Abstract
Epithelial-mesenchymal interactions underlie the foundation for ectodermal appendage formation. Signal molecules such as BMPs and WNTs mediate crosstalk between the two tissue layers and coordinate both the induction and morphogenesis of ectodermal appendages. Here we analyzed the function of two BMP downstream transcription factors, Msx2 and Foxn1, in nail differentiation. First we show that Msx2 function is required during onychocyte (nail cell) terminal differentiation. Second, the Msx2/Foxn1/hair keratin pathway controlling hair differentiation is also conserved during onychocyte differentiation. Finally, the Msx2−/−;Foxn1−/− double mutant nails exhibit a more severe phenotype than either single mutant including nail bed hyperplasia. Together, our data implicate important functions for Msx2 and Foxn1 in regulating differentiation of the keratogenous zone, proliferation of distal nail matrix cells and organization of the nail bed.
Keywords: transcription factor, differentiation, hyperplasia, keratogenous zone, nail bed, Msx2, Foxn1
INTRODUCTION
Development of skin and its appendages requires intricate interactions between the epidermis and the underlying mesenchyme. Skin appendages, such as hair, nail, feather, scale and mammary gland, share similar developmental mechanisms (Chuong, 1998). Nail primordia start to appear on the dorsal surface of the developing digits overlying the distal phalanges on embryonic day (E) 14.5-15 and E15.5 of the mouse fore- and hind-limbs, respectively (Kaufman et al., 2003). The nail unit is composed of keratinized stratified epithelium and the underlying supporting mesenchyme. The epithelial compartment is an extension of the dorsal epidermis and contains several structures (Frank et al., 1999; Lin and Kopan, 2003). The nail fold extends from the epidermis and folds inward to cover the proximal nail plate. The nail fold is followed by the nail matrix which contains proliferating keratinocytes. Decendents from these keratinocytes grow along a distally-oriented diagonal axis (Zaias, 1990). Once these cells exit the cell cycle, they enter the keratogenous zone dorsal to the matrix where they start to express hard keratins, undergo cell flattening and apoptosis, and deposit a fully cornified and hardened structure to the nail plate. The proximal nail matrix contributes to the dorsal nail plate whereas the distal nail matrix contributes to the ventral portion (Zaias, 1990). The nail plate contains mainly a subset of hard keratins that are expressed in hairs and 10-20% soft keratins as well (Lynch et al., 1986; Heid et al., 1988b; Heid et al., 1988a; Moll et al., 1988). Distal to the nail matrix is the nail bed, which contributes a few horn cells to the undersurface of the distal nail plate. The nail bed is composed of postmitotic keratinocytes arranged in a single basal cell layer and one or two layers of suprabasal cells (Mecklenburg et al., 2004). At the nail tip, the hyponychium, the epithelium characterized by its stratification and granular formation, connects the nail bed with the ventral epidermis of the digit. A schematic outline of the nail structure is presented in Figure 1. Unlike hair follicles, which undergo cyclic growth and regression, nails grow continuously and reach equilibrium between proliferation and wear-and-tear during adulthood.
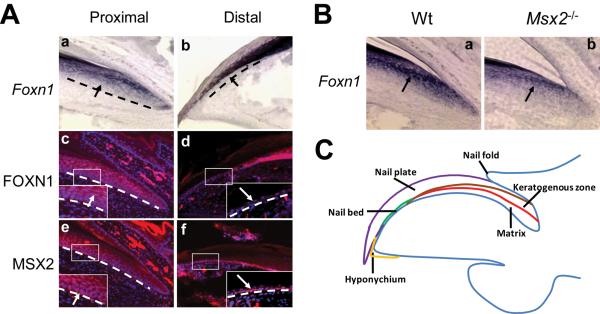
Figure 1. Msx2 regulates Foxn1 expression in the nail unit.
(A) By both in-situ hybridization (a, b) and immunohistochemistry (c, d), Foxn1 is weakly expressed in nail matrix cells excluding the basal layer and is dramatically upregulated in the keratogenous zone (A.a, c, inset, arrows). No expression is detected in the nail bed (A.b, d, inset, arrows). MSX2 protein is weakly expressed in the proximal nail unit and elevated in the more distal matrix including the basal layer, the keratogenous zone (A.e, inset, arrow) and the nail bed (A.f, inset, arrow). Proximal and distal indicate the nail unit near the phalanx and digit tip, respectively. Black and white dashed lines demarcate the border between epithelial structures and the underlying mesenchyme. (B) Foxn1 expression level is reduced in Msx2 mutant nails, as evidenced by in-situ hybridization (B.a, b, arrow). Digit 2 from the hind limbs is used for this assay. In all panels, the nail tips are orientated towards the left side. (C) A schematic outline of the nail unit.
Disrupting axis information for normal dorsal-ventral patterning of the limb also perturbs nail development. Wnt-7a-deficient mouse nails are truncated and display ventral structures on the dorsal side of the distal paws (Parr and McMahon, 1995; Cygan et al., 1997; Kawakami et al., 2000). Mice and humans with mutations in LMX1B exhibit nail plate dystrophy, known as the nail-patella syndrome (Dreyer et al., 1998; Ma et al., 1998; Vollrath et al., 1998; Dreyer et al., 2000). Loss of En-1 in the ventral limb ectoderm results in dorsalization of ventral structures and formation of ectopic nail plate on the ventral side of the digit (Loomis et al., 1996; Cygan et al., 1997; Loomis et al., 1998). Perturbation of nail homeostasis by gene loss- or gain-of-function may result in nail abnormalities. Mutations in parathyroid hormone-related peptide (PTHrP), Hoxc13, K17 or hr lead to mice with malformed nails (Foley et al., 1998; Godwin and Capecchi, 1998; McGowan et al., 2002; Ma et al., 2003; Pruett et al., 2004). Ectopic expression of activated Notch1 in the keratogenous zone results in hyperproliferation in the transgenic nail matrix and consequently longer nails (Lin and Kopan, 2003). Mutation in human FOXN1, a winged-helix transcription factor, leads to nail dystrophy (Pignata et al., 1996; Frank et al., 1999; Auricchio et al., 2005)(Pignata et al., 1996; Frank et al., 1999; Auricchio et al., 2005). In mouse, loss of Foxn1 results in altered keratogenous zone differentiation and decreased keratin and sulfur content, resulting in thin, weak and broken nail plates (Meier et al., 1999; Mecklenburg et al., 2004). The mammalian Msx homeobox genes are involved in epithelial-mesenchymal interactions during organogenesis (Davidson, 1995). Msx1 mutant mice have defective and thinner nail plates (Jumlongras et al., 2001). Msx2-deficient mice display enlarged nail plates (Satokata et al., 2000). Despite the known functions of these genes during nail morphogenesis, the genetic relationship between these genes in controlling nail homeostasis is unclear.
Both Msx2 and Foxn1 are targets of BMP signaling and upstream of Notch1 during hair differentiation (Kulessa et al., 2000; Andl et al., 2004). Our previous studies demonstrated that these two transcription factors function largely in parallel downstream of BMP in hair differentiation (Cai et al., 2009). In this study, we characterized the Msx2 mutant nail phenotype and investigated the combined role of Msx2 and Foxn1 in nail homeostasis. Our data show that Msx2 and Foxn1 are required for expression of certain hair keratins in the keratogenous zone. Loss of both genes causes distal matrix hyperproliferation, resulting in nail bed hyperplasia.
MATERIALS AND METHODS
Mice and genotyping
Msx2 mutant mice were generated previously and maintained on a CD-1 background (Satokata et al., 2000). Nude (Foxn1 mutant) mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained on a BALB/c background. Msx2−/−;Foxn1−/− double mutant mice were generated as described (Cai et al., 2009). Completely hairless mice were recognized as double mutants and confirmed by genotyping the Msx2 locus and by amplification and subsequent sequencing of the mutated Foxn1 gene. Despite the mixed genetic background, the nail phenotype was 100% penetrant in Msx2−/−;Foxn1−/− (n=25), as well as in Msx2−/− (n=34) and Foxn1−/− (n=32) mice.
In-situ hybridization
Postnatal day 7 (P7) mouse hind limb digits 2-4 were collected, fixed overnight in 4% paraformaldehyde in PBS, embedded in paraffin and sectioned at 10 μm. Digoxigenin (DIG)-UTP-labeled cRNA probes were generated from the following templates: pBSK plasmid containing the Foxn1 cDNA sequence (Nehls et al., 1994) and pNM1.2 plasmid containing the Krt33b cDNA sequence (Meier et al., 1999). To prepare cRNA probes for basic hair keratin mHb6 (Krt86), total RNA was isolated from P7 wild type mouse back skin and first strand cDNA was synthesized. PCR was then performed with primers as follow: forward primer, 5′-GAGGAGCAGAGGTTGTGTGAG-3′; reverse primer, 5′-CGGATCCTCCAAGATCCTAAG-3′. PCR products were subcloned into PCR4-TOPO (Invitrogen Life technologies, Carlsbad, CA) and antisense probe was synthesized. In-situ hybridization was carried out as previously described (Ma et al., 1998). Signals were visualized using anti-DIG antibody coupled to alkaline phosphatase (AP) conjugate (Roche, Indianapolis, IN) and AP substrates NBT and BCIP (Sigma, St. Louis, MO).
Immunohistochemistry
P7 digit sections were prepared at 5 μm as described above. Primary antibodies used were mouse anti-acidic hair keratin (AE13) (Lynch et al., 1986) (1:100), rabbit anti-MSX2 (M-70, Santa Cruz Biotechnology, Santa Cruz, CA) (1:250), anti-K14 (Covance, Emeryville, CA) (1:1000), anti-Ki67 (Novocastra, Newcastle, UK) (1:1000),anti-PAI2 (Koch et al., 1998) (1;1000) and goat anti-FOXN1 (G-20, Santa Cruz Biotechnology) (1:100). Secondary antibodies used were fluorescein-coupled goat anti-mouse IgG (Jackson Laboratory, West Grove, PA), Alexa594-coupled anti-rabbit IgG (Molecular Probes, Eugene, OR) and Alexa647-coupled anti-goat IgG (Molecular Probes). Sections were counterstained with bis-benzimide (Sigma, St. Louis, MO).
Histology and cell proliferation assay
For histological analysis, 10 μm P7 digit sections were stained with Hematoxylin and Eosin. For cell proliferation assays, Ki67-positive cells were detected by antibody staining and photographed. The number of Ki67-positive cells per boxed area was counted. On average, four sections per digit from three mice of each genotype were analyzed.
Scanning Electron Microscopy
Samples were fixed in 3% glutaraldehyde and 4% PFA in PBS (Ph 7.4) for at least 2 days. SEM analysis was then carried out as previously described (Lin et al., 2008).
RESULTS
Msx2 regulates Foxn1 expression in the nail unit
Previous studies showed that Msx2 was expressed in the nail matrix and nail bed of P7 digits (Han et al., 2003). Foxn1 mRNA is expressed in suprabasal layers of the nail matrix and greatly elevated in the keratogenous zone but not in the nail bed (Meier et al., 1999) (Fig. 1A.a, b). We compared the expression pattern of these two proteins in P7 digits by immunohistochemistry. Consistent with their mRNA localizations, we observed nuclear FOXN1 protein staining in the nail matrix not including the basal layer. The staining was absent in the nail bed (Fig. 1A.c, d). MSX2 protein was detected in the nail bed and the nail matrix, including the basal layer (Fig. 1A.e, f). Thus the expression of the two transcription factors overlaps in the upper nail matrix. Since Foxn1 expression is reduced in Msx2 mutant hair follicles (Ma et al., 2003), we asked whether this regulation is conserved in nails. In-situ hybridization revealed that indeed Foxn1 expression is downregulated in Msx2 mutant nails (Fig. 1B.a, b), while Msx2 expression is not changed in Foxn1 mutant nails (data not shown), suggesting that Msx2 resides upstream of Foxn1 during both hair follicle and nail differentiation.
Nail defects in Msx2, Foxn1 single and double mutants
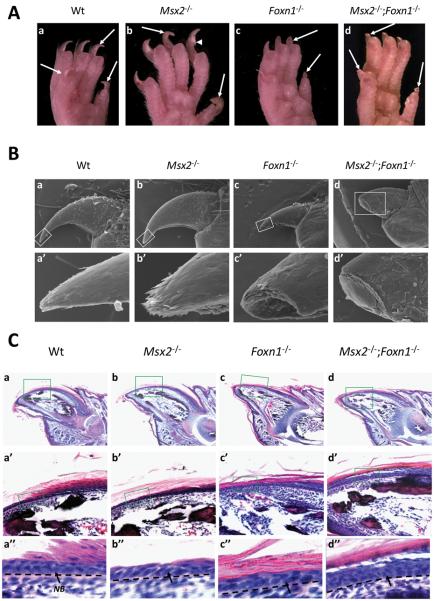
Previous study described elongated nails in Msx2 mutant mice (Satokata et al., 2000). We also observed that Msx2 mutant nails on hind limbs were longer and more fragile than those of wild type littermates (Fig. 2A.a, b). Cracks were frequently observed in Msx2 mutant nails (Fig. 2A.b, arrowhead). However, in contrast to Foxn1 mutant nails which were broken and exhibited blunt ends (Mecklenburg et al., 2004) (Fig. 2A.c), Msx2 mutant nail plates possessed a sharpened nail tip. To address the function of both Msx2 and Foxn1 in nail differentiation, double mutants were generated and their nails appeared to have a more severe phenotype compared to those of either Msx2 or Foxn1 single mutants in that nails were broken just beyond the hyponychium, even more proximal than Foxn1 mutant nails (Fig. 2A.d). The nail phenotype was 100% penetrant in either Msx2 single mutant (n=34) or Foxn1 single mutant (n=32) mice, as well as in Msx2−/−;Foxn1−/− mice (n=25). Scanning electron microscopy confirmed the morphological defects in various mutant nails and also revealed an irregular surface and squames (dead and flattened cells) on the double mutant nail plates (Fig. 2B.a-b, a′-b′).
Figure 2. Morphology and histology of wild type and various mutant nails.
(A) Morphology of adult wild type and various mutant nails from hind limbs. The nail plates of adult wild type mice are smooth, hard and with sharp tips (A.a). Msx2 mutant nails from the hind legs are longer than those of the wild type littermates but are frequently cracked (A.b, arrow head). Foxn1 mutant nails are broken in most of the mutant digits and exhibit blunt ends (A.c). Msx2 and Foxn1 double mutants still grow nails, which break beyond the hyponychium and also form blunt ends (A.d). (B) Scanning EM of 5-week old wild type and various mutant nails. The nail plates of adult wild type mice are smooth and with sharp tips (B.a, a′). No significant alteration is observed in a morphologically normal Msx2 mutant nail plate (B.b, b′). Foxn1 mutant nails are broken and exhibit blunt ends (B.c, c′). Msx2 and Foxn1 double mutant nails exhibit a rugged surface and irregular squames and break beyond the hyponychium (B.d, d′). (C) Histology of P7 wild type and various mutant nails. In both Msx2 and Foxn1 single and double mutant nails, the matrix cells were normal compared to wild type (C.a-d). The wild type nail bed (NB) is composed of a single basal layer and one or two suprabasal layers of postmitotic keratinocytes (C.a′, a″). Neither Msx2 nor Foxn1 single mutants show significant changes in nail bed structure (C.b′, b″, c′, c″). However, the double mutant nail bed undergoes hyperplasia and forms multilayers of cells with no visible transition from the nail matrix to the nail bed (C.d′, d″). In (B) and (C), a′-d′ and a″-d″ are higher magnifications of boxes in a-d. Black dashed lines demarcate the border between the nail bed and the underlying mesenchyme. Digit 4 from the hind limbs is used for this assay. In all panels, the nail tips are orientated towards the left side.
The long nail phenotype in Msx2 mutants could result from an increased proliferation in the nail matrix cells or decreased wear-and-tear. We analyzed proliferation of the nail matrix cells by Ki67 staining and similar results were obtained in Msx2 mutant and wild type littermates, suggesting that the longer nails were not due to increased proliferation of the nail matrix cells (131.2±20.78 in Msx2 mutants and 118.67±19.32 in wild type, n=3, p=0.19, t-test). Thus the long nail phenotype of Msx2 mutant mice most likely reflects a decreased wear-and-tear rate. On the other hand, we observed that the Msx2 mutant nails were frequently broken suggesting a weakened nail plate (Fig. 2A.b). This is further supported by the reduced expression of some acidic and basic keratins in Msx2 mutant keratogenous zone (Fig. 3), suggesting that the Msx2 mutant nail plate is weakened and the decreased wear-and-tear may be associated with behavioral changes of Msx2 mutant mice.
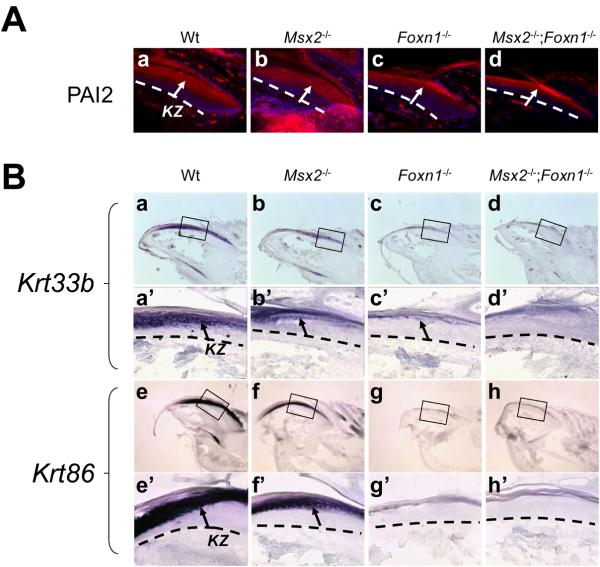
Figure 3. Abnormal differentiation of the keratogenous zone in various mutant nails.
(A) Plasminogen activator inhibitor type 2 (PAI2) is normally expressed in the keratogenous zone of the wild type nails (A.a, arrow). Neither the expression pattern nor the level of PAI2 is changed in the keratogenous zone of any of mutants (A.b-d, arrows). (B) Krt33b is normally detected in the keratogenous zone (KZ) of wild type nails (B.a, a′, arrow). In Msx2 mutant, its expression is reduced (B.b, b′, arrow). Krt33b expression is still detectable in Foxn1 mutant nails but lower than that in Msx2 mutant nails (B.c, c′, arrow). In the double mutant nails, no Krt33b expression is detected (B.d, d′). Krt86 mRNAs are also detected in the keratogenous zone of wild type nails (B.e, e′, arrow). Its expression is reduced in Msx2 mutants (B.f, f′, arrow), but not detectable in either Foxn1 mutant (B.g, g′) or double mutant nails (B.h, h′). In (B), a′-d′ and e′-h′ are higher magnifications of boxes in a-d and e-h, respectively. Black and White dashed lines demarcate the border between epithelial structures and the underlying mesenchyme. Digit 3 from the hind limbs is used for this assay. In all panels, the nail tips are orientated towards the left side.
To investigate mutant nail pathology, we made longitudinal sections of various mutant nails and stained them with hematoxylin and eosin. By histological examination, the nail matrix of all mutants appeared normal compared to that of wild type (Fig. 2C.a-d). However, marked changes were observed in the double mutant nail bed. The wild type nail bed is composed of postmitotic keratinocytes which form a single basal layer and a suprabasal layer (Fig. 2C.a′, a″). Msx2 and Foxn1 single mutants did not exhibit any overt changes in the nail bed structure (Fig. 2C.b, b′, c, c′). In contrast, the double mutant nail bed contained more than three layers of cells (Fig. 2C. d, d″).
Msx2 and Foxn1 regulate keratin expression in the keratogenous zone
The keratogenous zone expresses hard keratins, some of which are also present in the hair (Lynch et al., 1986; Heid et al., 1988b; Heid et al., 1988a; Moll et al., 1988). We first examined whether the keratogenous zone was established in various mutant nails by immunohistochemistry with a keratogenous zone marker, plasminogen activator inhibitor type 2 (PAI2) (Koch et al., 1998). Neither the pattern nor the level of PAI2 expression was changed in any of the mutant keratogenous zone, indicating that the keratogenous zone formed normally in these mutants (Fig. 3A.a-d). To assess onychocyte differentiation in various mutant nails, we stained for the expression of specific keratins by in-situ hybridization in the keratogenous zone. Krt33b is an acidic hair keratin that is regulated by both Msx2 and Foxn1 in the hair cortex (Meier et al., 1999; Ma et al., 2003) and by Foxn1 in the nail keratogenous zone (Meier et al., 1999). Krt33b expression was also downregulated in Msx2 mutant nails suggesting that it also resides downstream of Msx2 in the nail (Fig. 3B.a, a′, b, b′). Unlike in the hair follicle where Krt33b transcripts were absent in Foxn1 mutants (Meier et al., 1999), Krt33b transcript was still detectable in Foxn1 mutant nails with an expression lower than that in Msx2 mutants (Fig. 3B.c, c′). However, no Krt33b expression was detected in the double mutant nails, indicating a combinatorial effect of Msx2 and Foxn1 on Krt33b expression (Fig. 3B.d, d′). Krt86 is a basic hair keratin that is specifically expressed in the hair cortex and regulated by both Msx2 and Foxn1 (Schorpp et al., 2000). In nails, Krt86 mRNAs were detected in the keratogenous zone (Fig. 3B.e, e′). Its expression was visibly reduced in Msx2 mutants (Fig. 3B.f, f′), and not detected in either Foxn1 mutant or double mutant nails (Fig. 3B.g, g′, h, h′). These results indicate that Msx2 and Foxn1 together control keratin expression and onychocyte differentiation in the keratogenous zone.
Distal matrix hyperproliferation and nail bed hyperplasia in the double mutant
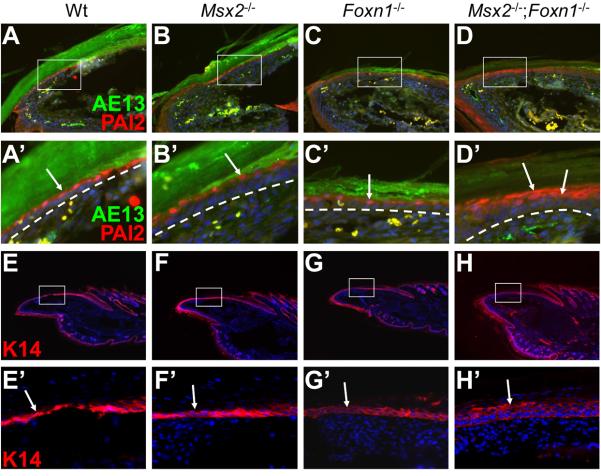
Since histological analyses revealed that the double mutant nail bed was composed of multiple layers of cells and that there was no clear transition from the matrix to the nail bed, we investigated the cellular and molecular basis of this phenotype. PAI2 antibody stains the suprabasal layer of the nail bed epithelium. In wild type, Msx2 mutant and Foxn1 mutant nails, only one cell layer in the nail bed was labeled by PAI2 antibody (Fig. 4A-C, A′-C′). In contrast, PAI2 was detected in 2 to 3 layers of cells in the double mutant nail bed (Fig. 4D, D′), clearly demonstrating that loss of Msx2 and Foxn1 resulted in nail bed hyperplasia. K14 staining confirmed this observation. K14 is normally expressed both in the nail matrix and in the nail bed epithelium including basal and suprabasal cells (Fig. 4E, E′). Only two layers of cells were stained in wild type, Msx2 and Foxn1 single mutant nail beds (Fig. 4F, F′, G, G′). However, more than three layers of cells were K14-positive in the double mutant nail bed (Fig. 4H, H′). These results indicate that Msx2 and Foxn1 together are required to maintain normal nail bed tissue homeostasis.
Figure 4. Hyperplastic nail bed in Msx2−/−;Foxn1−/− double mutant mice.
In addition to the keratogenous zone, PAI2 is also expressed in the suprabasal layer of the nail bed epithelium (A, A′, arrow). In Msx2 and Foxn1 single mutant nails, only one layer of cells in the nail bed is labeled, similar to wild type (B, B′, C, C′, arrows). However, PAI2 is detected in 2 to 3 layers of suprabasal cells in the double mutant nail bed (D, D′, arrows). AE13 staining marks the overlying nail plate (A-D, A′-D′). K14 is normally expressed in the nail matrix and the nail bed including both basal and suprabasal layers. In wild type, Msx2 and Foxn1 single mutant nail bed, K14 antibody stains two layers of cells. (E-G, E′-G′, arrows). However, more than three layers of cells are stained in the double mutant nail bed (H, H′, arrow). A′-D′ and E′-H′ are higher magnifications of boxes in A-D and E-H, respectively. White dashed lines demarcate the border between the nail bed and the underlying mesenchyme. Digit 4 of the hind limbs is used for assay. In all panels, the nail tips are orientated towards the left side.
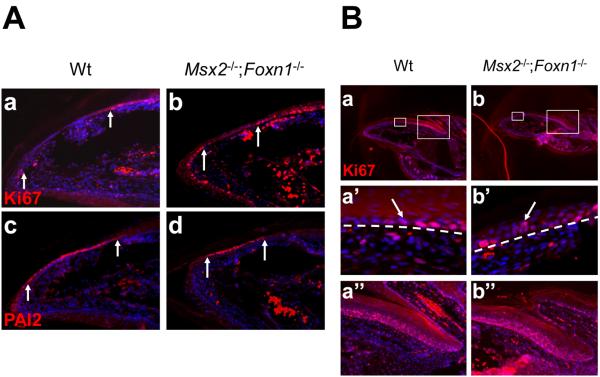
The keratinocytes in the nail bed are postmitotic and they originate from the matrix and migrate towards the hyponychium. The nail bed hyperplasia phenotype in the double mutant may result either from ectopic cell proliferation in the nail bed or from hyperproliferation of the adjacent nail matrix cells. To differentiate these two possibilities, we examined cell proliferation in and around the nail bed with an antibody against Ki67 and marked the nail bed region with PAI2 staining on adjacent sections. In wild type nails, Ki67-positive cells were detected in the distal matrix and the stratified hyponychium (Fig. 5A.a). The domain devoid of Ki67 staining (in-between the two arrows) is the PAI2-positive nail bed outlined on the adjacent section (Fig. 5A.c). Therefore Ki67 and PAI2 staining can demarcate the boundaries between nail matrix and nail bed, and between nail bed and hyponychium. Using this criterion, these boundaries were properly maintained in the double mutant nails and no ectopic cell proliferation was detected in the double mutant nail bed (Fig. 5A.b, d). However, in the double mutant distal matrix we observed more than one layer of proliferating cells (Ki67+) compared to a single layer in the wild type (Fig. 5B. a, a′, b, b′). This phenotype was unique to the double mutant and not observed in either single mutant (data not shown). Moreover, this phenotype was restricted to the distal matrix as the number of Ki67+ cells in the proximal matrix did not change in the double mutants, compared to wild type (119.45±12.63 in double mutants and 118.67±19.32 in wild type, n=3, p=0.33, t-test) (Fig. 5B.e, f). Altogether, these results indicate that the nail bed hyperplasia phenotype in Msx2−/−;Foxn1−/− double mutants likely results from hyperproliferation of the distal matrix cells and not from ectopic nail bed cell proliferation.
Figure 5. Double mutant nail bed hyperplasia results from distal matrix hyperproliferation.
(A) Ki67 antibody labels all cycling cells and Ki67+ cells are detected in the distal matrix and stratified hyponychium of wild type nails (A.a). The PAI2 positive nail bed is Ki67 negative revealed on adjacent sections (in-between two white arrows) (A.c). Similar to wild type, no Ki67 positive cell is detected in the double mutant PAI2 positive nail bed (A.b, d). (B) Under low magnification, no gross change in cell proliferation is observed in the double mutant nails (B.a, b). However, in the distal matrix, more than one layer of proliferating cells is labeled in the double mutant, as compared to a single layer in wild type (B.a′, b′, arrows). In the proximal matrix, the total number of proliferating cells (Ki67+) is not changed (B.a″, b″). In (B), a′, b′ and a″, b″ are higher magnifications of distal and proximal boxes in a and b, respectively. White dashed lines demarcate the border between the distal nail matrix and the underlying mesenchyme. Digit 2 from the hind limbs is used for this assay. In all panels, the nail tips are orientated towards the left side.
DISCUSSION
Roles of Msx2 and Foxn1 in nail differentiation
Previous studies have noted longer nails in Msx2 mutants and broken nails in Foxn1 mutants (Satokata et al., 2000; Mecklenburg et al., 2004). In this paper, we further characterized Msx2 mutant nail phenotype and also examined the combined role of Msx2 and Foxn1 in maintaining nail homeostasis. Msx2 mutant mice possess longer and fragile nails with sharpened tips. Since no change in matrix cell proliferation was observed in Msx2 mutant nails, the Msx2 mutant long nail phenotype may be due to decreased wear-and-tear. Frequent cracks observed in Msx2 mutant nails are indicative of a weakened mutant nail plate. Indeed, Msx2 mutant nail onychocytes in the keratogenous zone were poorly differentiated. They produced significantly less keratin as characterized by reduced Krt33b and Krt86 stainings. As hard keratins are major components of the nail plate, insufficient keratin synthesis should greatly affect its texture and thickness. The expression of these specific keratins is also reduced in Msx2 mutant hair follicles (Ma et al., 2003), suggesting a conserved role for Msx2 in regulating hard keratin expression in skin appendages, such as hair follicles and nails.
Foxn1 mutant nails exhibit a more severe phenotype than those of Msx2 mutants. Their nail plates are much thinner and easily broken. This phenotype is the result of even less hard keratin expression in Foxn1 mutant nails than in Msx2 mutant nails. Likewise, Foxn1 is also required for hard keratin expression during hair differentiation and it functions downstream of Msx2 (Meier et al., 1999; Ma et al., 2003). Here we show that Foxn1 expression is also downregulated in Msx2 mutant nails, suggesting that the Msx2/Foxn1/hair keratin pathway may represent a general pathway controlling epithelial appendage differentiation. On the other hand, the double mutant nails exhibit a phenotype more severe than that of either single mutant. Consistently, Krt33b expression is absent in the double mutant nails but is present in the single mutants. In addition, only the double mutant nail bed exhibited hyperplasia but not the single mutants. Together these results suggest that Msx2 and Foxn1 do not appear to reside in a simple epistatic pathway but may also have redundant and possibly parallel functions during nail homeostasis recapitulating the observation in the hair follicle (Cai et al., 2009). Nonetheless, it is clear that the major function of Msx2 and Foxn1 is to regulate onychocyte differentiation in the keratogenous zone.
Msx2 and Foxn1's role in maintaining nail bed homeostasis
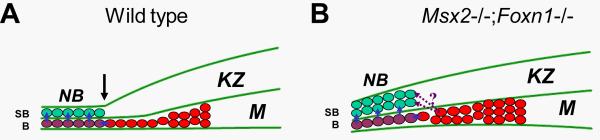
The wild type nail bed is composed of a single basal layer and one or two suprabasal layers consisting of postmitotic keratinocytes. Neither the Msx2 nor Foxn1 mutant showed any nail bed phenotype. In contrast, the double mutant nail bed undergoes hyperplasia and produces multiple layers of cells, suggesting that Msx2 and Foxn1 also play redundant roles in nail bed homeostasis. Staining with PAI2 which normally marks the suprabasal layer(s) of the nail bed showed that more than one cell layer in the double mutant nail bed is PAI2 positive, in agreement with histological observation. Consistently, K14 antibody which stains both the basal and suprabasal cells in wild type nail bed labeled more than three cell layers in the double mutant nail bed. One possibility is that the hyperplastic nail bed results from ectopic proliferation of nail bed cells. However, immunohistochemistry of Ki67 and PAI2 on adjacent sections showed that the double mutant nail bed cells were still postmitotic, arguing against this possibility. On the other hand, the hyperplastic nail bed may result from hyperproliferation of the distal matrix cells. This is indeed the case as the distal nail matrix in the double mutant mice undergoes hyperproliferation leading to more than one layer of Ki67+ cells as compared to only one layer in wild type or Msx2, Foxn1 single mutants. This effect on matrix cell proliferation seems to be restricted to the double mutant distal matrix as the proximal matrix appears not affected. In the skin epidermis, FOXN1 is expressed in the suprabasal layer immediately above the basal layer and functions to promote differentiation of suprabasal keratinocytes (Lee et al., 1999). In the absence of Foxn1, suprabasal cells ectopically express K5, a basal cell marker, which reflects a hyperplastic skin epidermis. Interestingly, since Msx2 is not expressed in the interfollicular epidermis, in essence, the basal layer of Foxn1 mutant epidermis thus resembles Msx2−/−;Foxn1−/− double mutant distal nail matrix: both exhibit hyperplasia and both show expansion of cells positive for basal layer-specific keratins into suprabasal layers (K5 in skin and K14 in nails). These results indicate that in the distal nail matrix, Msx2 and Foxn1 have redundant functions in restricting cycling cells to the basal layer and in maintaining homeostasis of the distal nail matrix and the nail bed. Currently it is believed that the suprabasal cells in the nail bed come from the underlying basal cells, which originally come from the distal matrix cells (Zaias, 1990) (Fig. 6A). In the absence of both Msx2 and Foxn1, more distal nail matrix cells undergo proliferation, such that more postmitotic cells are produced at the matrix-nail bed boundary, migrate into the nail bed and cause nail bed hyperplasia. However, it is not clear at present how this is accomplished at the cellular level. Since Msx2 have been implicated in suppressing expression of the adhesion molecule cdh6 and in regulating migration of neural crest cells (Ishii et al., 2005), it is possible that similar effects on cell-cell adhesion may also contribute to the nail bed phenotype in Msx2−/−;Foxn1−/− double mutant nails. Presumably the basal layer in the double mutant distal matrix will continue to provide postmitotic cells to the nail bed basal layer and the suprabasal proliferating cells will contribute to the suprabasal layers of the nail bed leading to nail bed hyperplasia (Fig. 6B). But we cannot exclude the possibility that the suprabasal proliferating cells can also contribute to nail bed basal cells.
Figure 6. A model for nail bed hyerplasia.
(A) In wild type nails, the distal matrix is composed of one layer of proliferating cells (red) migrating toward the basal layer of the nail bed epithelium. After they exit the matrix, they become postmitotic and enter the nail bed. In the nail bed, some basal cells (purple) detach from the basement membrane, migrate upward and differentiate into suprabasal cells (green) which contribute to the horny layer of the nail plate. (B) In double mutant nails, the distal matrix undergoes hyperproliferation. More cells enter the nail bed, either directly or indirectly contribute to the multilayered suprabasal cells in the nail bed, resulting in nail bed hyperplasia. B, basal layer; KZ, keratogenous zone; M, matrix; NB, nail bed; SB, suprabasal layer.
Studies in hair follicles showed that Msx2 and Fonx1 are downstream targets of BMP signaling (Kulessa et al., 2000; Andl et al., 2004). It is possible that the Msx2−/−;Foxn1−/− double mutant nail phenotype reflects perturbed BMP signaling in the nail. Although the nail phenotype in BMPR1A-deficient has not been described, constitutive activation of Akt signaling leads to suppression of Bmp4 signaling in the hair follicle and nail bed hyperplasia similar to that in Msx2−/−;Foxn1−/− double mutants (Segrelles et al., 2008). These results suggest that BMP signaling may also function upstream of Msx2 and Foxn1 in the nail and maintains nail homeostasis through Msx2 and Foxn1. The role of BMP signaling in nails needs to be addressed in future studies.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant ES014482. We thank G. M. Veith for help with SEM, Dr. Pamela Jensen for PAI2 antibody, Dr. Tung-Tien Sun for AE13 antibody and Dr. Raphael Kopan for K14 and Ki67 antibodies.
REFERENCES
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, 3rd, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Auricchio L, Adriani M, Frank J, Busiello R, Christiano A, Pignata C. Nail dystrophy associated with a heterozygous mutation of the nude/SCID human FOXN1 (WHN) gene. Arch Dermatol. 2005;141:647–648. doi: 10.1001/archderm.141.5.647. [DOI] [PubMed] [Google Scholar]
- Cai J, Lee J, Kopan R, Ma L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Dev Biol. 2009;326:420–430. doi: 10.1016/j.ydbio.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience; Austin, TX: 1998. [Google Scholar]
- Cygan JA, Johnson RL, McMahon AP. Novel regulatory interactions revealed by studies of murine limb pattern in Wnt-7a and En-1 mutants. Development. 1997;124:5021–5032. doi: 10.1242/dev.124.24.5021. [DOI] [PubMed] [Google Scholar]
- Davidson D. The function and evolution of Msx genes: pointers and paradoxes. Trends Genet. 1995;11:405–411. doi: 10.1016/s0168-9525(00)89124-6. [DOI] [PubMed] [Google Scholar]
- Dreyer SD, Morello R, German MS, Zabel B, Winterpacht A, Lunstrum GP, Horton WA, Oberg KC, Lee B. LMX1B transactivation and expression in nail-patella syndrome. Hum Mol Genet. 2000;9:1067–1074. doi: 10.1093/hmg/9.7.1067. [DOI] [PubMed] [Google Scholar]
- Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, Cole W, Johnson RL, Lee B. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47–50. doi: 10.1038/ng0598-47. [DOI] [PubMed] [Google Scholar]
- Foley J, Longely BJ, Wysolmerski JJ, Dreyer BE, Broadus AE, Philbrick WM. PTHrP regulates epidermal differentiation in adult mice. J Invest Dermatol. 1998;111:1122–1128. doi: 10.1046/j.1523-1747.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- Frank J, Pignata C, Panteleyev AA, Prowse DM, Baden H, Weiner L, Gaetaniello L, Ahmad W, Pozzi N, Cserhalmi-Friedman PB, Aita VM, Uyttendaele H, Gordon D, Ott J, Brissette JL, Christiano AM. Exposing the human nude phenotype. Nature. 1999;398:473–474. doi: 10.1038/18997. [DOI] [PubMed] [Google Scholar]
- Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12:11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Yang X, Farrington JE, Muneoka K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development. 2003;130:5123–5132. doi: 10.1242/dev.00710. [DOI] [PubMed] [Google Scholar]
- Heid HW, Moll I, Franke WW. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. I. Human and bovine hair follicles. Differentiation. 1988a;37:137–157. doi: 10.1111/j.1432-0436.1988.tb00805.x. [DOI] [PubMed] [Google Scholar]
- Heid HW, Moll I, Franke WW. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. II. Concomitant and mutually exclusive synthesis of trichocytic and epithelial cytokeratins in diverse human and bovine tissues (hair follicle, nail bed and matrix, lingual papilla, thymic reticulum) Differentiation. 1988b;37:215–230. doi: 10.1111/j.1432-0436.1988.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Ishii M, Han J, Yen HY, Sucov HM, Chai Y, Maxson RE. Combined deficiencies of Msx1 and Msx2 cause impaired patterning and survival of the cranial neural crest. Development. 2005;132:4937–4950. doi: 10.1242/dev.02072. [DOI] [PubMed] [Google Scholar]
- Jumlongras D, Bei M, Stimson JM, Wang WF, DePalma SR, Seidman CE, Felbor U, Maas R, Seidman JG, Olsen BR. A nonsense mutation in MSX1 causes Witkop syndrome. Am J Hum Genet. 2001;69:67–74. doi: 10.1086/321271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, Dai X, Alegre ML, Fuchs E. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Wada N, Nishimatsu S, Nohno T. Involvement of frizzled-10 in Wnt-7a signaling during chick limb development. Dev Growth Differ. 2000;42:561–569. doi: 10.1046/j.1440-169x.2000.00545.x. [DOI] [PubMed] [Google Scholar]
- Koch PJ, Mahoney MG, Cotsarelis G, Rothenberger K, Lavker RM, Stanley JR. Desmoglein 3 anchors telogen hair in the follicle. J Cell Sci. 1998;111(Pt 17):2529–2537. doi: 10.1242/jcs.111.17.2529. [DOI] [PubMed] [Google Scholar]
- Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. Embo J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Prowse DM, Brissette JL. Association between mouse nude gene expression and the initiation of epithelial terminal differentiation. Dev Biol. 1999;208:362–374. doi: 10.1006/dbio.1999.9221. [DOI] [PubMed] [Google Scholar]
- Lin C, Yin Y, Long F, Ma L. Tissue-specific requirements of beta-catenin in external genitalia development. Development. 2008;135:2815–2825. doi: 10.1242/dev.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Kopan R. Long-range, nonautonomous effects of activated Notch1 on tissue homeostasis in the nail. Dev Biol. 2003;263:343–359. doi: 10.1016/j.ydbio.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, Joyner AL. The mouse Engrailed-1 gene and ventral limb patterning. Nature. 1996;382:360–363. doi: 10.1038/382360a0. [DOI] [PubMed] [Google Scholar]
- Loomis CA, Kimmel RA, Tong CX, Michaud J, Joyner AL. Analysis of the genetic pathway leading to formation of ectopic apical ectodermal ridges in mouse Engrailed-1 mutant limbs. Development. 1998;125:1137–1148. doi: 10.1242/dev.125.6.1137. [DOI] [PubMed] [Google Scholar]
- Lynch MH, O'Guin WM, Hardy C, Mak L, Sun TT. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J Cell Biol. 1986;103:2593–2606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, Liu YH, Muller-Rover S, Peters H, Sundberg JP, Maxson R, Maas RL, Chuong CM. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–389. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- McGowan KM, Tong X, Colucci-Guyon E, Langa F, Babinet C, Coulombe PA. Keratin 17 null mice exhibit age- and strain-dependent alopecia. Genes Dev. 2002;16:1412–1422. doi: 10.1101/gad.979502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenburg L, Paus R, Halata Z, Bechtold LS, Fleckman P, Sundberg JP. FOXN1 is critical for onycholemmal terminal differentiation in nude (Foxn1) mice. J Invest Dermatol. 2004;123:1001–1011. doi: 10.1111/j.0022-202X.2004.23442.x. [DOI] [PubMed] [Google Scholar]
- Meier N, Dear TN, Boehm T. Whn and mHa3 are components of the genetic hierarchy controlling hair follicle differentiation. Mech Dev. 1999;89:215–221. doi: 10.1016/s0925-4773(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Moll I, Heid HW, Franke WW, Moll R. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. III. Hair and nail formation during human fetal development. Differentiation. 1988;39:167–184. doi: 10.1111/j.1432-0436.1988.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374:350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Perrin C, Langbein L, Schweizer J. Expression of hair keratins in the adult nail unit: an immunohistochemical analysis of the onychogenesis in the proximal nail fold, matrix and nail bed. Br J Dermatol. 2004;151:362–371. doi: 10.1111/j.1365-2133.2004.06108.x. [DOI] [PubMed] [Google Scholar]
- Pignata C, Fiore M, Guzzetta V, Castaldo A, Sebastio G, Porta F, Guarino A. Congenital Alopecia and nail dystrophy associated with severe functional T-cell immunodeficiency in two sibs. Am J Med Genet. 1996;65:167–170. doi: 10.1002/(SICI)1096-8628(19961016)65:2<167::AID-AJMG17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Pruett ND, Tkatchenko TV, Jave-Suarez L, Jacobs DF, Potter CS, Tkatchenko AV, Schweizer J, Awgulewitsch A. Krtap16, characterization of a new hair keratin-associated protein (KAP) gene complex on mouse chromosome 16 and evidence for regulation by Hoxc13. J Biol Chem. 2004;279:51524–51533. doi: 10.1074/jbc.M404331200. [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Schorpp M, Schlake T, Kreamalmeyer D, Allen PM, Boehm T. Genetically separable determinants of hair keratin gene expression. Dev Dyn. 2000;218:537–543. doi: 10.1002/1097-0177(200007)218:3<537::AID-DVDY1007>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Segrelles C, Moral M, Lorz C, Santos M, Lu J, Cascallana JL, Lara MF, Carbajal S, Martinez-Cruz AB, Garcia-Escudero R, Beltran L, Segovia JC, Bravo A, Digiovanni J, Paramio JM. Constitutively Active Akt Induces Ectodermal Defects and Impaired Bone Morphogenetic Protein Signaling. Mol Biol Cell. 2008;19:137–149. doi: 10.1091/mbc.E07-08-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D, Jaramillo-Babb VL, Clough MV, McIntosh I, Scott KM, Lichter PR, Richards JE. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091–1098. doi: 10.1093/hmg/7.7.1091. [DOI] [PubMed] [Google Scholar]
- Zaias N, editor. The Nail in Health and Disease. Appleton and Lange; East Norwalk, CT: 1990. [Google Scholar]