Abstract
This review is concerned with understanding how vasodilation initiated from local sites in the tissue can spread to encompass multiple branches of the resistance vasculature. Within tissues, arteriolar networks control the distribution and magnitude of capillary perfusion. Vasodilation arising from the microcirculation can ‘ascend’ into feed arteries that control blood flow into arteriolar networks. Thus distal segments of the resistance network signal proximal segments to dilate and thereby increase total oxygen supply to parenchymal cells. August Krogh proposed that innervation of capillaries provided the mechanism for a spreading vasodilatory response. With greater understanding of the ultrastructural organization of resistance networks, an alternative explanation has emerged: Electrical signaling from cell to cell along the vessel wall through gap junctions. Hyperpolarization originates from ion channel activation at the site of stimulation with the endothelium serving as the predominant cellular pathway for signal conduction along the vessel wall. As hyperpolarization travels, it is transmitted into surrounding smooth muscle cells through myoendothelial coupling to promote relaxation. Conducted vasodilation encompasses greater distances than can be explained by passive decay and understanding such behavior is the focus of current research efforts. In the context of athletic performance, the ability of vasodilation to ascend into feed arteries is essential to achieving peak levels of muscle blood flow. Conducted vasodilation is tempered by sympathetic neuroeffector signaling when governing muscle blood flow at rest and during exercise. Impairment of conduction during aging and in diseased states can limit physical work capacity by restricting muscle blood flow.
Keywords: ascending vasodilation, blood flow control, endothelium, exercise, gap junctions, ion channels, vascular smooth muscle
Introduction
In the intact vasculature, the largest pressure drop and thus highest resistance to flow occurs along arterioles and the feed arteries from which they originate (Bohlen et al., 1977, Fronek and Zweifach, 1975, Davis et al., 1986, Welsh and Segal, 1996). With blood flow to skeletal muscle coupled to local metabolic demand (Gorczynski et al., 1978, Laughlin and Armstrong, 1982), dilation along and among branches of resistance networks governs blood flow distribution and magnitude. For substantive changes in blood flow to occur, vasodilation must be coordinated among daughter and parent arterioles (Kurjiaka and Segal, 1995a) as well as their proximal feed arteries (Folkow et al., 1971, Segal and Duling, 1986a, Williams and Segal, 1993, Segal and Jacobs, 2001) as enabled by ascending vasodilation (Figure 1). These network-wide responses are facilitated by electrical and chemical signaling between and along endothelial cells (ECs) and smooth muscle cells (SMCs) comprising the vessel wall. Following a historical perspective on how ascending vasodilation and conducted vasodilation (CVD) have come to be recognized as a mechanism of blood flow control (Table 1), this review explores the underlying signaling events. In brief, CVD entails the initiation of SMC relaxation from a discrete location within the resistance network and the ensuing spread of vasodilation along the vessel wall via intercellular communication through gap junctions (Segal and Duling, 1986b, Figueroa and Duling, 2009, Schmidt et al., 2008) (Figure 2). Our emphasis here is on summarizing current understanding while identifying future directions for this active field of investigation. Our focus on CVD does not preclude the significance of complementary mechanisms of blood flow control (e.g., flow-dependent and metabolic vasodilation) and considerable insight is gained by considering how branches of vascular resistance networks (feed arteries and the arterioles they supply) coordinate their responses through intercellular communication.
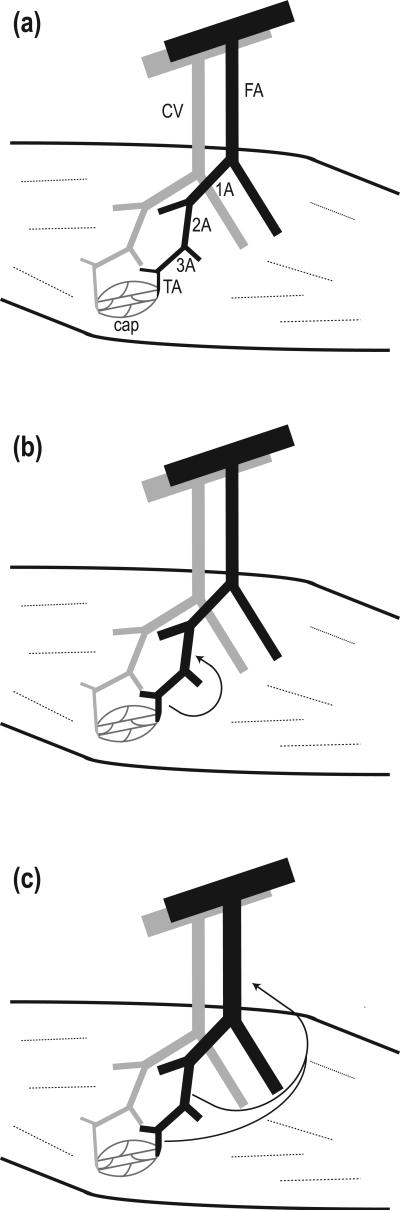
Figure 1. Ascending vasodilation in a resistance network controlling blood flow to skeletal muscle.
(a) Depiction (not to scale) of conduit artery and vein giving rise to feed artery (FA) and collecting vein (CV). Once the FA branch enters skeletal muscle it gives rise to first-order (1A), second-order (2A), third-order (3A) and terminal (TA) arterioles shown in black. A capillary network (cap) arises from the TA and converges into venules shown in parallel (gray) to arterioles. (b) Vasodilation originating from TA and 3A ascends into proximal 2A (arrow), contributing to an increase in local blood flow and capillary red cell perfusion. (c) Vasodilation ascends from intramuscular arterioles into the FA external to the muscle, contributing to an increase in total blood flow entering the muscle.
Table 1.
Evolution of Conducted (Ascending) Vasodilation
| Authors | Key Observation | Species | Tissue/preparation | Experimental Condition |
|---|---|---|---|---|
| Krogh et al., 1922 | Spreading vasodilation of arterioles via axon reflex to local chemical stimulus. | frog | hindlimb web | in vivo |
| Hilton, 1959 | Femoral artery dilation to contraction of lower leg muscles or distal infusion of ACh. | cat | hindlimb skeletal muscle | in vivo |
| Duling and Berne, 1970 | Propagated vasodilation of arterioles in response to ACh but not to other vasoactive substances. | hamster | cheek pouch | in vivo |
| Folkow et al., 1971 | Muscle contraction elicits ascending vasodilation from distal arterioles into proximal feed arteries. | cat | hindlimb skeletal muscle | in vivo |
| Hirst and Neild, 1978 | Electrical coupling among cells along the arteriolar wall. | guinea pig | submucosa | in vitro |
| Segal and Duling, 1986 | CVD via signaling through gap junctions along arteriolar ECs and SMCs | hamster | cheek pouch | in vivo |
| Segal, 1991 | CVD along terminal arterioles increases capillary red blood cell perfusion. | hamster | cremaster muscle | in vivo |
| Dietrich 1989 | Capillary stimulation alters capillary perfusion (arteriolar control) of red blood cells. | Rat | Mesentery | in vivo |
| Segal and Bény, 1992 | Homocellular dye coupling along arteriolar endothelium with hyperpolarization to ACh. | hamster | cheek pouch | in vivo |
| Kurjiaka and Segal, 1995 | CVD into parent arteriole increases blood flow into daughter arterioles. | hamster | cremaster muscle | in vivo |
| Kurjiaka and Segal, 1995 | Sympathetic nerve activation inhibits CVD along skeletal muscle arterioles. | hamster | cremaster muscle | in vivo |
| Little et al., 1995 | Myoendothelial dye coupling between arteriolar ECs and SMCs. | hamster | cheek pouch arterioles | in vitro |
| Little et al., 1995 | Cx40 and Cx43 expressed in arteriolar ECs and SMCs. | hamster, rat | cheek pouch, brain cremaster | in vivo |
| Xia et al., 1995 | Myoendothelial electrical coupling between arteriolar ECs and SMCs. | hamster | cheek pouch arterioles | in vitro |
| Dora et al., 1997 | Myoendothelial Ca2+ coupling: Elevated Ca2+ in SMCs stimulates NO production in ECs. | hamster | cheek pouch arterioles | in vitro |
| Doyle and Duling, 1997 | Conducted vasodilation includes NO-dependent and NO-independent components. | hamster | cheek pouch arterioles | in vitro |
| Berg et al., 1997 | Contracting muscle fiber bundles evokes dilation of supplying arteriolar branches. | hamster | cremaster muscle | in vivo |
| Welsh and Segal, 1998 | Endothelium and SMC layers provide parallel conduction pathways along arterioles. | hamster | cheek pouch | in vivo |
| de Wit et al., 1999 | EDHF/KCa contribute to CVD along skeletal muscle arterioles. | hamster | cremaster muscle | in vivo |
| Emerson and Segal, 2000 | Endothelium as primary cellular pathway for CVD. | hamster | retractor muscle feed arteries | in vitro |
| Emerson and Segal, 2000 | Bidirectional electrical coupling between ECs and SMCs. | hamster | retractor muscle feed arteries | in vitro |
| Hungerford et al., 2000 | Conducted vasoconstriction decays more rapidly than CVD along skeletal muscle arterioles. | mouse | cremaster muscle | in vivo |
| de Wit et al, 2000 | Cx40 deletion impairs CVD in skeletal muscle arterioles. | mouse | cremaster muscle | in vivo |
| Segal and Jacobs, 2001 | Endothelium integrity is required for ascending vasodilation to skeletal muscle contractions. | hamster | retractor muscle | in vivo |
| Crane et al., 2004 | Additional hyperpolarization contributing to CVD in arteriolar networks. | hamster | cheek pouch | in vivo |
| Diep et al., 2005 | Computational modeling of EC and SMC biophysical properties underlying CVD. | hamster | computer model | in silico |
| Jantzi et al., 2006 | Role for KIR channels to facilitate CVD | hamster | retractor muscle feed arteries | in vitro |
| Domeier and Segal, 2007 | Initiation of CVD via KCa; resolution of “fast” and “slow” components of CVD. | hamster | retractor muscle feed arteries | in vitro |
| Figueroa and Duling, 2008 | Resolution of passive and regenerative components of CVD in skeletal muscle arterioles. | mouse | cremaster muscle | In vivo |
| Wolfle et al, 2009 | Manipulating KCa channels alters CVD in skeletal muscle arterioles. | mouse | cremaster muscle | in vivo |
Citations listed in chronological order highlight initial key findings in the evolution of conducted/ascending vasodilation; in vivo refers to tissue preparations studied in anesthetized animals; in vitro refers to isolated vessels studied in a tissue chamber; in silico refers to computational modeling. Abbreviations: ACh, acetylcholine; Cx40, connexin40; CVD, conducted vasodilation; EC, endothelial cell; EDHF, endothelium-derived hyperpolarizing factor; NO, nitric oxide; SMC, smooth muscle cell.
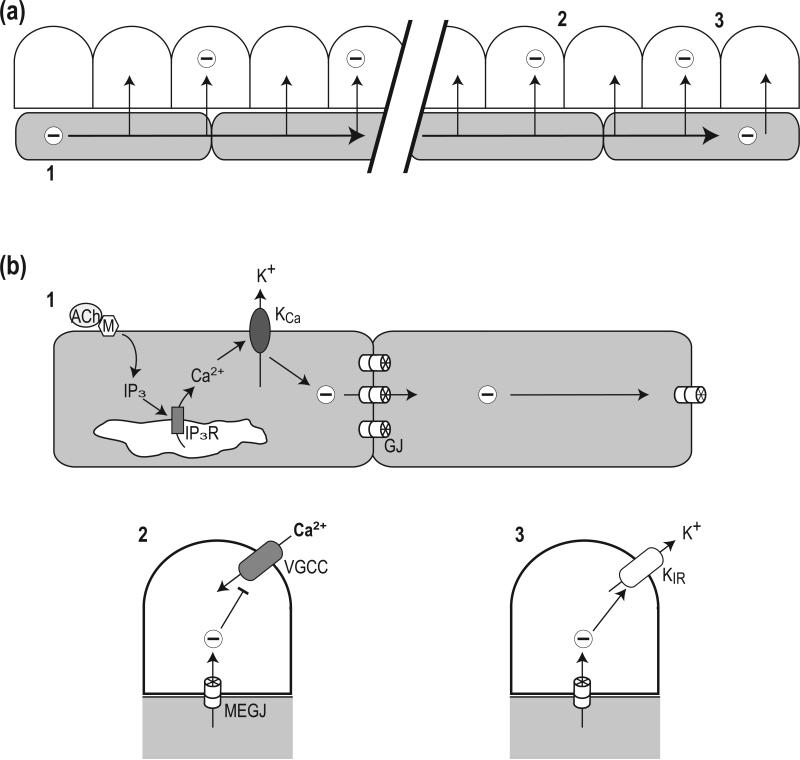
Figure 2. Initiation and conduction of vasodilation.
Depiction of longitudinal section of arteriolar wall (top) with endothelial cells in gray and smooth muscle cells in white (not to scale). The internal elastic lamina located between respective cell layers is omitted for clarity. (a) Integrated schematic depicting the initiation and conduction of hyperpolarization and vasodilation. Labels 1, 2, and 3 correspond to events shown with greater detail in respective components of panel (b). Hyperpolarization (-) is conducted along ECs (shaded with horizontal arrows) and into consecutive SMCs (vertical arrows). (b) Details of respective events indicated in upper panel. 1: Binding of ACh to a muscarinic receptor (M) triggers production of inositol 1,4,5-trisphosphate (IP3) in the cytoplasm which binds to its receptor (IP3R), releasing Ca2+ from the endoplasmic reticulum to stimulate Ca2+-activated potassium channels (KCa) in the plasma membrane. The efflux of K+ produces hyperpolarization which is conducted from EC to EC through homocellular gap junctions (GJ). 2: Along the vessel wall, hyperpolarization travels through myoendothelial gap junctions (MEGJ) to hyperpolarize SMCs and cause vasodilation by closing voltage gated Ca2+ channels (VGCC) to reduce Ca2+ entry. 3: The conduction of hyperpolarization can be augmented through activation of inward rectifying K+ channels (KIR), which may also occur along the endothelium (not depicted).
An historical perspective
August Krogh and coworkers first reported remote vasodilation to a local chemical stimulus (Krogh et al., 1922). After placing a crystal of silver nitrate near one toe in the web of the frog hindlimb, vasodilation was observed in capillaries and arterioles between adjacent toes. These remote dilations could not be explained by diffusion and spreading vasodilation was attributed to the stimulation of a network of nerve fibrils coursing through the tissue (Krogh et al., 1922). Later studies confirmed the existence of cholinergic neurogenic vasodilation in the frog microcirculation (Siggins and Weitsen, 1971) and the hypothesis of neurally-mediated spreading vasodilation persisted for many years. Indeed, ensuing work in the frog concluded that antidromic vasodilation arising from hindleg skeletal muscle was mediated by activating fine myelinated nerve fibers (Khayutin et al., 1991). During this period, alternative explanations were evolving in other laboratories using different experimental approaches to explain the ability of vasodilator signals originating within the distal microcirculation to encompass the proximal arterial supply (Figure 1).
In the femoral artery of cats, ascending dilations were recorded in response to muscle contractions of the lower leg and to injection of acetylcholine (ACh), bradykinin, histamine or nicotine into a distal branch of the arterial supply (Hilton, 1959). Ascending vasodilations persisted following ablation of autonomic nerves but were eliminated after cutting (and reconnecting) the femoral artery, leading to the conclusion that the conducting elements were the SMCs of the vessel media (Hilton, 1959). This conclusion was supported by observations on the electrical excitability of smooth muscle preparations and their ability to conduct signals through low-resistance cellular pathways (subsequently identified as gap junctions), particularly when excited by stretch or chemical transmitters (Burnstock and Prosser, 1960). Such behavior was reinforced independently by the finding that intercellular propagation among SMCs was necessary for coordinated vasomotion along the rat portal vein (Johansson and Ljung, 1967). However, during the period that these properties of large conduit vessels were being defined, there was little progress in understanding signal transmission in the resistance microvasculature.
The involvement of somatic neural pathways was implicated in ascending dilation of the cat femoral artery when these responses were blocked by cutting the sciatic nerve or with large doses of nicotine (Hilton, 1959). To investigate such behavior in the mammalian microcirculation, arterioles in the hamster cheek pouch preparation were studied in response to microiontophoresis of vasoactive agents onto an arteriole from a micropipette (Duling and Berne, 1970). Whereas ACh, histamine, K+, H+ or eledoisin initiated dilation at the site of stimulation, only ACh was found to elicit dilation bidirectionally along arterioles for distances of up to 2 mm. When muscle fibers of the cheek were stimulated, dilations were observed to spread for distances of up to 1.5 mm. Because treatment with local anesthetics (lidocaine or procaine) blocked the spread of vasodilation, it was suggested that such responses were of neural origin (Duling and Berne, 1970). Nevertheless, the low velocity of propagation (0.02 cm/s) appeared to be inconsistent with propagation along nerve fibers. Thus it remained unclear whether neural propagation could explain the ability of vasodilation to travel along arterioles over much greater distances than could be explained by simple diffusion of a vasoactive substance.
Nearly fifteen years elapsed before new experiments were undertaken in the hamster cheek pouch microcirculation to investigate the mechanism by which vasodilation could spread along arterioles. Using tetrodotoxin to inhibit neural propagation, arteriolar segments ~500 μm long were occluded at each end to prevent changes in blood flow or transmural pressure (Segal and Duling, 1986b). Remarkably, in response to ACh microiontophoresis, vasodilation initiated beyond one end of the segment produced vasodilation beyond the other end of the occluded segment. These findings demonstrated that, independent of neural activity, a vasodilatory signal was conducted along the arteriole by a mechanism of intercellular communication and that this mechanism could coordinate diameter changes along resistance vessels controlling tissue blood flow (Segal and Duling, 1986b).
The nature of conducted vasodilation
Conduction vs. propagation
To provide mechanistic insight into the nature of conducted vasomotor responses, ensuing experiments delivered inhibitory agents to a segment of an arteriole via micropipette positioned midway between the site of initiating vasodilation (with ACh) and remote locations observed 1-2 mm upstream. It should be recognized that while conduction is bidirectional along arterioles (Duling and Berne, 1970, Budel et al., 2003), remote sites have characteristically been observed upstream to avoid the possibility of convection of vasoactive agonists in the flow stream directly affecting regions of interest. Local treatment of an arteriole with atropine (a muscarinic receptor antagonist) delivered from a micropipette eliminated responses to ACh at the treated site but had no effect on the ability of vasodilation to travel through the site when initiated further along the arteriole (Segal and Duling, 1989). This finding indicated that while ACh readily initiates CVD (Figure 2), cholinergic signaling was not involved in mediating the spread of vasodilation. Complementary experiments delivered a depolarizing solution of KCl between the ACh stimulus and the observation site and demonstrated that vasodilation conducted up to but not beyond the region of KCl treatment, thereby implicating hyperpolarization as the signal transmitted along the vessel wall (Segal and Duling, 1989)(Figure 2a). Similar treatment with purported uncouplers of gap junctions also attenuated CVD at remote sites. These observations collectively suggested that CVD was intrinsic to the arteriolar wall with the spread of hyperpolarization mediated by gap junctions coupling SMCs, ECs or both (Segal and Duling, 1989)(Figure 2b).
Once it became apparent that intercellular communication rather than action potentials was the mechanism of signal transmission along the vessel wall, the term “conduction” was recognized as more accurate than “propagation” when describing remote vasodilation in response to local stimulation of arterioles (Segal and Duling, 1989). As developed below, subsequent studies have consistently reinforced this interpretation. It should be noted that vasoconstriction [e.g., in response to KCl, norepinephrine (NE) or electrical stimulation] can also travel along arterioles by intercellular conduction (Gustafsson and Holstein-Rathlou, 1999, Segal et al., 1989, Segal and Duling, 1989, Steinhausen et al., 1997, Hungerford et al., 2000, de Wit et al., 2000, Figueroa et al., 2003). While conducted vasoconstriction has been shown to interact with and attenuate CVD (Segal et al., 1989), it appears less robust over distance and more variable than CVD (Segal et al., 1999, Gustafsson and Holstein-Rathlou, 1999, de Wit et al., 2000, Hungerford et al., 2000, Kumer et al., 2000, Figueroa et al., 2003). Moreover, the present discussion presents CVD as a mechanism for increasing tissue blood flow; e.g., to exercising skeletal muscle (Figure 1). Thus a key feature of CVD is the ability for spatially distinct sites of stimulation within arteriolar networks to summate (Segal et al., 1989, Rivers, 1997b) in parent vessels. In such fashion, additive effects originating within arteriolar networks effectively promote ascending dilation into their parent feed arteries, thereby increasing the total delivery of blood flowing into arteriolar networks (Folkow et al., 1971, Segal and Duling, 1986b, Williams and Segal, 1993, Segal and Jacobs, 2001).
Intercellular signaling through gap junctions
The expression and role of gap junctions in the microvascular wall is summarized here briefly in the context of CVD. Gap junction channels couple adjacent cells and consist of two connexon hemichannels in series (Unger et al., 1999). Each hemichannel consists of six connexin (Cx) protein subunits and the Cx isoforms identified in blood vessels are Cx37, Cx40, Cx43 and Cx45 (Little et al., 1995a, Hill et al., 2002, Sandow et al., 2003, Looft-Wilson et al., 2004, Figueroa et al., 2004, Hakim et al., 2008, Figueroa and Duling, 2009). Each neighboring cell contributes a respective hemichannel and these hemichannels dock with each other to form a continuous pore (diameter, ~9 nm) between cells that is shielded from the extracellular space (Unger et al., 1999). Gap junctions are typically organized into plaques identified as pentalaminar electron-dense regions between cells (Sandow and Hill, 2000, Sandow et al., 2003) that allow direct passage of ions and small molecules (< 1KDa; e.g., Ca2+ and IP3) between coupled cells.
Cell-to-cell coupling through gap junctions within a single type of vascular cell (EC-EC or SMC-SMC) is recognized as homocellular coupling. Whereas gap junctions surround the borders of ECs in feed arteries and arterioles and are readily observed with immunostaining, they are more difficult to discern in surrounding SMC (Sandow et al., 2003, Looft-Wilson et al., 2004, Hakim et al., 2008). Further, heterocellular (myoendothelial) gap junctions (Figure 2a) enable electrical coupling and Ca2+ signaling and between respective cell layers of the microvessel wall (Little et al., 1995b, Xia et al., 1995, Emerson and Segal, 2000a, Yashiro and Duling, 2000, Yamamoto et al., 2001, Heberlein et al., 2009) as well as in vascular cell co-culture (Isakson and Duling, 2005, Isakson et al., 2007). In mouse mesenteric arteries, Ca2+ “pulsars” were found to be localized in membrane domains of ECs that project through the internal elastic lamina (IEL) to adjacent SMC and co-localized with intermediate-conductance Ca2+-sensitive potassium channels (IKCa), suggesting that myoendothelial junctions form spatially-restricted intercellular signaling domains in the vessel wall (Ledoux et al., 2008). This interpretation is consistent with ultrastructural localization of IKCa with Cx37 and Cx40 at myoendothelial junctions in the rat mesenteric artery (Sandow et al., 2006). In hamster cheek pouch and cremaster muscle arterioles responding to SMC activation (e.g., with phenylephrine), the subsequent rise in intracellular Ca2+ of underlying ECs may thereby provide negative feedback to attenuate vasoconstriction, e.g. by activating nitric oxide synthase or KCa channels to attenuate vasoconstriction (Dora et al., 1997, Yashiro and Duling, 2000).
Directionality in myoendothelial signaling (e.g., from SMCs to ECs or vice versa) may well reflect the differential expression and regulation of Cx isoforms in respective connexons as well as in the expression and localization of associated signaling complexes (Isakson et al., 2007, Kansui et al., 2008, Figueroa et al., 2004, Heberlein et al., 2009, Ledoux et al., 2006). Further, the absence of dye coupling between neighboring cells does not preclude intercellular transfer of ions (i.e., electrical coupling) or small molecules because results from dye coupling experiments can vary with the structure and charge of the dye being studied (Ransom and Kettenmann, 1990, Segal and Beny, 1992, Little et al., 1995b) as well as the profile of Cx expression. The advent of genetic knock-out technology in the mouse has implicated a crucial role for Cx40 in CVD. Whereas deletion of Cx37 did not attenuate CVD to ACh (Figueroa and Duling, 2008), deletion of Cx 40 attenuated CVD to both ACh and electrical stimulation in addition to producing hypertension (de Wit et al., 2000, Figueroa et al., 2003). Replacing Cx40 with Cx45 attenuated hypertension but CVD was still impaired (Wolfle et al., 2007). Such findings suggest that respective Cx isoforms, their regulation [e.g., by phosphorylation (Lampe et al., 2000)], and/or the signaling complexes with which they associate may all serve to contribute unique properties to CVD in vivo.
Cellular pathway(s) for conduction
Ultrastructural studies of resistance networks in the fascia of rabbit skeletal muscles (Rhodin, 1967) illustrated that the media of arterioles is comprised of a single layer of SMC wrapped circumferentially around the intima, with respective cell layers separated by the IEL. Perforations in the IEL enable cells in respective layers to send projections that physically contact each other through myoendothelial junctions (MEJs) (Rhodin, 1967, Heberlein et al., 2009). Morphological analysis of arterioles and feed arteries from tissues of the hamster and rat have provided more detailed information regarding how ECs and SMCs are organized along resistance microvessels (Haas and Duling, 1997, Sandow et al., 2003). Individual arteriolar EC are 5-7 μm wide and can exceed 100 μm in length, whereas individual SMCs (when relaxed) are of similar width and nearly as long. These dimensions enable individual ECs to lie subjacent to and directly interact with ~20 SMCs along the vessel media and individual SMCs to interact with a similar number of ECs as they circumscribe the intima (Emerson and Segal, 2000a, Haas and Duling, 1997). The feed arteries which give rise to arterioles have similar wall morphology (Emerson and Segal, 2000b, Sandow et al., 2003).
In accord with intercellular dye transfer following microinjection (Little et al., 1995b, Segal and Beny, 1992, Welsh and Segal, 1998) robust gap junction expression (Hakim et al., 2008, Looft-Wilson et al., 2004, Wolfle et al., 2007) provides low coupling resistance between longitudinally-oriented ECs (Haas and Duling, 1997) and favors the endothelium as the predominant cellular pathway for conducting vasoactive signals along the vessel wall (Figure 2a). Indeed, the monolayer of ECs surrounded by a single layer of SMCs establishes a structural precedent for longitudinal signaling along the vessel axis. In contrast, larger conduit arteries have multiple lamella of SMC that can dissipate electrical signals circumferentially (i.e., in a radial direction) and thereby attenuate conduction along the vessel axis (Beny, 1999).
Functional studies confirm that the integrity of the endothelium is integral to CVD. Selective disruption of ECs within a discrete segment of hamster retractor muscle feed arteries studied in vivo (Segal and Jacobs, 2001) or in vitro (Emerson and Segal, 2000b) prevented vasodilation from conducting through the region of EC damage. In contrast, selective disruption of surrounding SMCs was without effect on CVD (Emerson and Segal, 2000b) as the signal was transmitted along the intact endothelium. Complementary studies in arterioles of the mouse cremaster muscle (Looft-Wilson et al., 2004) provided further evidence that integrity of the endothelium is required for effective CVD. Computational modeling accounting for the biophysical properties of individual ECs and SMCs illustrates that the two key features which enable the endothelium to function in this manner are their longitudinal orientation and their low resistance to electrical coupling (Diep et al., 2005). In turn, the high input resistance of SMCs enables charge transfer from the endothelium through myoendothelial gap junctions with little dissipation of the electrical signal along the vessel wall (Figure 2a). From this perspective and in light of earlier discussion, the more variable and less effective conduction of vasoconstriction can be attributed to an agonist activating SMC with little or no change in membrane potential [i.e., through Ca2+ sensitization vs. depolarization (Somlyo and Somlyo, 2003, Mufti et al.)], high coupling resistance (due to paucity of gap junctions) between adjacent SMCs together with their circumferential (vs. longitudinal) orientation, and the dissipation of charge into the underlying endothelium through myoendothelial coupling (Diep et al., 2005, Tran et al., 2009).
Myoendothelial coupling has been demonstrated consistently in isolated microvessel preparations studied in vitro (Xia et al., 1995, Emerson and Segal, 2000a, Yamamoto et al., 2001) however not all studies support a role for such direct signaling between respective cell layers. In arterioles controlling blood flow to the hamster cheek pouch, intracellular recording was performed with dye labeling to identify the cell type recorded from (Segal and Beny, 1992). Microiontophoresis of either phenylephrine or NE induced conducted depolarization and vasoconstriction. Remarkably, depolarization was confined to the SMC layer (Welsh and Segal, 1998). Conversely, finding that ACh induced conducted hyperpolarization in both ECs and SMCs suggested that, in the absence of myoendothelial coupling, respective cell layers provided parallel paths for conduction (Welsh and Segal, 1998). Such behavior was confirmed by selectively damaging discrete regions of either ECs or SMCs using light-dye treatment (Bartlett and Segal, 2000, Budel et al., 2003). Experiments performed on arterioles in the mouse cremaster muscle illustrated differences in membrane potential, responsiveness to KCa channel antagonists, and vasoactive agents between EC and SMC layers (Siegl et al., 2005) providing further evidence against myoendothelial coupling in arterioles controlling blood flow in vivo. By comparing CVD in response to ACh vs. adenosine in cremaster muscle arterioles of mice deficient in Cx40 and Cx37 and exposed to an array of ion channel antagonists, recent findings suggest that conducted responses to ACh preferentially conduct along the endothelium whereas smooth muscle serves as the signaling pathway for adenosine (de Wit, 2009). Given that myoendothelial junctions are present in arterioles and feed arteries (Emerson and Segal, 2000a, Heberlein et al., 2009, Rhodin, 1967, Sandow et al., 2003), and that myoendothelial coupling has been consistently reported in vitro (above), the apparent lack of myoendothelial coupling found in vivo (de Wit, 2009, Siegl et al., 2005, Welsh and Segal, 1998) suggests that myoendothelial coupling is regulated under physiological conditions and that such regulation may be altered during isolation and cannulation for in vitro studies. However definitive (and quite challenging) experiments remain to be performed to test this hypothesis.
Variability in the initiation of CVD
Of numerous agonists tested for their ability to initiate vasodilation (histamine, K+, H+ and eledoisin), the muscarinic receptor agonists ACh, methacoline and muscarine have most consistently induced CVD (Duling and Berne, 1970, Delashaw and Duling, 1991, Rivers, 1997a). A subset of agonists including bradykinin, substance P, papaverine, isoproterenol, and adenosine have been found to initiate CVD albeit with considerable variability. The nitric oxide (NO) donor sodium nitroprusside routinely produces vasodilation at the site of delivery without initiating CVD (Delashaw and Duling, 1991, Kurjiaka and Segal, 1995a, Hoepfl et al., 2002, Tallini et al., 2007).
Heterogeneity in the ability to initiate CVD may be attributed to two major factors. Foremost is whether vasomotor tone is present. When vasomotor tone is lacking, vessels are pre-contracted with agonists to study vasodilation but such treatment may alter the behavior of CVD (Haug and Segal, 2005). Nevertheless, as shown using dual microelectrodes for simultaneously injecting current in one cell while recording from a remote cell in arterioles from guinea pig submucosa, electrical signals can readily travel from cell to cell in the absence of a vasomotor response (Hirst and Neild, 1978). In feed arteries of the hamster retractor muscle studied at low transmural pressure [25 mmHg vs. 60-70 mmHg in vivo (Welsh and Segal, 1996)], the inability of ACh to evoke CVD in the absence of myogenic tone was overcome by activating SMCs with NE (Walker and Segal, 1998). However, perivascular sympathetic nerves can attenuate CVD through activating α-adrenoreceptors (Kurjiaka and Segal, 1995b, Haug et al., 2003, Haug and Segal, 2005, Moore et al., 2010). These findings suggest that adrenergic signaling can suppress intercellular conduction, e.g., by phosphorylation of connexin subunits to promote closure of gap junctions (Lampe and Lau, 2000) and/or by opening ion channels in SMCs to dissipate current (Jackson, 2005, Nelson et al., 1990). However, additional studies will be required to definitely resolve such effects in the microcirculation.
A second factor critical to determining whether a stimulus can evoke CVD is whether it can activate ion channels, thereby triggering sufficient ion flux to produce an electrical signal that can be transmitted along the vessel wall (Figure 2a). Indeed, direct injection of hyperpolarizing current into an EC of isolated pressurized feed arteries produced robust CVD independent of receptor activation (Emerson and Segal, 2001). Moreover, electrical stimulation has proven to be a useful tool for initiating conducted responses in a variety of experimental preparations (Emerson and Segal, 2001, Figueroa et al., 2003, Steinhausen et al., 1997) without activating membrane receptors and associated signaling events. Further insight is likely to be gained through more selective mechanisms of initiating and modulating the signaling events that underlie CVD.
Signaling pathways contributing to CVD
The preceding discussion implicates charge movement (i.e., electrical signaling) through gap junctions as the basis of initiating CVD. Acetylcholine has proven to be the most reliable agonist in this regard and its mechanism of action is well described. Upon binding to muscarinic (G-protein coupled) receptors on the endothelium, phospholipase activation generates inositol 1,4,5-trisphospate (IP3) to release Ca2+ from the endoplasmic reticulum (Figure 2b) (Himmel et al., 1993, Danthuluri et al., 1988) In turn, intermediate- and small-conductance Ca2+-activated potassium channels (IKCa and SKCa, respectively) results in hyperpolarization (Busse et al., 1988, Olesen et al., 1988, Sakai, 1990, Jackson, 2005, Ledoux et al., 2006, Domeier and Segal, 2007, Wolfle et al., 2009). The resulting flow of current along the endothelium and into SMCs promotes closure of voltage-gated Ca2+channels, relaxing SMCs to produce vasodilation (Ledoux et al., 2006, Nelson et al., 1990) (Figure 2b). Recent findings in mice illustrate a key role for IKCa (KCa3.1) in hyperpolarization to ACh and for maintaining CVD along the arteriolar wall (Wolfle et al., 2009). A complex interplay between voltage-dependent Na+ channels and Ca2+channels has also been proposed to contribute to CVD in mouse cremaster arterioles (Figueroa et al., 2007). However definitive functional measurements are required to substantiate roles for respective ion channels in mediating CVD. Nevertheless, the electrical nature of CVD explains how it can rapidly coordinate SMC relaxation along the vessel wall (Welsh and Segal, 1998, Emerson and Segal, 2000a, Diep et al., 2005, Wolfle et al., 2009).
Complementary signaling events contributing to CVD have been most apparent in response to activating muscarinic receptors on arteriolar ECs. In isolated arterioles of the hamster cheek pouch, the inhibition of nitric NO synthase revealed a component of CVD that was mediated by NO (Doyle and Duling, 1997). Further, the presence of a NO “wave” was illustrated for cheek pouch arterioles studied in vivo using selective local disruption of SMC or EC in combination with NO synthase inhibition (Budel et al., 2003). In isolated feed arteries of the hamster retractor muscle, the inhibition of IKCa and SKCa prevented the initiation of hyperpolarization, thereby revealing a Ca2+ wave that was triggered by ACh and traveled along the endothelium to release NO (Domeier and Segal, 2007, Uhrenholt et al., 2007), albeit at a slower conduction velocity and over shorter distances then encompassed by electrical signaling. Complementary studies in Cx40BAC-GCaMP2 mice suggest a similar role for Ca2+ waves along arterioles controlling blood flow to the mouse cremaster muscle (Tallini et al., 2007). Although the inhibition of NO synthase has been found to attenuate the duration of CVD (Segal et al., 1999, Hungerford et al., 2000, Domeier and Segal, 2007), enhanced NO production during sepsis (McKinnon et al., 2006) or histamine exposure (Payne et al., 2004) inhibited conducted responses in mouse cremaster arterioles. Thus while NO readily evokes local vasodilation and contributes to CVD, it does not elicit conducted responses by itself (Delashaw and Duling, 1991, Kurjiaka and Segal, 1995a, Hoepfl et al., 2002, Tallini et al., 2007). Further, when NO production is enhanced in response to inflammatory stimuli it can exert an inhibitory effect on conduction. Such differences in findings among studies indicate that additional experiments are required to define the precise role(s) of NO in cell-to-cell signaling along the vessel wall.
Independent investigators have implicated a role for endothelium-derived hyperpolarizing factor (EDHF) in contributing to CVD. Identified as a cytochrome P-450 metabolite of arachidonic acid (Campbell and Harder, 1999), inhibition of this pathway (e.g., with 17-ODYA or sulfaphenazole) significantly attenuate CVD along arterioles in the hamster cheek pouch (Welsh and Segal, 2000) as well as arterioles in the cremaster muscle of the hamster (de Wit et al., 1999, Hoepfl et al., 2002) and mouse (Hungerford et al., 2000). In light of the ability of elevated extracellular K+ (e.g., 10-15 mM) to promote hyperpolarization [e.g., by activating inward-rectifying K+ channels (Jackson, 2005)], K+ has also been implicated as an EDHF (Edwards et al., 1998). Alternatively, EDHF may actually reflect myoendothelial coupling (Emerson and Segal, 2000a, Yamamoto et al., 2001, Figueroa and Duling, 2009) or regulation thereof. Nevertheless, the preceding discussion collectively illustrates that multiple signaling pathways can contribute to CVD.
Passive decay vs. ion channel activation
Early studies of conduction illustrated that vasomotor responses at the site of stimulation were greater than those at remote sites and were attributed to direct actions of the stimulus locally with passive decay of the signal along the arteriolar wall (Segal et al., 1989, Segal and Duling, 1986b). Ensuing studies confirmed that vasoconstriction (and depolarization) decay with distance for reasons discussed earlier and the dissipation of depolarization along arterioles increases with network branching (Segal and Neild, 1996). In contrast, CVD (and hyperpolarization) encompass far greater distances than can be explained by a purely passive mechanism of conduction. In response to ACh, the length constant describing the decay of hyperpolarization was greater than that determined for intracellular injection of hyperpolarizing current (Emerson et al., 2002). In arterioles of the hamster cheek pouch, hyperpolarizing responses to ACh increased with distance whether recorded from SMC or EC, suggesting the contribution of “active” membrane processes (Crane et al., 2004). Recent studies in the mouse cremaster suggest that such behavior may be explained by the interaction between voltage-dependent ion channels in the endothelium (Figueroa et al., 2007). Complementary studies illustrated that Cx40 was integral to non-decremental CVD initiated by ACh, suggesting the activation of a mechanism for regenerative conduction (Figueroa and Duling, 2008). Recent findings indicate that Cx40 along with SKCa channels play a crucial role in active hyperemia (Milkau et al., 2010). Alternatively, non-decremental CVD along feed arteries of the hamster retractor muscle has been attributed to expression of inward rectifying K+ channels (KIR) in SMC (Jantzi et al., 2006). The ability of KIR to increase K+ efflux as the membrane hyperpolarizes (e.g., from a resting potential of ~-35 mV) (Jackson, 2005, Nelson et al., 1990) enables them to contribute additional hyperpolarizing current to that originating from the endothelium (e.g., in response to ACh; Figure 2b). Coupled with the ability of CVD triggered from multiple sites in daughter arterioles to sum together in parent vessels (Segal et al., 1989), such integrated behavior underscores the ability of vasodilation arising within arteriolar networks to ascend into and along feed arteries, thereby increasing total blood flow into exercising skeletal muscle (Segal and Jacobs, 2001, Folkow et al., 1971)
A Role for Capillaries in Conducted Responses
In support of Krogh's original studies concerned with the role of capillaries in spreading vasodilation of the frog web, an accumulating body of evidence has implicated capillaries as a site from which vasomotor responses in arterioles can be triggered. For example, focal stimulation of capillaries in the rat mesentery (Dietrich, 1989), rat tibialis anterior muscle or frog sartorius muscle (Dietrich and Tyml, 1992b) with NE released from a micropipette resulted in a reduction in capillary red blood cell velocity implying constriction of arterioles upstream. Complementary experiments (Dietrich and Tyml, 1992a) excluded roles for nerve propagation or venular- arteriolar diffusion of the agonist (Tigno et al., 1989, Hester, 1990), implicating the capillary itself as a pathway for communicating with arterioles. Subsequent studies of capillaries in the frog sartorius muscle confirmed this behavior while showing that ACh delivered from a micropipette increased capillary red blood cell velocity along with arteriolar diameter (Song and Tyml, 1993). Moreover, NE and ACh delivered simultaneously onto different capillaries fed by the same arteriole attenuated the response to ACh. This behavior is consistent with cancellation of conducted vasodilation and conducted vasoconstriction in parent arterioles when both responses were initiated simultaneously on paired daughter branches (Segal et al., 1989).
Using a voltage-sensitive dye (di-8-ANEPPS), capillaries in the hamster cheek pouch depolarized in response to KCl or phenylephrine and hyperpolarized in response to ACh when these agonists were ejected from micropipettes (Beach et al., 1998, McGahren et al., 1998). These observations suggest that changes in membrane potential serve as a mechanism by which capillaries communicate with arterioles in the local control of blood flow and oxygen delivery. In the hamster cremaster muscle, stimulating terminal arterioles that were constricted and devoid of flow under resting conditions resulted in red blood cell perfusion of dependent capillaries (Segal, 1991), illustrating a key role for CVD in capillary recruitment to enhance blood flow to skeletal muscle fibers. In turn, stimulating small bundles of muscle fibers in the vicinity of capillary “modules” was observed to produce dilation of arterioles upstream from the active myocytes (Berg et al., 1997, Cohen et al., 2000, Murrant and Sarelius, 2000), illustrating functional connectivity between active muscle fibers, their surrounding capillaries and the arterioles which control capillary perfusion.
Summary and Conclusion
The endothelium functions as a highly effective pathway for the conduction of electrical signals in the microcirculation. Vasoactive signals originating in capillaries can govern capillary blood flow by signaling to the arterioles from which they originate. Thus arteriolar networks can be viewed as integrating signals arising from their most distal branches and then generating responses that ascend into their parent feed arteries (Figure 1). Thus the ability to integrate vasoactive signals and coordinate vasomotor response reflects a myriad of intercellular signaling that is intrinsic to the resistance vasculature. Moreover, these signaling pathways underlie the ability to distribute and increase blood flow in accord with local metabolic demand. As shown for a variety of tissues including skeletal muscle (Segal and Duling, 1986a, Segal, 1991, Williams and Segal, 1993, Berg et al., 1997, Segal and Jacobs, 2001, Moore et al., 2010), brain (Dietrich et al., 2009, Iadecola et al., 1997, Ngai et al., 2007), kidney (Chen et al., 1995, Steinhausen et al., 1997), mesentery (Gustafsson and Holstein-Rathlou, 1999) and lymphatics (Zawieja et al., 1993), the conduction of vasomotor responses may serve as a universal mechanism of blood flow control in tissues throughout the body. Intercellular conduction is impaired in a variety of pathophysiological states (Lidington et al., 2002, de With et al., 2005, Rose et al., 2005, McKinnon et al., 2006), with aging (Bearden et al., 2004, Bearden et al., 2007, Jackson et al., 2010) and during enhanced sympathetic nerve activity (Haug and Segal, 2005) which is associated with aging, obesity and hypertension. Therefore continued efforts to understand the basic mechanisms behind CVD and its (patho)physiological regulation promise to have significant applications in health and disease.
Acknowledgements
The authors’ research is supported by grants R37-HL041026, R01-HL056786, R01-HL086483 (to S.S.S.), F32-HL097463 and T32-AR048523 (to P.B.) from the Heart, Lung and Blood Institute of the National Institutes of Health, United States Public Health Service.
Footnotes
Conflict of Interest
There are no conflicts of interest to declare by either author.
References
- Bartlett IS, Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H604–H612. doi: 10.1152/ajpheart.2000.278.2.H604. [DOI] [PubMed] [Google Scholar]
- Beach JM, McGahren ED, Duling BR. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am J Physiol Heart Circ Physiol. 1998;275:H1489–H1496. doi: 10.1152/ajpheart.1998.275.4.H1489. [DOI] [PubMed] [Google Scholar]
- Bearden SE, Linn E, Ashley BS, Looft-Wilson RC. Age-related changes in conducted vasodilation: effects of exercise training and role in functional hyperemia. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1717–R1721. doi: 10.1152/ajpregu.00827.2006. [DOI] [PubMed] [Google Scholar]
- Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561:535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beny JL. Information Networks in the Arterial Wall. News Physiol Sci. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol Heart Circ Physiol. 1997;272:H2693–H2700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- Bohlen HG, Gore RW, Hutchins PM. Comparison of microvascular pressures in normal and spontaneously hypertensive rats. Microvasc Res. 1977;13:125–130. doi: 10.1016/0026-2862(77)90121-2. [DOI] [PubMed] [Google Scholar]
- Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res. 2003;93:61–68. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Prosser CL. Conduction in smooth muscles: comparative electrical properties. Am J Physiol. 1960;199:553–559. doi: 10.1152/ajplegacy.1960.199.3.553. [DOI] [PubMed] [Google Scholar]
- Busse R, Fichtner H, Luckhoff A, Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol Heart Circ Physiol. 1988;255:H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res. 1999;84:484–488. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- Chen YM, Yip KP, Marsh DJ, Holstein-Rathlou NH. Magnitude of TGF-initiated nephron-nephron interactions is increased in SHR. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;269:F198–F204. doi: 10.1152/ajprenal.1995.269.2.F198. [DOI] [PubMed] [Google Scholar]
- Cohen KD, Berg BR, Sarelius IH. Remote arteriolar dilations in response to muscle contraction under capillaries. Am J Physiol Heart Circ Physiol. 2000;278:H1916–H1923. doi: 10.1152/ajpheart.2000.278.6.H1916. [DOI] [PubMed] [Google Scholar]
- Crane GJ, Neild TO, Segal SS. Contribution of Active Membrane Processes to Conducted Hyperpolarization in Arterioles of Hamster Cheek Pouch. Microcirculation. 2004;11:425–433. doi: 10.1080/10739680490457836. [DOI] [PubMed] [Google Scholar]
- Danthuluri NR, Cybulsky MI, Brock TA. ACh-induced calcium transients in primary cultures of rabbit aortic endothelial cells. Am J Physiol Heart Circ Physiol. 1988;255:H1549–H1553. doi: 10.1152/ajpheart.1988.255.6.H1549. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Ferrer PN, Gore RW. Vascular anatomy and hydrostatic pressure profile in the hamster cheek pouch. Am J Physiol Heart Circ Physiol. 1986;250:H291–H303. doi: 10.1152/ajpheart.1986.250.2.H291. [DOI] [PubMed] [Google Scholar]
- de Wit C. Different pathways with distinct properties conduct dilations in the microcirculation in vivo. Cardiovasc Res. 2009;85:604–613. doi: 10.1093/cvr/cvp340. [DOI] [PubMed] [Google Scholar]
- de Wit C, Esser N, Lehr HA, Bolz SS, Pohl U. Pentobarbital-sensitive EDHF comediates ACh-induced arteriolar dilation in the hamster microcirculation. Am J Physiol Heart Circ Physiol. 1999;276:H1527–H1534. doi: 10.1152/ajpheart.1999.276.5.H1527. [DOI] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- de With MC, Haug SJ, Brigitte van der Heijden EP, Segal SS. Ischemia-reperfusion impairs ascending vasodilation in feed arteries of hamster skeletal muscle. Microcirculation. 2005;12:551–561. doi: 10.1080/10739680500253451. [DOI] [PubMed] [Google Scholar]
- Delashaw JB, Duling BR. Heterogeneity in conducted arteriolar vasomotor response is agonist dependent. Am J Physiol Heart Circ Physiol. 1991;260:H1276–H1282. doi: 10.1152/ajpheart.1991.260.4.H1276. [DOI] [PubMed] [Google Scholar]
- Diep HK, Vigmond EJ, Segal SS, Welsh DG. Defining electrical communication in skeletal muscle resistance arteries: a computational approach. J Physiol. 2005;568:267–81. doi: 10.1113/jphysiol.2005.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich HH. Effect of locally applied epinephrine and norepinephrine on blood flow and diameter in capillaries of rat mesentery. Microvasc Res. 1989;38:125–135. doi: 10.1016/0026-2862(89)90021-6. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Horiuchi T, Xiang C, Hongo K, Falck JR, Dacey RG., Jr. Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vasc Res. 2009;46:253–264. doi: 10.1159/000167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich HH, Tyml K. Capillary as a communicating medium in the microvasculature. Microvasc Res. 1992a;43:87–99. doi: 10.1016/0026-2862(92)90008-d. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Tyml K. Microvascular flow response to localized application of norepinephrine on capillaries in rat and frog skeletal muscle. Microvasc Res. 1992b;43:73–86. doi: 10.1016/0026-2862(92)90007-c. [DOI] [PubMed] [Google Scholar]
- Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MP, Duling BR. Acetylcholine induces conducted vasodilation by nitric oxide-dependent and -independent mechanisms. Am J Physiol Heart Circ Physiol. 1997;272:H1364–H1371. doi: 10.1152/ajpheart.1997.272.3.H1364. [DOI] [PubMed] [Google Scholar]
- Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res. 1970;26:163–70. doi: 10.1161/01.res.26.2.163. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Neild TO, Segal SS. Conduction of hyperpolarization along hamster feed arteries: augmentation by acetylcholine. Am J Physiol Heart Circ Physiol. 2002;283:H102–H109. doi: 10.1152/ajpheart.00038.2002. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000a;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000b;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–H167. doi: 10.1152/ajpheart.2001.280.1.H160. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol. 2007;293:H1371–H1383. doi: 10.1152/ajpheart.01368.2006. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295:H2001–H2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology. 2004;19:277–284. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- Folkow B, Sonnenschein RR, Wright DL. Loci of neurogenic and metabolic effects on precapillary vessels of skeletal muscle. Acta Physiol Scand. 1971;81:459–471. doi: 10.1111/j.1748-1716.1971.tb04924.x. [DOI] [PubMed] [Google Scholar]
- Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol. 1975;228:791–796. doi: 10.1152/ajplegacy.1975.228.3.791. [DOI] [PubMed] [Google Scholar]
- Gorczynski RJ, Klitzman B, Duling BR. Interrelations between contracting striated muscle and precapillary microvessels. Am J Physiol Heart Circ Physiol. 1978;235:H494–H504. doi: 10.1152/ajpheart.1978.235.5.H494. [DOI] [PubMed] [Google Scholar]
- Gustafsson F, Holstein-Rathlou NH. Angiotensin II modulates conducted vasoconstriction to norepinephrine and local electrical stimulation in rat mesenteric arterioles. Cardiovasc Res. 1999;44:176–184. doi: 10.1016/s0008-6363(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- Hakim CH, Jackson WF, Segal SS. Connexin Isoform Expression in Smooth Muscle Cells and Endothelial Cells of Hamster Cheek Pouch Arterioles and Retractor Feed Arteries. Microcirculation. 2008;15:503–514. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of α1- and α2-adrenoreceptor activation. J Physiol. 2005;563:541–555. doi: 10.1113/jphysiol.2004.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug SJ, Welsh DG, Segal SS. Sympathetic nerves inhibit conducted vasodilatation along feed arteries during passive stretch of hamster skeletal muscle. J Physiol. 2003;552:273–282. doi: 10.1113/jphysiol.2003.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation. 2009;16:307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RL. Venular-arteriolar diffusion of adenosine in hamster cremaster microcirculation. Am J Physiol Heart Circ Physiol. 1990;258:H1918–H1924. doi: 10.1152/ajpheart.1990.258.6.H1918. [DOI] [PubMed] [Google Scholar]
- Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol. 2002;29:620–625. doi: 10.1046/j.1440-1681.2002.03699.x. [DOI] [PubMed] [Google Scholar]
- Hilton SM. A peripheral arterial conducting mechanism underlying dilatation of the femoral artery and concerned in functional vasodilatation in skeletal muscle. J Physiol. 1959;149:93–111. doi: 10.1113/jphysiol.1959.sp006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfl B, Rodenwaldt B, Pohl U, De Wit C. EDHF, but not NO or prostaglandins, is critical to evoke a conducted dilation upon ACh in hamster arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H996–H1004. doi: 10.1152/ajpheart.01082.2001. [DOI] [PubMed] [Google Scholar]
- Hungerford JE, Sessa WC, Segal SS. Vasomotor control in arterioles of the mouse cremaster muscle. FASEB J. 2000;14:197–207. doi: 10.1096/fasebj.14.1.197. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol. 2010;588:2269–2282. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–27. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291:H1319–H1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- Johansson B, Ljung B. Spread of excitation in the smooth muscle of the rat portal vein. Acta Physiol Scand. 1967;70:312–322. doi: 10.1111/j.1748-1716.1967.tb03631.x. [DOI] [PubMed] [Google Scholar]
- Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayutin VM, Sonina RS, Frolenkov GI, Zizin IM. Antidromic vasodilation in frog: identification of the nerve fiber types involved. Pflugers Arch. 1991;419:508–513. doi: 10.1007/BF00370797. [DOI] [PubMed] [Google Scholar]
- Krogh A, Harrop GA, Rehberg PB. Studies on the physiology of capillaries. III. The innervation of the blood vessels in the hind legs of the frog. J Physiol. 1922;56:179–189. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumer SC, Damon DN, Duling BR. Patterns of conducted vasomotor response in the mouse. Microvasc Res. 2000;59:310–315. doi: 10.1006/mvre.1999.2198. [DOI] [PubMed] [Google Scholar]
- Kurjiaka DT, Segal SS. Conducted vasodilation elevates flow in arteriole networks of hamster striated muscle. Am J Physiol Heart Circ Physiol. 1995a;269:H1723–1728. doi: 10.1152/ajpheart.1995.269.5.H1723. [DOI] [PubMed] [Google Scholar]
- Kurjiaka DT, Segal SS. Interaction between conducted vasodilation and sympathetic nerve activation in arterioles of hamster striated muscle. Circ Res. 1995b;76:885–891. doi: 10.1161/01.res.76.5.885. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Lidington D, Tyml K, Ouellette Y. Lipopolysaccharide-induced reductions in cellular coupling correlate with tyrosine phosphorylation of connexin 43. J Cell Physiol. 2002;193:373–379. doi: 10.1002/jcp.10179. [DOI] [PubMed] [Google Scholar]
- Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995a;268:H729–39. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995b;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97:1152–11158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- McGahren ED, Beach JM, Duling BR. Capillaries demonstrate changes in membrane potential in response to pharmacological stimuli. Am J Physiol Heart Circ Physiol. 1998;274:H60–H65. doi: 10.1152/ajpheart.1998.274.1.H60. [DOI] [PubMed] [Google Scholar]
- McKinnon RL, Lidington D, Bolon M, Ouellette Y, Kidder GM, Tyml K. Reduced arteriolar conducted vasoconstriction in septic mouse cremaster muscle is mediated by nNOS-derived NO. Cardiovasc Res. 2006;69:236–244. doi: 10.1016/j.cardiores.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Milkau M, Kohler R, de Wit C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J. 2010;24:3572–3579. doi: 10.1096/fj.10-158956. [DOI] [PubMed] [Google Scholar]
- Moore AW, Bearden SE, Segal SS. Regional activation of rapid onset vasodilatation in mouse skeletal muscle: Regulation through α-adrenoreceptors. J Physiol. 2010;588:3321–3331. doi: 10.1113/jphysiol.2010.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti RE, Brett SE, Tran CH, Abd El-Rahman R, Anfinogenova Y, El-Yazbi A, Cole WC, Jones PP, Chen SR, Welsh DG. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol. 588:3983–4005. doi: 10.1113/jphysiol.2010.193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Local and remote arteriolar dilations initiated by skeletal muscle contraction. Am J Physiol Heart Circ Physiol. 2000;279:H2285–H2294. doi: 10.1152/ajpheart.2000.279.5.H2285. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Nguyen TS, Meno JR, Britz GW. Postischemic augmentation of conducted dilation in cerebral arterioles. Stroke. 2007;38:124–130. doi: 10.1161/01.STR.0000252157.93998.47. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Davies PF, Clapham DE. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988;62:1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Payne GW, Madri JA, Sessa WC, Segal SS. Histamine inhibits conducted vasodilation through endothelium-derived NO production in arterioles of mouse skeletal muscle. FASEB J. 2004;18:280–286. doi: 10.1096/fj.03-0752com. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Kettenmann H. Electrical coupling, without dye coupling, between mammalian astrocytes and oligodendrocytes in cell culture. Glia. 1990;3:258–266. doi: 10.1002/glia.440030405. [DOI] [PubMed] [Google Scholar]
- Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Rivers RJ. Components of methacholine-initiated conducted vasodilation are unaffected by arteriolar pressure. Am J Physiol Heart Circ Physiol. 1997a;272:H2895–H901. doi: 10.1152/ajpheart.1997.272.6.H2895. [DOI] [PubMed] [Google Scholar]
- Rivers RJ. Cumulative conducted vasodilation within a single arteriole and the maximum conducted response. Am J Physiol Heart Circ Physiol. 1997b;273:H310–H316. doi: 10.1152/ajpheart.1997.273.1.H310. [DOI] [PubMed] [Google Scholar]
- Rose K, Ouellette Y, Bolon M, Tyml K. Hypoxia/reoxygenation reduces microvascular endothelial cell coupling by a tyrosine and MAP kinase dependent pathway. J Cell Physiol. 2005;204:131–138. doi: 10.1002/jcp.20283. [DOI] [PubMed] [Google Scholar]
- Sakai T. Acetylcholine induces Ca-dependent K currents in rabbit endothelial cells. Jpn J Pharmacol. 1990;53:235–246. doi: 10.1254/jjp.53.235. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res. 2003;60:643–653. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt VJ, Wolfle SE, Boettcher M, de Wit C. Gap junctions synchronize vascular tone within the microcirculation. Pharmacol Rep. 2008;60:68–74. [PubMed] [Google Scholar]
- Segal SS. Microvascular recruitment in hamster striated muscle: role for conducted vasodilation. Am J Physiol Heart Circ Physiol. 1991;261:H181–H189. doi: 10.1152/ajpheart.1991.261.1.H181. [DOI] [PubMed] [Google Scholar]
- Segal SS, Beny JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol Heart Circ Physiol. 1992;263:H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- Segal SS, Damon DN, Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol Heart Circ Physiol. 1989;256:H832–H837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Communication between feed arteries and microvessels in hamster striated muscle: segmental vascular responses are functionally coordinated. Circ Res. 1986a;59:283–290. doi: 10.1161/01.res.59.3.283. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986b;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol Heart Circ Physiol. 1989;256:H838–H845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS, Neild TO. Conducted depolarization in arteriole networks of the guinea-pig small intestine: effect of branching of signal dissipation. J Physiol. 1996;496:229–244. doi: 10.1113/jphysiol.1996.sp021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS, Welsh DG, Kurjiaka DT. Spread of vasodilatation and vasoconstriction along feed arteries and arterioles of hamster skeletal muscle. J Physiol. 1999;516:283–291. doi: 10.1111/j.1469-7793.1999.283aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Weitsen HA. Cytochemical and physiological evidence for cholinergic, neurogenic vasodilation of amphibian arterioles and precapillary sphincters. I. Light microscopy. Microvasc Res. 1971;3:308–322. doi: 10.1016/0026-2862(71)90056-2. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Song H, Tyml K. Evidence for sensing and integration of biological signals by the capillary network. Am J Physiol Heart Circ Physiol. 1993;265:H1235–H1242. doi: 10.1152/ajpheart.1993.265.4.H1235. [DOI] [PubMed] [Google Scholar]
- Steinhausen M, Endlich K, Nobiling R, Parekh N, Schutt F. Electrically induced vasomotor responses and their propagation in rat renal vessels in vivo. J Physiol. 1997;505:493–501. doi: 10.1111/j.1469-7793.1997.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res. 2007;101:1300–1309. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- Tigno XT, Ley K, Pries AR, Gaehtgens P. Venulo-arteriolar communication and propagated response. A possible mechanism for local control of blood flow. Pflugers Arch. 1989;414:450–456. doi: 10.1007/BF00585056. [DOI] [PubMed] [Google Scholar]
- Tran CH, Vigmond EJ, Plane F, Welsh DG. Mechanistic basis of differential conduction in skeletal muscle arteries. J Physiol. 2009;587:1301–1318. doi: 10.1113/jphysiol.2008.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1634–H1640. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- Walker BD, Segal SS. Role of smooth muscle activation in conduction of vasodilation along isolated hamster feed arteries. J Vasc Res. 1998;35:405–412. doi: 10.1159/000025611. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circ Res. 1996;79:551–559. doi: 10.1161/01.res.79.3.551. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Role of EDHF in conduction of vasodilation along hamster cheek pouch arterioles in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1832–H1839. doi: 10.1152/ajpheart.2000.278.6.H1832. [DOI] [PubMed] [Google Scholar]
- Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res. 2007;101:1292–1299. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- Wolfle SE, Schmidt VJ, Hoyer J, Kohler R, de Wit C. Prominent role of KCa3.1 in endothelium-derived hyperpolarizing factor-type dilations and conducted responses in the microcirculation in vivo. Cardiovasc Res. 2009;82:476–483. doi: 10.1093/cvr/cvp060. [DOI] [PubMed] [Google Scholar]
- Xia J, Little TL, Duling BR. Cellular pathways of the conducted electrical response in arterioles of hamster cheek pouch in vitro. Am J Physiol Heart Circ Physiol. 1995;269:H2031–H2038. doi: 10.1152/ajpheart.1995.269.6.H2031. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol. 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro Y, Duling BR. Integrated Ca2+ signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol Heart Circ Physiol. 1993;264:H1283–H1291. doi: 10.1152/ajpheart.1993.264.4.H1283. [DOI] [PubMed] [Google Scholar]