobin on blood pressure and vasoconstriction. J Appl Physiol. 2009;107:1548–1558. doi: 10.1152/japplphysiol.00622.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buehler PW, Zhou Y, Cabrales P, Jia Y, Sun G, Harris DR, Tsai AG, Intaglietta M, Palmer AF. Synthesis, biophysical properties and pharmacokinetics of ultrahigh molecular weight tense and relaxed state polymerized bovine hemoglobins. Biomaterials. 2010;31:3723–3735. doi: 10.1016/j.biomaterials.2010.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabrales P, Zhou Y, Harris DR, Palmer AF. Tissue oxygenation after exchange transfusion with ultrahigh-molecular-weight tense- and relaxed-state polymerized bovine hemoglobins. Am J Physiol Heart Circ Physiol. 2010;298:H1062–71. doi: 10.1152/ajpheart.01022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonini E, Brunori M. Kinetics of reactions of hemoglobin and myoglobin with ligands. In: Neuberger A, Tatum EL, editors. Hemoglobin and myoglobin in their reactions with ligands. North-Holland Publishing Company; Amsterdam: 1971. pp. 188–218. [Google Scholar]
- 39.Alayash AI, Summers AG, Wood F, Jia Y. Effects of glutaraldehyde polymerization on oxygen transport and redox properties of bovine hemoglobin. Arch Biochem Biophys. 2001;391:225–34. doi: 10.1006/abbi.2001.2426. [DOI] [PubMed] [Google Scholar]
- 40.Isbell TS, Koenitzer JR, Crawford JH, White CR, Kraus DW, Patel RP. Assessing NO-dependent vasodilatation using vessel bioassays at defined oxygen tensions. Methods Enzymol. 2005;396:553–68. doi: 10.1016/S0076-6879(05)96047-3. [DOI] [PubMed] [Google Scholar]
- 41.Faulkner KM, Bonaventura C, Crumbliss AL. A spectroelectrochemical method for evaluating factors which regulate the redox potential of hemoglobins. Inorganica Chimica Acta. 1994;226:187–194. [Google Scholar]
- 42.Taboy CH, Bonaventura C, Crumbliss AL. Anaerobic oxidations of myoglobin and hemoglobin by spectroelectrochemistry. Methods Enzymol. 2002;353:187–209. doi: 10.1016/s0076-6879(02)53048-2. [DOI] [PubMed] [Google Scholar]
- 43.Dorman SC, Harrington JP, Martin MS, Johnson TV. Determination of the formal reduction potential of Lumbricus terrestris hemoglobin using thin layer spectroelectrochemistry. J Inorg Biochem. 2004;98:185–8. doi: 10.1016/j.jinorgbio.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Cassoly R, Gibson Q. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol. 1975;91:301–13. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- 45.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 46.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO*-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40:3385–95. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 47.Patel RP. Biochemical aspects of the reaction of hemoglobin and NO: implications for Hb-based blood substitutes. Free Radic Biol Med. 2000;28:1518–25. doi: 10.1016/s0891-5849(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 48.Alzawahra WF, Talukder MA, Liu X, Samouilov A, Zweier JL. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am J Physiol Heart Circ Physiol. 2008;295:H499–508. doi: 10.1152/ajpheart.00374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingram TE, Pinder AG, Milsom AB, Rogers SC, Thomas DE, James PE. Blood vessel specific vaso-activity to nitrite under normoxic and hypoxic conditions. Adv Exp Med Biol. 2009;645:21–5. doi: 10.1007/978-0-387-85998-9_4. [DOI] [PubMed] [Google Scholar]
- 50.Rifkind JM, Nagababu E, Cao Z, Barbiro-Michaely E, Mayevsky A. Nitrite-induced improved blood circulation associated with an increase in a pool of RBC-NO with no bioactivity. Adv Exp Med Biol. 2009;645:27–34. doi: 10.1007/978-0-387-85998-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–7. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 52.Rogers SC, Khalatbari A, Datta BN, Ellery S, Paul V, Frenneaux MP, James PE. NO metabolite flux across the human coronary circulation. Cardiovasc Res. 2007;75:434–41. doi: 10.1016/j.cardiores.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 53.McNulty PH, Scott S, Kehoe V, Kozak M, Sinoway LI, Li J. Nitrite consumption in ischemic rat heart catalyzed by distinct blood-borne and tissue factors. Am J Physiol Heart Circ Physiol. 2008;295:H2143–8. doi: 10.1152/ajpheart.00050.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan MA, Frasier LM, Stewart JC, Mack CM, Gough MS, Graves BT, Apostolakos MJ, Doolin KP, Darling DC, Frampton MW, Pietropaoli AP. Artery-to-vein differences in nitric oxide metabolites are diminished in sepsis. Crit Care Med. 2010;38:1069–77. doi: 10.1097/CCM.0b013e3181d16a3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu C, Mikulski R, Swenson ER, Silverman DN. Reactions of nitrite with hemoglobin measured by membrane inlet mass spectrometry. Free Radic Biol Med. 2009;46:14–9. doi: 10.1016/j.freeradbiomed.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–94. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 58.Perissinotti LL, Marti MA, Doctorovich F, Luque FJ, Estrin DA. A microscopic study of the deoxyhemoglobin-catalyzed generation of nitric oxide from nitrite anion. Biochemistry. 2008;47:9793–802. doi: 10.1021/bi801104c. [DOI] [PubMed] [Google Scholar]
- 59.Yi J, Safo MK, Richter-Addo GB. The nitrite anion binds to human hemoglobin via the uncommon O-nitrito mode. Biochemistry. 2008;47:8247–9. doi: 10.1021/bi801015c. [DOI] [PubMed] [Google Scholar]


