Abstract
Objective
To determine whether extending the interval between chemoradiation (CRT) and surgery, and administering additional chemotherapy during the waiting period has an impact on tumor response, CRT-related toxicity and surgical complications in patients with advanced rectal cancer.
Background
Locally advanced rectal cancer is usually treated with pre-operative CRT followed by surgery approximately 6 weeks later. The Timing of Rectal Cancer Response to Chemoradiation Consortium designed a prospective, multi-center, Phase II clinical trial to investigate extending the interval between CRT and surgery, and administering additional chemotherapy during the waiting period. Here, we present preliminary results of this trial, reporting the tumor response, CRT-related toxicity and surgical complications.
Methods
Stage II and III rectal cancer patients were treated concurrently with 5-Fluorouracil (FU) and radiation for 5–6 weeks. Patients in study group (SG) 1 underwent total mesorectal excision (TME) 6 weeks later. Patients in SG2 with evidence of a clinical response 4 weeks after CRT received 2 cycles of modified FOLFOX-6 (mFOLFOX-6) followed by TME 3–5 weeks later. Tumor response, CRT-related toxicity and surgical complications were recorded.
Results
144 patients were accrued. 136 (66, SG1; 70, SG2) were evaluated for CRT-related toxicity. 127 (60, SG1; 67, SG2) were assessed for tumor response and surgical complications. A similar proportion of patients completed CRT per protocol in both SGs, but the cumulative dose of sensitizing 5-FU and radiation was higher in SG2. CRT-related toxicity was comparable between SGs. Average time from CRT-to-surgery was 6 (SG1) and 11 weeks (SG2). Pathologic complete response (pCR) was 18% (SG1) and 25% (SG2). Post-operative complications were similar between SGs.
Conclusions
Intense neoadjuvant therapy consisting of CRT followed by additional chemotherapy (mFOLFOX-6), and delaying surgery may result in a modest increase in pCR rate without increasing complications in patients undergoing TME for locally advanced rectal cancer.
This study is registered with ClinicalTrials.org Identifier: NCT00335816
Introduction
Pre-operative neoadjuvant chemoradiation (CRT) combined with total mesorectal excision (TME) is the mainstay of treatment in patients with locally advanced rectal cancer as it results in a high rate of local disease control, sphincter-preservation and patient survival.1 In recent years tumor response to CRT has also emerged as a potentially important predictor of local tumor control and patient survival.2 Several studies are now investigating a change in surgical approach for patients with tumors that respond to CRT.3 Strategies to improve pathologic complete response (pCR) may be clinically important.
Surgery in rectal cancer patients has routinely been performed approximately 6 weeks after completion of neoadjuvant therapy to allow the tumor sufficient time to respond to CRT and the patient adequate time to recover from any CRT-related toxicity. An emerging body of data suggests that the response to CRT in patients with rectal cancer is time-dependent, and complete tumor regression may take months.4 Thus, extending the interval between CRT and surgery may increase the proportion of patients achieving a pCR. However, surgeons have been reluctant to delay surgery beyond 6–8 weeks due to the concern that radiation-induced pelvic fibrosis may increase the technical difficulty of the operation and increase the risk of surgical complications and loco-regional recurrence.
To investigate this, the Timing of Rectal Cancer Response to Chemoradiation Consortium designed a series of sequential Phase II trials or study groups (SGs), each with additional cycles of chemotherapy after CRT and a longer interval between CRT and surgery. Here, we present the preliminary results from SG1, patients who had TME at the standard interval after CRT and SG2, patients who had TME after a longer interval and received two cycles of chemotherapy (mFOLFOX-6) during the waiting period. We report the pathologic complete response (pCR) rate, CRT and mFOLFOX-6-related adverse events (AEs) and surgical complications.
Patients and methods
Study design
The study was a non-randomized, multi-center, Phase II clinical trial. Each SG was designed with a progressively longer CRT-to-surgery time interval, allowing administration of additional cycles of mFOLFOX-6 chemotherapy before surgery, thus increasing the time interval between completion of neoadjuvant treatment and surgery in each subsequent group. The design within each SG was a single-arm Simon’s two-stage minimax design5 (Figure 1) in which the threshold for determining patient accrual in SG2 was dependent on the empirical pCR rate observed in SG1. We set the expected pCR rate in SG1 at 15% based on best available information. We estimated the sample size in SG2 based on a 5% increase in the pCR rate (to 20%), with a Type I Error of 5% and power of 90%. Based on these parameters, the original patient accrual target for SG1 and SG2 was 62 and 74 patients, respectively, including an estimated drop-out rate of 15%. A central Institutional Review Board (IRB) and the IRB at each participating institution approved the study protocol. All patients provided written informed consent before entering the trial.
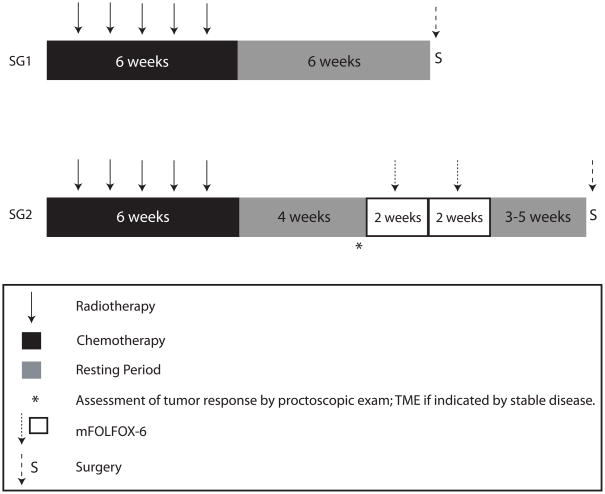
Figure 1. Treatment schema for the trial.
CRT: 5-FU was given at 225 mg/m2/day for 7 days/week in continuous infusion throughout radiation therapy. Radiation therapy was given once a day for 5 days at 1.8 Gy/day for a total of 45 Gy, given in 25 fractions. A minimum boost of 5.4 Gy was required. Surgery was performed following the principles of sharp-mesorectal excision. mFOLFOX-6 chemotherapy: Racemic leucovorin (LV) was given at 200 mg/m2 or 400 mg/m2 in a 2-hour infusion on day 1. Oxaliplatin was given at 85 mg/m2 in a 2-hour infusion concurrent with LV on day 1, followed by bolus 5-FU 400 mg/m2 on day 1 and a 46-hour infusion of 5-FU 2,400 mg/m2 (mFOLFOX-6) with two weeks between cycles. Resting period was defined as the interval between treatments.
Patient eligibility
Patients with clinical stage II (T3-4, N0) or stage III (any T, N1-2) invasive adenocarcinoma of the rectum with a distal tumor border within 12 cm of the anal verge, as measured on rigid proctoscopic examination were eligible for study. Local staging was performed by either Endorectal Ultrasound (ERUS) or Phased-Array MRI. In addition, prior to treatment patients underwent a full colonoscopy, CT scan of the abdomen and pelvis, and chest x-ray or chest CT scan. Patients were required to have an Eastern Collaborative Oncology Group Performance Status (ECOG PS) of 0 or 1, or a comparable Karnofsky performance status. Patients with a history of pelvic radiation, polyposis syndromes, inflammatory bowel disease, locally recurrent rectal cancer, metastatic disease or other primary tumors within the previous 5 years were not eligible. Patients with significant cardiac disease, seizure disorders, neurological disease or psychiatric conditions, renal, hepatic or bone marrow dysfunction were also ineligible.
Treatment protocol
Patients in both SGs were treated with 5-Fluorouracil (FU); 225 mg/m2/day by continuous IV infusion over 24 hours, 7 days/week throughout radiation. Radiation therapy was given once a day at 1.8 Gy/day, 5 days/week (excluding weekends), for a total of 45.0 Gy, given in 25 fractions, followed by a minimum boost of 5.4 Gy. In cases where the entire small bowel could be excluded from the final cone down, a second boost of 3.6 Gy (54.0 Gy total cumulative dose) was given. A linear accelerator using a minimum 6 MV energy in 3–4 fields was delivered. Intensity-modulated radiation therapy (IMRT) was permitted if approved by the supervising radiation oncologist. 5-FU and radiation dose modifications were permitted for drug-related toxicities.
Patients in SG1 had surgery within 6–8 weeks of finishing CRT. Patients in SG2 were evaluated for tumor response approximately 4 weeks after completing CRT. Clinical response was defined according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.6 Patients with signs of stable disease or disease progression compared to baseline staging had surgery without further delay. Patients with a clinical partial response (cPR) or clinical complete response (cCR) received two cycles of mFOLFOX-6 (racemic leucovorin, LV), 200 mg/m2 or 400 mg/m2 (per investigator discretion) on day 1, plus oxaliplatin, 85 mg/m2 in a 2-hour infusion on day 1, followed by bolus 5-FU 400 mg/m2 on day 1 and a 46-hour infusion of 5-FU 2,400 mg/m2 before surgery; 2 cycles (Figure 1). Patients in SG2 had surgery within 3–5 weeks of the last cycle of mFOLFOX-6.
Surgery was performed according to the principles of sharp mesorectal excision. Specimens were evaluated according to the recommendations of the Association of Directors of Anatomic and Surgical Pathology.7 Post-operative chemotherapy was not dictated by the trial and was performed at the discretion of the treating physician.
Patient evaluation
To ensure that patients with CRT-resistant tumors were not put at risk of disease progression during the longer CRT-to-surgery interval (introduced in SG2), an interim evaluation using the same imaging tests employed for initial staging (ERUS or Phased-Array MRI) was performed 4 weeks after finishing CRT. Clinical response was initially defined according to RECIST guidelines.6 Patients with progressive or stable disease after CRT did not receive additional pre-operative chemotherapy (mFOLFOX-6) and had surgery without further delay 6 weeks after completing CRT. Seven of the first 15 patients assessed in SG2 were diagnosed with stable disease based on MRI or ERUS, and underwent surgery 6 weeks after completing CRT. However, following surgery it was revealed that all of these patients achieved at least a pathologic partial response (pPR), including one patient who had a pCR. Given the inaccuracy of the imaging studies to assess clinical response at 4 weeks, the protocol was amended and assessment of response was subsequently performed by proctoscopic exam (Figure 1). This clinical approach proved to be more reliable for all subsequent patients in SG2, evident by the fact that none of the patients considered to have a partial response by proctoscopic exam had stable disease at the time of surgery.
Adverse events during CRT and mFOLFOX-6 treatment were measured using the Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. Information related to surgical procedures was collected prospectively. The degree of pelvic fibrosis and surgical difficulty were assessed using arbitrary scales ranging from 1 to 10, where 1 represented the lowest and 10 the highest degree of pelvic fibrosis and surgical difficulty, as judged by the surgeon. For both SGs, blood loss information was collected at all participating sites, while operative time was collected only at high accrual centers.
The study pathologist reviewed pathology reports and slides from the diagnostic biopsy and surgical specimens to confirm diagnosis of adenocarcinoma and pathologic staging was performed at treating institutions. Pathologic complete response and tumor regression grade (TRG) were defined according to the American Joint Committee on Cancer (AJCC) guidelines.8 Pathologic complete response was defined as the absence of tumor cells in the surgical specimen, both at the primary tumor site and regional lymph nodes. Conflicting results were resolved by consensus. Post-operative complications were graded according to the Clavien-Dindo classification.9
Statistical considerations
The primary end-point of the study was to compare the rate of pCR to CRT for stage II and stage III rectal cancers at different CRT-to-surgery intervals. Given the trial design, the study was not powered to perform statistical inference between treatment groups as might be considered in a larger multi-arm trial. P-values are presented with the understanding that they are a secondary consideration to the statistical estimates. Unless otherwise specified, p-values are based on the two-sided Fisher’s Exact test or un-pooled two-sample t-tests.
Results
Patients
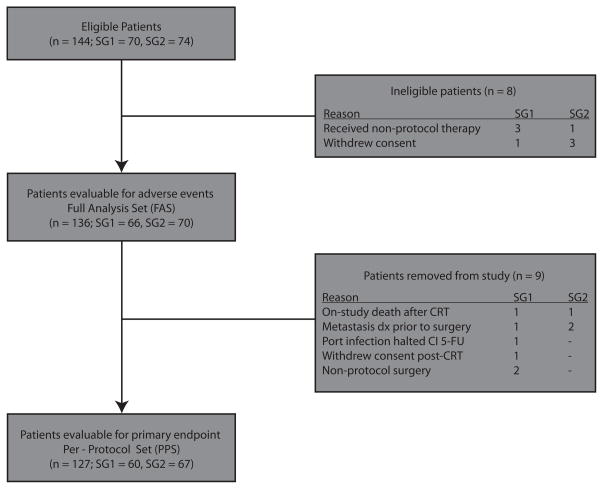
A total of 144 patients, 70 in SG1 and 74 in SG2, initially met eligibility criteria and were registered to the trial. Eight patients, (4 in each SG), were then excluded because they either withdrew consent before CRT or received non-protocol sensitizing chemotherapy. All remaining patients (66, SG1 and 70, SG2) were included in analysis of AEs related to neoadjuvant therapy. They represent the full analysis set (FAS). Nine additional patients were removed from study. The remaining 127 patients (60, SG1 and 67, SG2) represent the per-protocol set (PPS) and were included in the primary end-point analysis (Figure 2).
Figure 2. Patient attrition.
Abbreviations: Dx = diagnosis.
Patient demographics and tumor characteristics for patients in the FAS are shown in Table 1. Patients in SG2 were younger, but had more stage III and circumferential tumors compared to SG1. Other tumor characteristics such as tumor size and distance from the anal verge were not different between SGs.
Table 1.
Patient demographics and baseline tumor characteristics for the FAS†
| Demographic/Characteristic | SG1 n = 66 |
SG2 n = 70 |
p-value |
|---|---|---|---|
| Age, yearsψ | 61 ± 12 | 56 ± 11 | 0.0181 |
|
| |||
| Female | 26 (39%) | 32 (46%) | 0.4912 |
|
| |||
| ECOG PS 0 | 56 (85%) | 61 (87%) | 0.8062 |
|
| |||
| Clinical Stage | 0.0531 | ||
| II | 22 (33%) | 13 (19%) | |
| III | 44 (67%) | 57 (81%) | |
|
| |||
| Pre-CRT T Stage | 0.1996 | ||
| T2 | 2 (3%) | 7 (10%) | |
| T3 | 63 (95%) | 62 (89%) | |
| T4 | 1 (2%) | 1 (1%) | |
|
| |||
| Pre-CRT N Stage | 0.0854 | ||
| N0 | 22 (33%) | 13 (18%) | |
| N1 | 42 (64%) | 53 (76%) | |
| N2 | 1 (2%) | 4 (6%) | |
| NX | 1 (2%) | 0 (0%) | |
|
| |||
| Distance from anal verge (cm)ψ | 6.9 ± 3.1 | 6.6 ± 3.1 | 0.5340 |
|
| |||
| Size (cm)* ψ | 4.4 ± 1.6 | 4.8 ± 2.0 | 0.2401 |
|
| |||
| Circumferential tumors | 12 (18%) | 25 (36%) | - |
Non-circumferential tumors;
Mean ± standard deviation;
Full analysis set as described in Figure 2. Abbreviations: ECOG PS - Eastern Collaborative Oncology Group Performance Status.
Treatment compliance and adverse events
Treatment compliance for the FAS is presented in Table 2. The proportion of patients completing sensitizing chemotherapy per protocol was similar in both SGs, but more patients in SG2 completed radiation per protocol compared to SG1. The cumulative dose of sensitizing 5-FU and radiation was also higher in SG2 compared to SG1.
Table 2.
Chemotherapy and radiation intervention for the FAS†
| Treatment | SG1 n = 66 |
SG2 n = 70 |
p-value |
|---|---|---|---|
| 5-FU continuous infusion cum. dose × 103 (mg/m2)ψ | 9.5 ± 1.3 | 10.1 ± 1.2 | 0.0034 |
| Radiotherapy cumulative dose (Gy)ψ | 51 ± 0.3 | 52 ± 0.2 | 0.0008 |
| Chemotherapy completed per protocol | 55 (83%) | 66 (94%) | 0.0553 |
| Radiotherapy completed per protocol | 54 (82%) | 69 (99%) | 0.0009 |
Mean ± standard deviation;
FAS: Full Analysis Set as described in Figure 2. Abbreviations: cum. – cumulative.
Adverse event information for the FAS is presented in Table 3. The proportion of patients who developed Grade 3+ AEs during CRT was similar between SGs; 15 out of 66 (23%) patients in SG1 and 11 out of 70 (16%) patients in SG2 (p=0.6822). One patient in SG1 died 4 weeks after finishing CRT from a sudden cardiac arrest. One patient in SG2 with chronic pulmonary disease died 4 weeks after completing CRT, before starting mFOLFOX-6. The most common AE reported in both SGs was gastrointestinal complications.
Table 3.
Adverse events attributed to CRT for the FAS†
| CRT-associated AEs | SG1 n = 66 |
SG2 n = 70 |
|---|---|---|
| Maximum AE grade | ||
| Grade 3 | 15 (23%) | 11 (16%) |
| Grade 4 | 0 (0%) | 0 (0%) |
| Grade 5 | 1 (1%) | 1 (1%) |
|
| ||
| Most common adverse event | ||
| Gastrointestinal | 7 (11%) | 7 (10%) |
| Pain – Gastrointestinal | 5 (8%) | 1 (1%) |
| Vascular | 2 (3%) | 2 (3%) |
| Infection | 2 (3%) | 2 (3%) |
| Constitutional Symptoms | 3 (5%) | 2 (3%) |
Full Analysis Set as described in Figure 2.
Nine of the 67 PPS patients in SG2 did not receive mFOLFOX-6; 7 patients were diagnosed with stable disease at the interim evaluation 4 weeks after CRT completion, 1 patient was ineligible due to AEs during CRT, and 1 patient decided against further chemotherapy before surgery. These patients underwent surgery 6 weeks after completing CRT but are included in SG2 for the tumor response analysis. A total of 55 patients received 2 cycles of mFOLFOX-6; 2 patients received only 1 cycle due to toxicity and 1 patient received 3 cycles.
Five out of 67 (7%) patients in SG2 had Grade 3+ AEs related to mFOLFOX-6. These were myocardial infarction after completing the first cycle of mFOLFOX-6 (n=1), superior vena cava syndrome (n=1), severe abdominal pain (n=1), vomiting (n=1), and upper extremity thrombosis related to a PICC line (n=1).
Surgery and pathology
Surgical results are summarized in Table 4. The average time interval between CRT and surgery was 6 weeks for patients in SG1 and 11 weeks in SG2. The proportion of patients who had a sphincter-saving procedure, a diverting stoma, and an R0 resection, as well as the average number of lymph nodes analyzed in surgical specimens, were similar in both SGs. The average pelvic fibrosis score was higher in SG2 compared to SG1, but surgical difficulty was similar in both groups. For the patients in SG2 that received 2 cycles of mFOLFOX-6 (n=55), surgical difficulty (4.9 ± 2.7; mean ± standard deviation) and pelvic fibrosis (3.7 ± 2.6; mean ± standard deviation) were comparable to SG2 overall. There was a trend toward longer operative time and blood loss for patients in SG2 compared to SG1.
Table 4.
Surgical results for the PPS†
| Surgical results | SG1 n = 60 |
SG2 n = 67 |
p-value |
|---|---|---|---|
| Days from start of CRT to surgery | 100 ± 31 | 119 ± 20 | < 0.0001 |
| Days from end of CRT to surgery | 40 ± 8 | 77 ± 16 | < 0.0001 |
| Sphincter-saving surgery | 46 (77%) | 50 (75%) | 0.8382 |
| Ileostomy | 38 (63%) | 43 (64%) | 1.000 |
| R0 resection | 58 (97%) | 64 (96%) | 1.000 |
| Lymph nodes examined | 13 ± 7 | 15 ± 7 | 0.3341 |
| Pelvic fibrosis* | 2.4 ± 1.7 | 4.0 ± 2.6 | 0.0003 |
| Surgical technical difficulty** | 4.5 ± 2.7 | 5.1 ± 2.7 | 0.2220 |
| Estimated blood loss (ml) | 263 ± 235 | 328 ± 117 | 0.1224 |
| Operating time (minutes) | 249 ± 55 | 314 ± 317 | 0.3310 |
Scale for pelvic fibrosis ranges from 1 (none) to 10 (maximal);
Scale for surgical difficulty ranges from 1 (easy) to 10 (difficult);
Per-Protocol Set as described in Figure 2. Note: Mean ± standard deviation shown.
Tumor response
All 127 PPS patients were assessable for pathologic response (Table 5). Six out of 60 patients (10%) in SG1, but no patients in SG2, had stable disease. The differences in overall pathologic response was statistically significant between SG1 and SG2 (p=0.0217). The pathologic T stage, but not the N stage, was also significantly different between SGs (p=0.0008). The proportion of patients with a pCR was higher in SG2 (25%) compared to SG1 (18%) but the difference did not reach statistical significance. The pCR rate for the patients in SG2 that received 2 cycles of mFOLFOX-6 was also 25% (14 out of 55 patients).
Table 5.
Pathologic response rate following surgery for the PPS†
| Tumor response | SG1 n = 60 |
SG2 n = 67 |
p-value |
|---|---|---|---|
| Pathologic tumor response | 0.0217 | ||
| pCR | 11(18%) | 17(25%) | |
| pPR | 43 (72%) | 50 (75%) | |
| pSD | 6 (10%) | 0 (0%) | |
|
| |||
| Pathologic T stage | 0.0008 | ||
| T0 | 14 (23%) | 21 (31%) | |
| Tis | 2 (3%) | 1 (2%) | |
| T1 | 3 (5%) | 4 (6%) | |
| T2 | 15 (25%) | 19 (28%) | |
| T3 | 26 (43%) | 19 (28%) | |
| T4 | 0 (0%) | 2 (3%) | |
|
| |||
| Pathologic N stage | 0.6606 | ||
| N0 | 45 (75%) | 50 (75%) | |
| N1 | 7 (12%) | 10 (15%) | |
| N2 | 8 (13%) | 7 (10%) | |
Per-Protocol Set as described in Figure 2. Abbreviations: pCR - Pathologic complete response; pPR - Pathologic partial response; pSD - Stable disease.
Three patients (5%) in SG1 and two (3%) in SG2 were diagnosed with metastatic disease during treatment. One patient in SG1 with a normal liver on CT scan before CRT was diagnosed with liver metastasis after CRT and never had surgery for the primary tumor. Two additional patients in SG1, one with a mildly suspicious small attenuation lesion in the dome in the liver on CT scan before CRT and one with a completely normal CT scan before CRT, were diagnosed with liver metastasis during surgery. Both were included in the PPS. Two patients in SG2 with small abnormalities on the chest CT scan obtained before CRT were diagnosed with lung metastasis after CRT and had non-protocol therapy.
Surgical complications
There was no peri-operative mortality in either SG. However, 51 of the 127 PPS patients (40%) developed post-operative complications. The proportion of patients developing any postoperative complications was similar in both SGs; 24 out of 60 (40%) in SG1, and 27 out of 67 (40%) in SG2. For the patients in SG2 that received 2 cycles of mFOLFOX-6, 21 out of 55 (38%) patients developed any post-operative complications and 3 out of 55 (5%) patients reported at least one Grade 3+ complication. The most common Grade 3 and 4 complications for SG1 and SG2 are shown in Table 6.
Table 6.
Grade 3+ surgical complications for the PPS†
| Surgical complication | SG1 n = 60 |
SG2 n = 67 |
|---|---|---|
| Obstruction/ileus | 7 (12%) | 7 (9%) |
| Superficial SSI | 5 (8%) | 7 (9%) |
| Urinary retention | 3 (5%) | 8 (12%) |
| Organ Specific SSI (Leak or Abscess) | 6 (10%) | 4 (6%) |
| Urinary tract infection | 2 (3%) | 3 (4%) |
| Ileostomy related | 3 (5%) | 1 (1%) |
| Atelectasis | 2 (3%) | 1 (1%) |
| Other | 6 (10%) | 5 (7%) |
Per-Protocol Set as described in Figure 2. Abbreviations: SSI - Surgical Site Infection. Note: Some patients had more than one complication.
Discussion
Preliminary results of SG1 and SG2 from the Timing of Rectal Cancer Response to Chemoradiation study indicate that giving two cycles of chemotherapy (mFOLFOX-6) after CRT, and increasing the time interval between completion of CRT and TME (to an average of 11 weeks) may increase the pCR rate without significantly increasing CRT- or chemotherapy-related AEs, operative difficulty or post-operative complications, compared to traditional neoadjuvant CRT and an average waiting time of 6 weeks. Whether our observed increase in tumor response is attributable to the additional neoadjuvant chemotherapy or the longer CRT-to-surgery interval can not be conclusively determined by this trial.
Several retrospective case series have investigated the time interval between neoadjuvant therapy and surgery as a predictor of tumor response.10–12 Tulchinsky, et al assessed whether the time interval between neoadjuvant CRT and surgery affected operative and post-operative morbidity, pCR rate and disease recurrence in 132 patients with locally advanced rectal cancer treated with CRT and surgery. They found that patients operated on more than 7 weeks after CRT had a pCR rate of 35%, compared to 17% for patients operated on less than 7 weeks after CRT (p=0.03). The rate of peri-operative complications was similar in both groups. A longer CRT-to-surgery interval was also associated with significantly better disease-free survival.10 Their results were supported by Moore, et al who found a 19% pCR rate in patients operated on more than 44 days after CRT compared to 12% in patients operated within 44 days of CRT,11 and by Kalady, et al who reported a 31% pCR rate in patients operated on more than 8 weeks after CRT compared to 16% in patients operated on within 8 weeks of CRT.12 In Kalady’s study, an extended interval from CRT-to-surgery was the only factor associated with a higher pCR. In contrast, other studies report no difference in pCR rates with longer CRT-to-surgery intervals using similar, though not identical criteria.13–15 These discrepancies may be explained by differences in CRT regimens, arbitrary selection of the CRT-to-surgery intervals, and likely different definitions of tumor response.
The Lyon R90-01 study, the only prospective trial in which patients with locally advanced rectal cancer treated with radiation therapy were randomly assigned to have surgery at two different time intervals following CRT, showed that a 6–8 week interval resulted in a higher response rate compared to a 2-week interval. Furthermore, at a median follow-up of 33 months, no differences were found in morbidity, local recurrence, or survival between the two groups, suggesting that a longer interval from radiation to surgery increases tumor down-staging, with no detrimental effect on toxicity or early clinical results.16,17 However, in this study patients did not receive sensitizing chemotherapy during radiation, and the interval between radiation and surgery was only 8 weeks, closer to the short interval chosen in our study.
The effect of a more intense neoadjuvant regimen consisting of capecitabine and oxaliplatin before and concurrent with pre-operative radiotherapy in patients with locally advanced rectal cancer was initially investigated by Koeberle, et al. Surgery was performed 5 weeks after CRT. Most patients (98%) had an R0 resection, and 23% had a pCR.18 Unfortunately in this study pCR was defined as a complete or near complete pathologic response, and therefore their results can not be compared to ours. More recently, Chua, et al reported their results in stage II and III rectal cancer patients who received 12 weeks of capecitabine and oxaliplatin followed by CRT using capecitabine as radio-sensitizer. Patients had surgery 47 days (median) after finishing CRT. Twenty-one (20%) patients had a pCR,19 a rate lower than the 25% observed among patients of SG2 in our trial who only received 4 weeks of neoadjuvant chemotherapy before surgery. Although the chemotherapy used in both trials was different, comparison of the results suggests that the increase in pCR rate observed in SG2 of our study may be attributable in part to the longer CRT-to-surgery interval.
Habr-Gama, et al also studied the effect of a more intense neoadjuvant regimen on tumor response by adding chemotherapy.20 In their trial, patients with T3 or T4 tumors were treated with bolus infusion 5-FU-based CRT (total radiation dose, 54.0 Gy) followed by 3 additional cycles of bolus 5-FU over 9 weeks. Tumor response was assessed by clinical exam at protocol completion. A total of 22 out of 29 (76%) patients entered in the study had a complete clinical response and did not undergo surgery; 4 patients had recurrence within 12 months and required additional treatment. In total, 18 out of 29 (62%) patients had a sustained clinical response and had avoided surgery for at least 12 months. However, the follow-up in this study is still too short and the possibility that clinically occult cancer cells may remain viable and lead to a delayed tumor relapse can not be excluded.
The impact of delaying surgery on surgical difficulty and post-operative morbidity is an important consideration before a longer surgery interval between CRT and surgery can be recommended. Our study shows that delaying surgery does not affect the proportion of patients having an R0 resection or a sphincter-saving procedure. Surgeons in our study reported more pelvic fibrosis in patients operated on 11 weeks compared to 6 weeks after CRT, but these results should be interpreted with caution because the surgeons were not blinded to the timing of surgery. Interestingly, the increase in fibrosis did not translate into a significant increase in technical difficulty of the operation and more importantly, addition of chemotherapy during the longer CRT-to-surgery interval, did not increase the risk of post-operative complications, confirming previous observations. 21
A concern of the opponents of a longer CRT-to-surgery interval is the possibility of tumor spread that may ultimately lead to impaired survival. The trial by Francois, et al suggested there was no difference in survival when the CRT-to-surgery interval was 8 weeks,16 but a trial by Supiot, et al suggested otherwise.22 They examined the influence of delayed surgery on survival after pre-operative radiotherapy in T2-T4, N0-N1 M0 rectal cancer and found that an interval of more than 16 weeks between diagnosis and surgery may reduce overall survival for patients treated with pre-operative radiation. Their conclusions were that surgery should be performed shortly after completion of radiation. However, neither of these studies used systemic chemotherapy during the longer CRT-to-surgery interval. In our trial, patients received some of the systemic chemotherapy before surgery. The impact of splitting chemotherapy before and after surgery will need a longer follow-up.
There are a number of limitations of our trial that deserve mention. First, the primary study objective was to compare the pCR rate to CRT for stage II and stage III rectal cancers at different CRT-to-surgery intervals. However, given the trial design of sequential SGs, the study was not powered to determine statistically significant differences in pCR rate between SG1 and SG2 as might be considered in a larger multi-arm trial. Second, there were differences in treatment compliance between SG1 and SG2. CRT and radiation dosage was higher in SG2 compared to SG1, which could account for the higher pCR rate. However, it did not seem to influence the complication rate. Thirdly, it is worth noting that there were differences in tumor stage between SG1 and SG2. A higher proportion of patients in SG2 had T2 tumors compared to SG1 and this may explain the higher pCR rate observed in SG2. However, slightly more patients in SG2 had positive lymph nodes on clinical exam compared to patients in SG1 so this could equally affect the pCR rate. Lastly, the results presented are preliminary and a longer follow-up is needed to determine long-term outcomes of this regimen on recurrence and survival.
In conclusion, our preliminary results suggest that adding chemotherapy after CRT and extending the interval between CRT and surgery is well-tolerated by most patients and may lead to an increase in pCR rate without increasing the risk of surgical complications. Studies are underway to assess the impact of adding additional cycles of chemotherapy and further extending the interval between CRT and surgery.
Acknowledgments
The authors thank Nicola Solomon, Ph.D., and Chip Reuben, M.S., for assistance in writing and editing the manuscript. This study was supported by the National Institutes of Health (NIH), National Cancer Institute (NCI) R01 Grant CA090559 (JGA).
Participating Investigators
Timing of Rectal Cancer Response to Chemoradiation Consortium: W. Donald Buie, MD, University of Calgary • Theodore Coutsoftides, MD, Center for Cancer Prevention and Treatment, St. Joseph Hospital • David Dietz, MD, Cleveland Clinic Foundation • Alessandro Fichera, MD, University of Chicago Medical Center • Daniel Herzig, MD, Knight Cancer Institute, Oregon Health & Science University • Steven Hunt, MD, Washington University (St. Louis, MO) • Peter Cataldo, MD and Neil Hyman, MD, University of Vermont • Jorge Marcet, MD, University of South Florida • Samuel Oommen, MD, John Muir Health (Concord, CA) • Thomas E. Read, MD, Lahey Clinic Medical Center, Tufts University School of Medicine • David Rothenberger, MD, University of Minnesota • Lee Smith, MD, Washington Hospital Center • Michael J. Stamos, MD, University of California, Irvine • Charles A. Ternent, MD, FACS, Colon & Rectal Surgery Inc. (Omaha, NE) • Madhulika G. Varma, MD, University of California, San Francisco.
Footnotes
Commercial Sponsorship
There was no commercial sponsorship provided for this study.
Financial Disclosure
This study was supported by the National Institutes of Health (NIH), National Cancer Institute (NCI) R01 Grant CA090559 (JGA). ClinicalTrials.org Identifier: NCT00335816.
Disclosure:
The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Maas M, Nelemans PJ, Valentino V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill BD, Brown G, Heald RJ, Cunningham D, Tait DM. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007;8(7):625–633. doi: 10.1016/S1470-2045(07)70202-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Cummings B, Catton P, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: long term outcome of 271 patients. Radiother Oncol. 2005;77(2):126–32. doi: 10.1016/j.radonc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumor assessment. Eur J Cancer. 2006;42(8):1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Jass JR, O’Brien MJ, Riddell RH, Snover DC. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Association of Directors of Anatomic and Surgical Pathology. Hum Pathol. 2007;38(4):537–545. doi: 10.1016/j.humpath.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 9.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 10.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15(10):2661–2667. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 11.Moore HG, Gittleman MD, Minsky B, et al. Rate of Pathologic complete response with increased interval between preoperative combine modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47(3):279–286. doi: 10.1007/s10350-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 12.Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive Factors of Pathologic Complete Response After Neoadjuvant Chemoradiation for Rectal Cancer. Ann Surg. 2009;250(4):582–589. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 13.Stein DE, Mahmoud NN, Anné PR, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum. 2003;46(4):448–453. doi: 10.1007/s10350-004-6579-0. [DOI] [PubMed] [Google Scholar]
- 14.Dolinsky CM, Mahmoud NN, Mick R, et al. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol. 2007;96(3):207–212. doi: 10.1002/jso.20815. [DOI] [PubMed] [Google Scholar]
- 15.Lim SB, Choi HS, Jeong SY, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg. 2008;248(2):243–251. doi: 10.1097/SLA.0b013e31817fc2a0. [DOI] [PubMed] [Google Scholar]
- 16.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396–2402. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 17.Glehen O, Chapet O, Adham M, et al. Long-term results of the Lyons R90-01 randomized trial of preoperative radiotherapy with delayed surgery and its effect on sphincter-saving surgery in rectal cancer. Br J Surg. 2003;90(8):996–998. doi: 10.1002/bjs.4162. [DOI] [PubMed] [Google Scholar]
- 18.Koeberle D, Burkhard R, von Moos R, et al. Phase II study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. Br J Cancer. 2008;98(7):1204–1209. doi: 10.1038/sj.bjc.6604297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(3):241–248. doi: 10.1016/S1470-2045(09)70381-X. [DOI] [PubMed] [Google Scholar]
- 20.Habr-Gama A, Perez RO, Proscurshim I, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(4):1181–1188. doi: 10.1016/j.ijrobp.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Kerr SF, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg. 2008;95(12):1534–1540. doi: 10.1002/bjs.6377. [DOI] [PubMed] [Google Scholar]
- 22.Supiot S, Bennouna J, Rio E, et al. Negative influence of delayed surgery on survival after preoperative radiotherapy in rectal cancer. Colorectal Dis. 2006;8(5):430–435. doi: 10.1111/j.1463-1318.2006.00990.x. [DOI] [PubMed] [Google Scholar]