Abstract
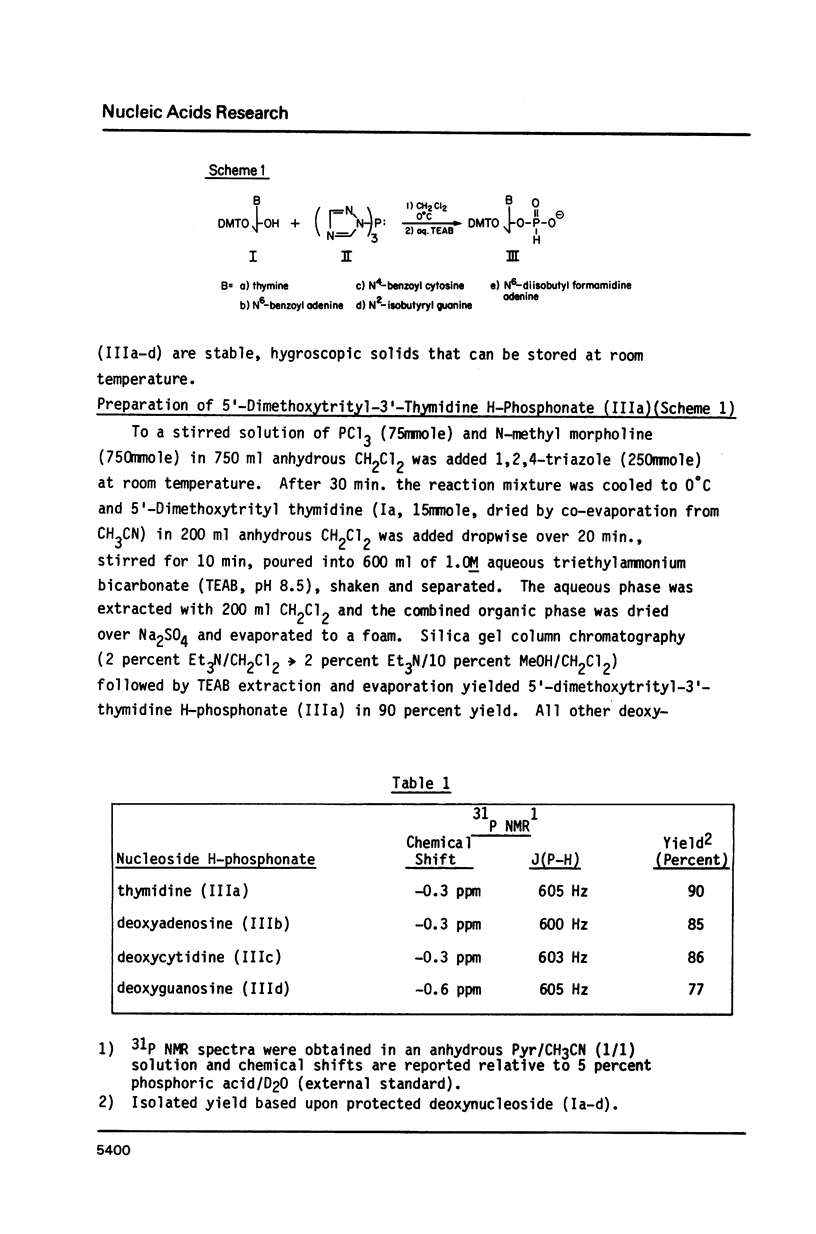
Deoxynucleoside H-phosphonates are used in the chemical synthesis of deoxyoligonucleotides up to 107 bases in length. The biological activity of the synthetic DNA is assessed by cloning into M13 and sequencing. An improved synthesis of protected deoxynucleoside H-phosphonates is also described.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Froehler B. C., Matteucci M. D. Dialkylformamidines: depurination resistant N6-protecting group for deoxyadenosine. Nucleic Acids Res. 1983 Nov 25;11(22):8031–8036. doi: 10.1093/nar/11.22.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Damha M. J., Ogilvie K. K. Modification of guanine bases by nucleoside phosphoramidite reagents during the solid phase synthesis of oligonucleotides. Nucleic Acids Res. 1985 Sep 25;13(18):6447–6465. doi: 10.1093/nar/13.18.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Letsinger R. L. Syringe method for stepwise chemical synthesis of oligonucleotides. Nucleic Acids Res. 1982 May 25;10(10):3249–3260. doi: 10.1093/nar/10.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]





