Abstract
Purpose
Anxiety is common in patients undergoing radiation therapy (RT) and in their family caregivers (FCs). Little is known about individual differences in anxiety trajectories during and after RT. This study aimed to identify distinct latent classes of oncology patients and their FCs based on self-reported anxiety symptoms from the beginning to four months after the completion of RT.
Method
Using growth mixture modeling (GMM), longitudinal changes in Spielberger State Anxiety Inventory (STAI-S) scores among 167 oncology outpatients with breast, prostate, lung, or brain cancer and 85 FCs were evaluated to determine distinct anxiety symptom profiles. STAI-S scores were assessed just prior to, throughout the course of, and for four months following RT (total of 7 assessments). Baseline trait anxiety and depressive symptoms (during and after RT) were also assessed.
Results
The GMM analysis identified three latent classes of oncology patients and FCs with distinct trajectories of state anxiety: Low Stable (n=93, 36.9%), Intermediate Decelerating (n=82, 32.5%), and High (n=77, 30.6%) classes. Younger participants, women, ethnic minorities, and those with children at home were more likely to be classified in the High anxiety class. Higher levels of trait anxiety and depressive symptoms, at the initiation of RT, were associated with being in the High anxiety class.
Conclusions
Subgroups of patients and FCs with high, intermediate, and low mean levels of anxiety during and after RT were identified with GMM. Additional research is needed to better understand the heterogeneity of symptom experiences as well as comorbid symptoms in patients and FCs.
Keywords: psycho-oncology, anxiety, radiation therapy, family caregivers, growth mixture modeling, depression
INTRODUCTION
Approximately 50% of cancer patients receive radiation therapy (RT), either alone or in combination with surgery or chemotherapy. Clinical experience suggests that RT is associated with significant levels of anxiety that is often underdetected and undertreated (Stiegelis et al., 2004). In fact, findings from a recent review of studies on psychological distress during RT (Stiegelis et al., 2004) suggest that 10% to 20% of patients experience clinically significant levels of anxiety at the initiation of RT.
Stiegelis and colleagues’ review (2004) highlighted the considerable heterogeneity in patients’ levels of anxiety at the initiation of RT. In addition, because of the paucity of longitudinal studies, little is known about changes in levels of anxiety during and after the completion of RT. Finally, virtually no information is available on the factors that predict which patients are at greatest risk for high levels of anxiety at the initiation of and during RT. Therefore, the present report used growth mixture modeling (GMM) to identify groups of oncology patients and their family caregivers (FCs) with distinct profiles of anxiety symptoms during and after the completion of RT.
Current state of research
Only three studies have evaluated for changes in anxiety symptoms in patients who underwent RT (Andersen and Tewfik, 1985; Chen et al., 2009; Munro and Potter, 1996). Anderson and Tewfik (1985) reported that in patients with low anxiety prior to RT, anxiety increased during RT. In contrast, in patients with high baseline anxiety, anxiety scores decreased during RT. Patients with moderate anxiety before treatment experienced little change in anxiety. These findings suggest that distinct subgroups of patients with anxiety symptoms may exist. However, this study had only two measurement points, did not evaluate patients after RT, and had a small sample size. Munro and Potter (1996) assessed 110 patients with breast, lung, or head and neck cancer before, during, and 4 weeks after RT. At RT initiation, 62% endorsed anxiety, whereas only 42% endorsed anxiety post-RT. However, the severity and impact of anxiety was not reported. In 40 patients with head and neck cancer, Chen and colleagues (2009) found that 23% reported mild anxiety, 10% moderate anxiety, and 7% severe anxiety at the initiation of RT; anxiety scores remained constant during and following RT. Given the paucity of longitudinal studies on anxiety in patients undergoing RT (Andersen and Tewfik, 1985; Chen et al., 2009; Munro and Potter, 1996), these studies’ limitations, and advances made in RT since the two earlier studies were published, a need exists to examine changes in anxiety symptoms over time in patients undergoing RT.
Rationale for Studying Both Patients and Family Caregivers
Family caregivers (FCs) provide tremendous amounts of care and support (Yabroff and Kim, 2009) and are themselves at risk for psychological distress and negative physical outcomes (Institute of Medicine, 2007). In fact, some studies have reported higher rates of depression and anxiety in FCs than in patients (Couper et al., 2006). Despite the prevalence and impact of these symptoms in both groups, most studies have examined patients’ and FCs’ symptoms separately, under the assumption that the type and severity of stressors faced by the two groups are different.
However, for several reasons, patients and FCs ought to be studied in combination in a subset of studies. First, while a traditional disease model would view cancer as a primary contributor to anxiety symptoms, because of disease- and treatment-related factors, more recent evidence has found that other variables, including demographic, dispositional, and personality-related factors, appear to be robust predictors of distress, generally, and depressive symptoms more specifically (Bower, 2008; Deshields et al., 2006). On the other hand, in these studies, disease-related variables explained a relatively modest amount of variance in levels of these symptoms (Bower, 2008; Deshields et al., 2006).
Second, as described in both the cancer and non-cancer literature (Gottlieb and Rooney, 2004; Kurtz et al., 1997), FCs’ unique personal backgrounds, resources, coping styles, and chronic medical conditions influence the caregiving experience. As described by the Stress Process model of caregiver stress (Pearlin et al., 1990), these individual FC characteristics—beyond disease- and caregiving-related stressors (e.g., physical and logistic demands)—should be considered in studies of caregiver stress.
Third, the approach described here reflects and is consistent with the “allostatic load” hypothesis of stress and adaptation (McEwen and Wingfield, 2010). In this model, stress, regardless of its source, is mediated through common biological pathways. If these pathways are perturbed repeatedly or over a long duration, and if coping mechanisms cannot help the individual adapt, any individual (i.e., a patient or a FC) may manifest what would be viewed as distress, depressive symptoms, or anxiety (McEwen, 2003). The allostatic load hypothesis incorporates genetic factors that affect traits and behaviors that relate to the individual’s ability to adapt to stressors.
Finally, support for combining patients and FCs comes from recent data that suggests that patients and FCs face similar levels of overall symptom burden (Fletcher et al., 2009; Fletcher et al., 2008; Miaskowski et al., 2008). For example, in an evaluation of fatigue in patients with prostate cancer (Miaskowski et al., 2008) and their FCs (Fletcher et al., 2009), we found that while the specific predictors of fatigue differed among patients and their FCs, fatigue severity levels over six months (i.e., the overall outcome) were equivalent in both groups. Thus, our approach to understanding symptoms views cancer as one among many stressors that may influence the incidence, course, and severity, of symptoms, including anxiety in both patients and their FCs.
Rationale for examining latent classes
In most of the psychological distress literature, group means are used to characterize changes in symptoms. However, examination of changes in group means over time (for example, with repeated measures analysis of variance (ANOVA), multilevel regression, or latent growth models), are not sensitive to unobserved systematic (i.e., “latent”) patterns of change in symptom severity. Individuals’ symptom experiences may resemble those of some individuals more than they resemble those of others. In other words, a closer examination of changes in symptoms over time may reveal subgroups of individuals whose symptom experiences are more similar to those of other individuals within that subgroup than to those of individuals in other subgroups.
Recent advances in longitudinal data analysis (e.g., Muthen and Muthen, 2000) permit an examination of such underlying or latent patterns of change in symptom severity over time. One such analytic method, growth mixture modelling (GMM), enables identification of subgroups of individuals, referred to as latent growth classes, whose symptoms share a similar trajectory over time. Although this type of analysis has been used to identify latent classes in the general population with distinct trajectories of anxiety and depression (Das-Munshi et al., 2008; Hunter et al., 2010), as well as in oncology patients with distinct distress trajectories (Helgeson et al., 2004; Henselmans et al., 2010; Lam et al., 2010), GMM has not been used to evaluate differences in anxiety trajectories in oncology patients and FCs.
To better characterize anxiety symptom trajectories of patients and FCs, the purposes of this study were to determine whether we could identify distinct latent classes of oncology patients and FCs based on self-reported anxiety symptoms from prior to the initiation of to four months after the completion of RT and to examine differences in demographic and clinical characteristics among these latent classes. Because depressive symptoms are common in patients and FCs (Swore Fletcher et al., 2008), and because anxiety and depression commonly co-occur in the general population (Das-Munshi et al., 2008) and in cancer patients (Frick et al., 2007), differences in depressive symptom levels over the course of the study were also evaluated among the latent anxiety classes. As the primary purpose of this GMM analysis was exploratory, the focus of this report is not on hypothesis testing. However, we did hypothesize that the distinct latent classes for anxiety that were identified would not be dependent on patient or FC status.
METHODS
Participants
This study, which focuses on anxiety symptoms, is part of a large, longitudinal study of symptoms in patients and their FCs (Aouizerat et al., 2009; Fletcher et al., 2009; Fletcher et al., 2008; Miaskowski et al., 2008). A total of 167 oncology outpatients with breast, prostate, lung, or brain cancer and 85 FCs participated in this longitudinal study. Patients were eligible to participate if they were >18 years of age; were able to read, write, and understand English; had a self-reported Karnofsky Performance Status (KPS) score of ≥ 60; and were scheduled to receive primary or adjuvant RT. Patients were excluded if they had metastatic disease, more than one cancer diagnosis, or a diagnosed sleep disorder.
Following recruitment, patients were asked to identify the person most involved in their care (i.e., their FC). FCs were eligible to participate if they: were >18 years of age; were able to read, write, and understand English; had a self-reported KPS score of ≥ 60; were living with the patient; and did not have a diagnosed sleep disorder. Participants were recruited from RT departments located in a Comprehensive Cancer Center and a community-based oncology program. Both sites’ institutional review boards approved this study.
Of 472 patients approached, 167 consented to participate. The major reasons for refusal were being too overwhelmed with their cancer experience or too busy. No differences were found in any demographic or clinical characteristics between patients who did and did not choose to participate.
Instruments
Instruments were completed by both patients and FCs at all timepoints. A demographic questionnaire provided information on age, marital status, living arrangements, education, ethnicity, employment status, number of comorbidities, and KPS score (Karnofsky, 1977).
Anxiety was measured using the Spielberger State-Trait Anxiety Inventories (STAI-S and STAI-T). Each inventory consists of 20 items that are rated from 1 to 4. Scores for each scale are summed and can range from 20 to 80. STAI-T measures an individual’s predisposition to anxiety determined by his/her personality. STAI-S measures an individual’s transitory emotional response to a stressful situation. The STAI-T and STAI-S have well established criterion and construct validity and internal reliability coefficients (Spielberger, 1983). In the current study, the Cronbach’s alphas for the STAI-T and STAI-S were 0.86 and 0.91, respectively.
Depressive symptoms were measured using the Center for Epidemiological Studies–Depression (CES-D) scale (Radloff, 1977), which consists of 20 items representing the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores of ≥ 16 indicating the need for clinical evaluation for major depression. The CES-D has well-established validity and reliability (Hann et al., 1999; Myers and Weissman, 1980). In the current study, the Cronbach’s alpha for the CES-D was 0.83.
Pain was assessed using a modification of the Brief Pain Inventory (BPI) (Daut et al., 1983). Patients and FCs were asked to indicate whether or not they had pain “other than every day kinds of pain”. Those who responded in the affirmative were asked to note the intensity of average and worst pain using a 0 (no pain) to 10 (worst possible pain) numerical rating scale, and to rate the number of days per week. Because over 50% of patients and FCs did not have pain, for the subsequent longitudinal analysis, pain was coded as present or absent.
Study procedures
Approximately 1 week prior to the start of RT (i.e. simulation visit when the measurements for RT are made), patients were invited to participate in the study. If the FC was present, a research nurse explained the study protocol to both the patient and FC, determined eligibility, and obtained written informed consent. FCs who were not present were contacted by phone to determine their interest in participation. These FCs completed the enrollment procedures at home.
At the time of the simulation visit (i.e., baseline), participants (patients and FCs) completed the demographic questionnaire, the STAI-T, the STAI-S, the CES-D, and the BPI. After the initiation of RT, patients and FCs completed the STAI-S and CES-D at 4 weeks post-initiation of RT, at the end of RT, and at 4, 8, 12, and 16 weeks after the completion of RT (i.e., 7 assessments over 6 months).
Statistical analyses
Descriptive statistics and frequency distributions were generated on the sample characteristics and symptom severity scores. Independent sample t-tests, ANOVA, and Chi-square analyses were done to evaluate for differences in demographic and clinical characteristics between patients and FCs, and among the GMM latent classes. Data were analyzed using SPSS Version 15.0 (SPSS, 2006) and Mplus Version 5.21 (Muthén and Muthén, 2009).
Unlike linear regression analysis or ANOVA, that employ the general linear model to estimate regression coefficients and differences between groups, latent variables models such as GMM employ maximum likelihood estimation. (While a detailed explanation of maximum likelihood estimation is beyond the scope of this article, more comprehensive explanations are provided in: Eliason, 1993; Hox, 2010; Singer and Willett, 2003). GMM with robust maximum likelihood estimation was used to identify latent classes (i.e., subgroups of participants) with distinct state anxiety (STAI-S) trajectories over the 6 months of the study (Muthen and Kaplan, 2004). “Robust” in this context means that the estimation is more accurate when the dependent variables are not normally distributed.
Traditional methods for evaluating change across time for a variable such as anxiety include repeated measures ANOVA, multilevel regression, and latent growth models (Bauer, 2003; Byrne and Crombie, 2003; Curran and Hussong, 2003; Duncan et al., 2006; Gueorguieva and Krystal, 2004; Singer and Willett, 2003). With each of these approaches, a single curve or change trajectory is estimated for the entire group. In contrast, the present analysis using GMM allows for the estimation of more than one growth curve which enables the more accurate identification of groups of individuals who change differently over time.
For the typical estimation of a growth curve or trajectory, initial status is called the intercept and represents the mean for the dependent variable at the beginning of the assessments (i.e., in this study – level of state anxiety at the initiation of RT). The slope represents the amount of change (i.e., in this study the amount of change in anxiety) predicted for each unit of time. More than one slope coefficient is determined when the change is not linear. In our study, changes in anxiety over time had both a linear and a non-linear (in this case, quadratic) component. Therefore, intercepts and linear and quadratic slopes for each latent class were estimated.
Intercept and linear slope variances were allowed to vary within classes - meaning that individuals had different initial levels of anxiety as well as some variation in the way that their scores changed over time within each identified subgroup or class. Therefore, intercepts and linear slopes in these models are called random effects. However, because our sample size was relatively small for this type of model, we were forced to treat quadratic slopes as fixed effects (i.e., quadratic slope variance was fixed at zero), because the models could not be estimated when quadratic slopes were treated as random effects, even for the simplest (two-class) model.
Traditional methods of longitudinal data analysis exclude cases that have missing data at any timepoint, which reduces the sample size and leads to biased (inaccurate) results. In contrast, the estimation technique we used, robust maximum likelihood, allows accurate estimation of the model retaining all participants, even when participants have some missing data, through the use of the Expectation-Maximization algorithm in Mplus Version 5.21. This method assumes that any missing data are ignorable (i.e., missing at random) (Muthen, 2002; Schafer and Graham, 2002).
We were concerned that patients and their FCs might experience similar anxiety levels at baseline and across time (i.e., a dyadic effect). Therefore, analyses were performed to confirm that any similarity in anxiety scores between pairs of patients and FCs (dyads) did not influence the results of the GMM analyses reported here. Specifically, to examine any possible dyadic effect, a 3-level analysis of the linear and quadratic growth trajectories of anxiety scores for patients, compared to FCs, was performed. For this data structure, the repeated measures of anxiety are at Level 1, individuals (patients or FCs) are at Level 2, and dyads (for 82 paired observations) are at Level 3. In addition, the possibility that the linear and quadratic change trajectories differed for patients compared to FCs was tested (these are referred to as cross-level interactions).
When estimating latent variable models for understanding the obtained data using GMM, competing models are compared with fit indices that help identify the “best” model, given the data. The model fit for the GMM reported here was assessed statistically by identifying the model with the lowest Bayesian Information Criterion (BIC), and by testing the “K” versus “K-1” class models to determine whether a model with K classes fit the data better than a model with K-1 classes with the parametric bootstrapped likelihood ratio test (BLRT) (Jung and Wickrama, 2008; Nylund et al., 2007). For example, we compared models with three latent classes (K) and two latent classes (K-1) to see which model fit the data better by comparing the BICs for the two models, and by testing with the BLRT whether the three-class model provided an improvement in fit over the two-class model.
Mixture models are known to produce solutions at local maxima (meaning that the solution achieved by one maximum likelihood estimation is not the best solution), so each model was fit with random starts to be sure that the solution for the model with the maximum log likelihood values was replicated (Muthen and Muthen, 1998–2008). In other words, each model was estimated many times (several hundred, the specific number depending on the complexity of the model) to be sure that the “best” solution was replicated. An additional measure of model fit, called “entropy” (i.e., the proportion of latent versus predicted class membership), was estimated for each solution, with ≥ .80 being preferred. Better-fitting models should produce higher entropy values (Muthen and Muthen, 1998–2008).
In addition, the best fitting model was visually inspected by plotting observed (actual) against model-predicted (estimated) values to determine whether the predicted (estimated) trajectories followed the observed (actual) trajectories for the classes, and to evaluate whether the predicted plots “made sense” theoretically and clinically (Muthen and Kaplan, 2004).
To test for differences in depression at baseline and over time among the latent anxiety classes, a linear mixed model analysis was done using the SPSS MIXED module. Post hoc contrasts were done to evaluate for differences among the GMM anxiety classes at baseline (i.e., intercept) as well as for GMM anxiety class × time interactions (i.e., Does the change over time in depression scores vary across the different GMM anxiety classes?).
Differences in demographic and clinical characteristics between patients and FCs were considered statistically significant at the p < 0.05 level. Post hoc contrasts were done using the Bonferroni procedure to control the overall family alpha level of the three possible pairwise contrasts for the three GMM anxiety classes at 0.05. For any one of the three pairwise contrasts, a p-value of < 0.017 (.05/3) was considered statistically significant.
RESULTS
Participant characteristics
As summarized in Table I, the majority of participants were female, Caucasian, and well educated. Patients and FCs differed only in terms of gender, marital status, and pain. Compared to the patients, a greater proportion of the FCs was female and married or partnered and a smaller proportion had pain.
Table I.
Baseline demographic and clinical characteristics of participants
| Characteristic | Total Sample n = 252 | Patients n = 167 | Family Caregivers n = 85 | Statistic |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 61.4 (11.3) | 60.9 (11.6) | 62.5 (10.5) | t=−1.03, p=0.305 |
| Education (years) | 15.9 (3.0) | 16.0 (2.9) | 15.8 (3.2) | t=0.56, p=0.575 |
| Number of comorbid conditions | 4.6 (2.7) | 4.8 (2.6) | 4.2 (2.9) | t=1.52, p=0.131 |
| KPS score | 92.0 (11.5) | 91.1 (11.9) | 93.7 (10.6) | t=−1.65, p=0.100 |
| Baseline STAI-T score | 33.8 (10.0) | 34.7 (9.7) | 34.1 (9.9) | t=0.49, p=0.484 |
| Baseline STAI-S score | 31.0 (10.8) | 30.9 (10.9) | 31.0 (10.7) | t=−0.062, p=0.951 |
| Baseline CES-D score | 8.8 (8.2) | 9.1 (8.7) | 8.3 (7.2) | t=0.79, p=0.429 |
| % | % | % | ||
| Gender | ||||
| Male | 46.2 | 55.4 | 28.2 | χ2=16.7, p<0.0001 |
| Female | 53.8 | 44.6 | 71.8 | |
| Race/ethnicity | ||||
| African American | 13.5 | 15.0 | 10.6 | |
| Asian/Pac. Island. | 6.3 | 7.2 | 4.7 | χ2=4.9, p=0.43 |
| Caucasian | 74.6 | 71.9 | 80.0 | |
| Other | 5.6 | 5.9 | 4.7 | |
| Marital status | ||||
| Married/partnered | 69.3 | 56.0 | 95.3 | χ2 = 40.8, p<0.0001 |
| Other | 30.7 | 44.0 | 4.7 | |
| Works for pay | 46.4 | 47.0 | 45.2 | χ2 = 0.07, p=0.80 |
| Kids at home | 17.0 | 17.0 | 16.9 | χ2 = 0.00, p=1.00 |
| Pain (% Yes) | 47.8 | 56.0 | 31.8 | χ2 = 13.23, p<0.0001 |
| Patient’s cancer diagnosis1 | ||||
| Breast cancer | 32.8 | 38.1 | 22.4 | |
| Prostate cancer | 53.8 | 48.8 | 63.5 | χ2 = 6.63, p=0.09 |
| Lung cancer | 5.9 | 7.1 | 8.2 | |
| Brain cancer | 7.5 | 6.0 | 5.9 | |
Abbreviations: SD = standard deviation; KPS = Karnofsky Performance Status; STAI-S = Spielberger State Anxiety Inventory; STAI–T = Spielberger Trait Anxiety Inventory; CES-D = Center for Epidemiological Studies – Depression scale
The percentages in Table I for the FCs refer to the percentage of FCs who were caregivers for patients with each diagnosis.
Results of GMM analysis
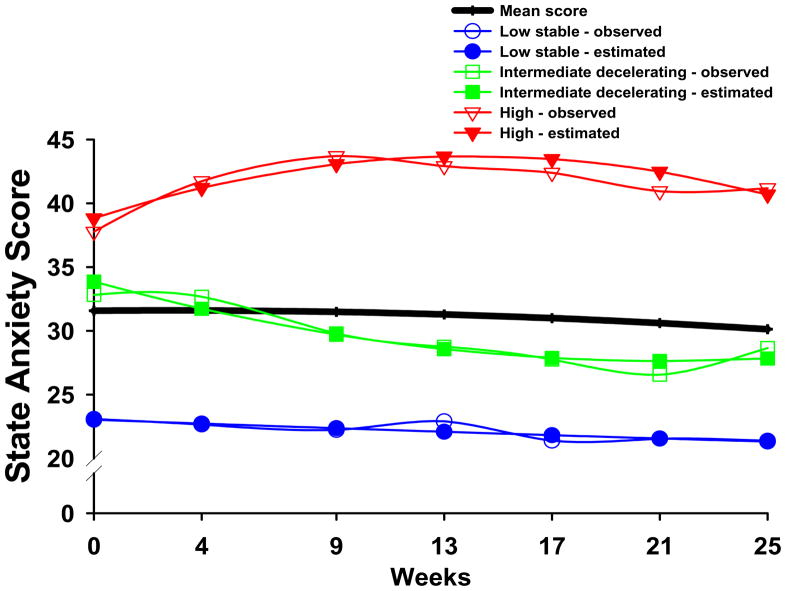
Three distinct latent classes of anxiety symptom trajectories were identified using GMM (see Figure 1). The fit indices for the various models are shown in Table II. As shown in Table II, a three-class model was selected because its BIC was smaller than the two-class and four-class models, and by comparison of the other fit indices. In addition, each class in the three-class model had a reasonable size and interpretability (Jung and Wickrama, 2008).
Figure 1.
STAI-S trajectories for observed (actual) scores and estimated (predicted) scores for participants in each of the latent classes, as well as the mean STAI-S scores for the total sample.
Table II.
Fit Indices for the State Anxiety Class Solutions
| GMM | LL | AIC | BIC | Entropy | BLRT |
|---|---|---|---|---|---|
| 1-Classa | −5526.794 | 11079.588 | 11125.471 | n/a | n/a |
| 2-Class | −5459.580 | 10955.159 | 11018.689 | 0.761 | 147.080* |
| 3-Classb | −5435.980 | 10915.960 | 10993.607 | 0.761 | 64.406* |
| 4-Class | −5432.905 | 10913.811 | 10998.517 | 0.751 | 49.908c |
p < .001
Latent growth curve with linear and quadratic components; χ2 = 49.599, 22 df, p < 0.001, CFI = 0.955, RMSEA = 0.071
3-class model was selected, based on its having the smallest BIC and a significant BLRT.
This is the Chi-squared value for both the BLRT and the VLMR. The VLMR test was not significant for the 4-class model. This test is known to be anti-conservative, so when it is not significant, it is firm evidence that the K-class model does not fit the data better than the K-1-class model. In this case, it means that the 4-class model is not better than the 3-class model. The BLRT could not be estimated reliably for the 4-class model; the best log-likelihood could not be replicated in most of the bootstrap draws, and the bootstrap draws did not converge in some runs even with a large number of random starts.
Abbreviations: AIC = Akaike Information Criteria; BIC = Bayesian Information Criterion; BLRT = bootstrapped likelihood ratio test; GMM = Growth mixture model; LL = log likelihood test; RMSEA = root mean square error of approximation; VLMR = Vuong-Lo-Mendell-Rubin test
The parameter estimates for the three latent classes are listed in Table III. The largest percentage of participants was classified into the Low Stable class (36.9%). These participants had total STAI-S scores that were low at baseline (mean 23.0), with a stable trajectory over the course of the study. Participants in the second largest class (i.e., Intermediate Decelerating class (32.5%)), had a mean baseline STAI-S score of 33.8 and symptom scores that decreased slightly over the course of the study. Participants in the third class (i.e, High class (30.6%)), had elevated STAI-S scores at baseline (mean 38.8) that increased and then very gradually decreased after the completion of RT.
Table III.
Parameter Estimates for Latent Classes for State Anxiety from 7 Assessmentsa
| Parameter Estimatesb | Low Stable (1) | Intermediate Decelerating (2) | High (3) |
|---|---|---|---|
| n = 93 (36.9%) | n = 82 (32.5%) | n = 77 (30.6%) | |
| Means | Mean (S.E.) | ||
| Intercept | 23.035*** (0.536) | 33.864*** (1.031) | 38.821*** (1.368) |
| Linear slope | −0.077 (0.070) | −0.587*** (0.165) | 0.696*** (0.184) |
| Quadratic slope | 0.000 (0.002) | 0.014* (0.006) | −0.025*** (0.007) |
|
Variances | |||
| Intercept | 0c | 2.595 (2.189) | 83.944*** (13.883) |
| Linear Slope | 0c | 0c | 0.138*** (0.035) |
p < .05,
p < .01,
p < .001
Trajectory group sizes are for classification of individuals based on their most likely latent class probabilities
Growth mixture model estimates were obtained with robust maximum likelihood
Fixed at zero
Abbreviations: S.E. = Standard error
Examination of possible patient/FC status effects and dyadic effects
No significant differences were found in patients’ and FCs’ baseline STAI-T or STAI-S scores. In addition, no significant differences were found in the proportions of patients and FCs among the three GMM classes described above (see Table IV). Furthermore, even after taking patient and FC status and dependency within dyads into account in the 3-level GMM, no significant differences were found in the parameter estimates (intercepts and slopes) for the three GMM trajectories that were identified in the 2-level GMM. Given the absence of any dyadic or status effect, the relatively small proportion of dyads in the total sample, and the preference for model parsimony, the results of the 2-level GMM are reported in this paper.
Table IV.
Differences in Baseline Demographic and Clinical Characteristics among the Three Latent Classes
| Characteristic | Low Stable (1) | Intermediate Decelerating (2) | High (3) | Statistic and Post hoc Comparisons* |
|---|---|---|---|---|
| n=93 (36.9%) | n=82 (32.5%) | n=77 (30.6%) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 64.9 (9.0) | 62.7 (11.5) | 52.0 (11.3) | F(2,249)=15.46; p<0.001 1,2>3 |
| Education (years) | 16.3 (3.0) | 15.7 (3.3) | 15.8 (2.7) | F(2,246)=1.24; p=0.290 |
| # Comorbid Conditions | 4.2 (2.6) | 4.6 (2.7) | 5.0 (2.8) | F(2,249)=1.81; p=0.165 |
| Weight (pounds) | 180.9 (38.2) | 174.5 (34.8) | 169.5 (43.1) | F(2,246)=1.81; p=0.166 |
| KPS Score | 96.1 (7.1) | 91.2 (11.7) | 87.5 (13.9) | F(2,243)=12.55; p<0.001 1>2,3 |
| STAI-S | 22.8 (4.1) | 33.0 (8.3) | 38.4 (12.1) | F(2,244)=72.58; p<0.001 1<2<3 |
| STAI-T | 26.2 (5.5) | 36.5 (8.1) | 40.8 (9.4) | F(2,247)=79.68; p<0.001 1<2<3 |
| CES-D Total | 3.7 (4.3) | 9.3 (7.7) | 14.4 (8.5) | F(2,243)=50.02; p<0.001 1<2<3 |
| n (%) | n (%) | n (%) | ||
| Gender (% female) | 39 (41.9%) | 42 (51.2%) | 54 (70.1%) | χ2=13.73; p=0.001 |
| Ethnicity (% White) | 81 (87.1%) | 57 (69.5%) | 50 (64.9%) | χ2=12.58; p=0.002 |
| Lives Alone (% yes) | 18 (29.0%) | 14 (26.4%) | 22 (42.3%) | χ2=3.52; p=0.172 |
| Married or partnered (% yes) | 72 (77.4%) | 57 (70.4%) | 45 (59.2%) | χ2=6.59; p=0.037 |
| Children at home (% yes) | 6 (8.0%) | 14 (18.9%) | 15 (24.2%) | χ2=6.88; p=0.032 |
| Older adult at home (% yes) | 0 (0%) | 3 (4.1%) | 4 (6.3%) | χ2=4.45; p=0.108 |
| Work for pay (% yes) | 42 (45.7%) | 41 (50.6%) | 32 (43.2%) | χ2=0.89; p=0.640 |
| Patient/FC (% Patient) | 62 (66.7%) | 53 (64.6%) | 52 (67.5%) | χ2=0.16; p=0.923 |
| Pain (% yes) | 35 (37.6%) | 35 (42.7%) | 51 (66.2%) | χ2=15.19; p=0.001 |
Abbreviations: FC = family caregiver; KPS = Karnofsky Performance Status; STAI-S = Spielberger State-Trait Anxiety Inventory – State subscale; STAI-T = Spielberger State-Trait Anxiety Inventory – Trait subscale; CES-D = Center for Epidemiological Studies – Depression scale
Post hoc comparisons: Numbers refer to latent classes, e.g., for Age: 1,2>3 represents Low Stable and Intermediate Decelerating classes were older than the High class. Only significant post hoc contrasts are shown.
Differences in demographic and clinical characteristics among the three latent classes
No differences were found among the three latent classes in the proportion of patients and FCs (Table IV). However, differences in age, gender, ethnicity, marital status, having children living at home, presence of pain, and baseline KPS scores were found among the three latent classes.
Participants in the High class were significantly younger than participants in the Low Stable and Intermediate Decelerating anxiety classes. While women made up 53.8% of the total sample, post hoc contrasts revealed that a significantly higher proportion of women were in the High class (70.1%) compared to the Low Stable (41.9%) and Intermediate Decelerating (51.2%) classes. In terms of ethnicity, while 74.6% of the total sample was white, post hoc contrasts revealed that significantly higher proportions of nonwhites were in the Intermediate Decelerating (30.5%) and High (35.1%) classes compared to the Low Stable class. In terms of marital status, a higher proportion of those in the High class (40.8%) were not married compared to those in the Low Stable class (22.6%). In addition, a significantly higher proportion (24.2%) of participants in the High class had children at home, compared to those in the Low Stable class (8.0%).
In terms of physical functioning, post-hoc contrasts demonstrated that individuals in the Intermediate Decelerating and High classes had lower KPS scores at baseline than those in the Low Stable class. In addition, the presence of pain was different among the three anxiety classes, with post hoc contrasts revealing that a greater proportion of those in the High class (66.2%) reported pain, compared to those in the Low Stable (37.6%) and Intermediate Decelerating (42.7%) classes.
Differences in baseline trait anxiety among the three latent classes
Significant differences in baseline STAI-T scores were found among the three latent classes. Participants in the High class reported the highest trait anxiety scores followed by those in the Intermediate Decelerating class, whose scores were significantly higher than those reported by the Low Stable class (all p < 0.002, Table IV).
Differences in baseline CES-D scores among the three latent classes
As shown in Table IV, significant differences were found among the three latent classes in baseline CES-D scores, with the High class reporting the worst depression scores, followed by the Intermediate Decelerating class, followed by the Low Stable class (all p < .001).
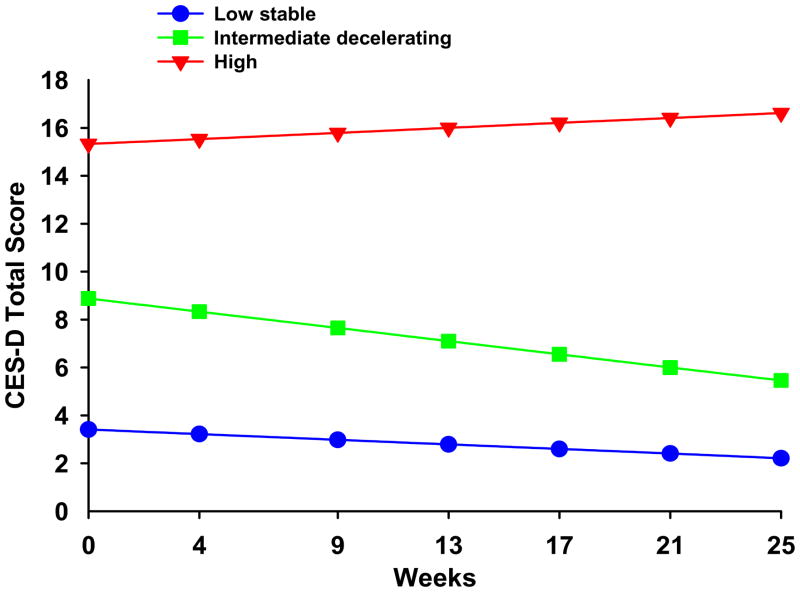
Differences in the trajectories of CES-D scores among the three latent classes
Differences in the trajectories of CES-D total scores are illustrated in Figure 2. Post hoc contrasts for the class by time interactions for changes over time in the CES-D score are summarized in the legend for Figure 2.
Figure 2.
Changes over time in Center for Epidemiologic Studies – Depression total scores for participants in each of the three latent anxiety classes. Post hoc contrasts for intercept 1 < 2 < 3a; and slopes 1a and 2b ≠ 3. (ap<0.0001, bp=0.011).
DISCUSSION
To our knowledge, this study is the first to use GMM to identify latent classes of oncology patients and FCs with distinct anxiety symptom trajectories during and following the patient’s RT. Our hypothesis that distinct latent classes would be independent of whether one was a patient or FC was supported. These findings have a number of implications for clinical practice and for symptom management research in oncology patients and FCs.
Implications for clinical practice
Although specific anxiety disorders (e.g., generalized anxiety disorder, adjustment disorders) were not diagnosed, a substantial subgroup of patients and FCs (i.e., High anxiety latent class) had mean STAI-S scores that were above the clinically meaningful cutoff score (i.e., ≥ 32.2) (Fletcher et al., 2008; Spielberger, 1983) throughout the study. In addition, these individuals had, on average, higher levels of depressive symptoms compared to the other two anxiety classes. These patients and FCs may represent a relatively high-risk group who warrant a more comprehensive assessment (e.g., evaluation by a mental health clinician) as well as individualized interventions for anxiety and/or depression.
Clinicians understandably seek efficient and reliable ways to identify patients or FCs at risk for anxiety, depression, or other clinically significant forms of distress. Whether the individuals identified by the GMM analysis as belonging to the High anxiety class would have been identified using brief screening tools [e.g., the Distress Thermometer, the GAD-7 (Spitzer et al., 2006)] cannot be inferred from our findings. Nevertheless, the distinguishing characteristics of the individuals in the High anxiety class suggest several possible risk factors for elevated anxiety in both patients and FCs during and after RT. Specifically, women, younger individuals, members of racial or ethnic minorities, those who were unmarried, and those with children at home were more likely to be classified in the High anxiety class. These age and gender differences are consistent with previous reports of predictors of anxiety (Stark et al., 2002) and of psychological distress more generally in cancer patients (Weisberg, 2009). The finding that unmarried participants were more likely to have higher anxiety levels suggests that social support may be protective, corroborating prior literature (Stark et al., 2002). However, higher anxiety in those with children at home may indicate that greater social responsibilities increase the risk for anxiety. Several studies have documented greater distress in patients with children at home (Bower, 2008; Deshields et al., 2006). Thus, clinicians should ask about, and be attuned to the possibility of, anxiety particularly in individuals with these characteristics. Asking about anxiety, rather than assuming that patients will volunteer their symptoms, is critical. FCs, in particular, may not voice their own distress, yet may suffer as much as or more than patients during and after this stressful experience (Couper et al., 2006).
Our findings suggest that not all patients or FCs are at increased risk for anxiety. Over 30% of participants (i.e., those in the Low Stable class) had, on average, low baseline STAI-S scores [i.e., below the 25th percentile compared to population norms (Spielberger, 1983)], and their mean STAI-S scores remained low throughout the study. This finding, which is consistent with previous cross-sectional studies of oncology patients and FCs (Fletcher et al., 2008; Stiegelis et al., 2004; Swore Fletcher et al., 2008), suggests that those individuals with low anxiety at the beginning of RT are unlikely to experience significantly increased anxiety during or after RT (i.e., a relatively protected group)—although these findings need to be replicated. Again, it would be useful to determine whether these individuals could be clearly identified early in the course of treatment using briefer measures than those used in this study.
The association between anxiety and depression underscores the importance of evaluating both of these symptoms as well as their co-occurrence clinically, given the deleterious effects of comorbid anxiety and depressive symptoms, even at subsyndromal levels (Das-Munshi et al., 2008).
Implications for symptom management research
The use of GMM permitted the identification of subgroups of patients and FCs with high, intermediate, and low mean levels of anxiety—subgroups not seen when inspecting mean STAIS scores over time. These subgroups are consistent with previous reports of substantial heterogeneity in patients’ and FCs’ experience of anxiety (Frick et al., 2007; Krischer and Xu, 2008; Stiegelis et al., 2004). The findings are novel in that anxiety was studied in both patients and FCs. However, the findings need to be replicated in other oncology populations - particularly in more homogeneous groups of patients with a particular cancer diagnosis and in FCs of patients with a single cancer diagnosis and/or at different points in the patients’ treatment trajectory.
Further research is needed to better understand comorbid symptom experiences in patients and FCs. Given that even subthreshold levels of comorbid anxiety and depression were shown to adversely affect health and functioning in the general population (Das-Munshi et al., 2008), in-depth examination of this relationship in cancer patients and FCs is warranted. Moreover, the finding that mean depressive symptom scores increased over time in the High anxiety group—a significantly different profile from that of both the Intermediate Decelerating and Low Stable classes—highlights the importance of studying clusters of symptoms over time, in order to understand driving relationships among individual symptoms or symptom domains.
In addition, further study of the relationship between anxiety profiles, functional status, and pain is warranted. The causal relationship between anxiety and physical function in oncology patients and FCs has not been clearly elucidated. It is possible that the lower functional status scores (albeit still high) of the High and Intermediate Decelerating classes compared to the Low Stable class may reflect underlying differences in other factors or traits, the effects of psychological distress on physical functioning (or vice versa), or differences in self-perceived functional status depending on psychological state. The association between pain and High anxiety class membership further highlights the need for a better understanding of the interrelationships between physical and psychological symptoms, including their influence on quality of life (Ferrans et al., 2005; Stiegelis et al., 2004).
Finally, this study paves the way for further work examining the relationship of traits to states. For instance, resilience, which is now believed to be more common than previously appreciated in adults who experience a significant trauma (e.g., a life-threatening illness) (Bonanno, 2004), may explain the finding of a latent class of individuals with low levels of anxiety. Resilience is a trait (an enduring aspect of one’s personality or coping style) that can be measured. Interestingly, in one study of patients undergoing RT, resilience was inversely related to the need for psychosocial support (Brix et al., 2008), although the specific relationship between resilience and anxiety symptoms was not assessed. Distinguishing the effects of traits from transient state reactions could help inform screening and intervention research.
This study has several limitations. Although valid and reliable self-report measures were used to assess anxiety and depressive symptoms, future work should employ structured diagnostic evaluations to assess previous and concurrent psychiatric conditions. Major reasons for enrollment refusal were being too overwhelmed with treatment or too busy which may have led to either underestimation or overestimation of anxiety symptoms. Also, unmeasured variables (e.g., other psychosocial stressors, side effects of treatment) may have influenced the results. Data are lacking on psychotropic medication use. Since many people are prescribed anxiolytics particularly benzodiazepines, during cancer treatment (Muriel et al., 2009), these medications could have affected symptom severity scores. Finally, although GMM is more robust with large samples, the present sample size (n=252) is at the lower limit necessary for meaningful, reliable estimates of latent classes (cf. Nylund et al., 2007; Tofighi and Enders, 2008). Finally, until these findings are replicated in future studies, caution is needed when interpreting these findings. Sample sizes should be large enough for confirmatory analyses of both the number and trajectories of the latent classes, as well as the characteristics that distinguish among the classes.
Despite these limitations, these novel GMM-derived results suggest that BOTH patients and FCs may be characterized by distinct anxiety profiles, and that approximately one-third (and up to two-thirds if subthreshold symptoms are considered) will experience clinically meaningful levels of anxiety. Important tasks for researchers going forward include identifying predictors of trajectories indicating persistently high levels of symptoms, elucidating additional factors (e.g., genetic, physiologic, environmental) that distinguish among the latent classes, and better appreciating the relationships among anxiety, depressive, and other symptom experiences over time in oncology patients and their FCs.
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (NR04835). Dr. Aouizerat is funded through the National Institutes of Health Roadmap for Medical Research Grant (KL2 RR624130). Dr. Dunn received funding from the Mount Zion Health Fund and the UCSF Academic Senate.
Footnotes
Conflict of Interest Statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen BL, Tewfik HH. Psychological reactions to radiation therapy: reconsideration of the adaptive aspects of anxiety. Journal of Personality and Social Psychology. 1985;48:1024–1032. doi: 10.1037//0022-3514.48.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Wara W, Swift P, Dunn LB, Miaskowski C. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research in Nursing. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Bauer DJ. Estimating multilevel linear models as structural equation models. Journal of Educational and Behavioral Statistics. 2003;28:135–167. [Google Scholar]
- Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. Journal of Clinical Oncology. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix C, Schleussner C, Fuller J, Roehrig B, Wendt TG, Strauss B. The need for psychosocial support and its determinants in a sample of patients undergoing radiooncological treatment of cancer. Journal of Psychosomatic Research. 2008;65:541–548. doi: 10.1016/j.jpsychores.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Byrne BM, Crombie G. Modeling and testing change: An introduction to the latent growth curve model. Understanding Statistics. 2003;2:177–203. [Google Scholar]
- Chen AM, Jennelle RL, Grady V, Tovar A, Bowen K, Simonin P, Tracy J, McCrudden D, Stella JR, Vijayakumar S. Prospective study of psychosocial distress among patients undergoing radiotherapy for head and neck cancer. International Journal of Radiation Oncology, Biology, Physics. 2009;73:187–193. doi: 10.1016/j.ijrobp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology. 2006;15:937–953. doi: 10.1002/pon.1031. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Hussong AM. The use of latent trajectory models in psychopathology research. Journal of Abnormal Psychology. 2003;112:526–544. doi: 10.1037/0021-843X.112.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das-Munshi J, Goldberg D, Bebbington PE, Bhugra DK, Brugha TS, Dewey ME, Jenkins R, Stewart R, Prince M. Public health significance of mixed anxiety and depression: beyond current classification. British Journal of Psychiatry. 2008;192:171–177. doi: 10.1192/bjp.bp.107.036707. [DOI] [PubMed] [Google Scholar]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- Deshields T, Tibbs T, Fan MY, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psychooncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Strycker LA. An Introduction to Latent Variable Growth Curve Modeling: Concepts, Issues, and Applications. 2. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. [Google Scholar]
- Eliason SR. Maximum likelihood estimation: Logic and practice. Sage University Papers Series: Quantitative Applications in the Social Sciences. Sage Publications, Inc; Thousand Oaks, CA: 1993. [Google Scholar]
- Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. Journal of Nursing Scholarship. 2005;37:336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- Fletcher BA, Schumacher KL, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C. Trajectories of fatigue in family caregivers of patients undergoing radiation therapy for prostate cancer. Research in Nursing and Health. 2009;32:125–139. doi: 10.1002/nur.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Lee K, Aouizerat B, Swift P, Wara W, Miaskowski CA. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. Journal of Clinical Oncology. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- Frick E, Tyroller M, Panzer M. Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. European Journal of Cancer Care (Engl) 2007;16:130–136. doi: 10.1111/j.1365-2354.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb BH, Rooney JA. Coping effectiveness: determinants and relevance to the mental health and affect of family caregivers of persons with dementia. Aging and Mental Health. 2004;8:364–373. doi: 10.1080/13607860410001709719. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) Journal of Psychosomatic Research. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychology. 2004;23:3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychology. 2010;29:160–168. doi: 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- Hox JJ. Multilevel Analysis: Techniques and Applications. 2. Routledge; New York: 2010. [Google Scholar]
- Hunter AM, Muthen BO, Cook IA, Leuchter AF. Antidepressant response trajectories and quantitative electroencephalography (QEEG) biomarkers in major depressive disorder. Journal of Psychiatric Research. 2010;44:90–98. doi: 10.1016/j.jpsychires.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. The National Academies Press; Washington, DC: 2007. [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- Karnofsky D. Performance Scale. In: Kennealy GT, Mitchell MS, editors. Factors that Influence the Therapeutic Repsone in Cancer: A Comprehensive Treatise. Plenum Press; New York: 1977. pp. 97–101. [Google Scholar]
- Krischer MM, Xu P. Determinants of psychological functioning in patients undergoing radiotherapy: a descriptive study. Journal of Psychosocial Oncology. 2008;26:1–13. doi: 10.1080/07347330802359552. [DOI] [PubMed] [Google Scholar]
- Kurtz ME, Kurtz JC, Given CW, Given B. Predictors of postbereavement depressive symptomatology among family caregivers of cancer patients. Supportive Care in Cancer. 1997;5:53–60. doi: 10.1007/BF01681962. [DOI] [PubMed] [Google Scholar]
- Lam WW, Bonanno GA, Mancini AD, Ho S, Chan M, Hung WK, Or A, Fielding R. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psychooncology. 2010;19:1044–51. doi: 10.1002/pon.1658. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. What is in a name? Integrating homeostasis, allostasis and stress. Hormones and Behavior. 2010;57:105–111. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Paul SM, Cooper BA, Lee K, Dodd M, West C, Aouizerat BE, Swift PS, Wara W. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. Journal of Pain and Symptom Management. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro AJ, Potter S. A quantitative approach to the distress caused by symptoms in patients treated with radical radiotherapy. British Journal of Cancer. 1996;74:640–647. doi: 10.1038/bjc.1996.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel AC, Hwang VS, Kornblith A, Greer J, Greenberg DB, Temel J, Schapira L, Pirl W. Management of psychosocial distress by oncologists. Psychiatric Services. 2009;60:1132–1134. doi: 10.1176/appi.ps.60.8.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcoholism: Clinical and Experimental Research. 2000;24:882–891. [PubMed] [Google Scholar]
- Muthen BO. Beyond SEM: General latent variable modeling. Behaviormetrika. 2002;29:81–117. [Google Scholar]
- Muthen BO, Kaplan DW. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. The Sage Handbook of Quantitative Methodology for the Social Sciences. Sage Publications; Newbury Park, CA: 2004. pp. 345–368. [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. 5. Muthen & Muthen; Los Angeles: 1998–2008. [Google Scholar]
- Muthén LK, Muthén BO. Mplus (Version 5.21) Muthén & Muthén; Los Angeles: 2009. [Google Scholar]
- Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. American Journal of Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS for Windows (Version 15) SPSS, Inc; Chicago, IL: 2006. [Google Scholar]
- Stark D, Kiely M, Smith A, Velikova G, House A, Selby P. Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. Journal of Clinical Oncology. 2002;20:3137–3148. doi: 10.1200/JCO.2002.08.549. [DOI] [PubMed] [Google Scholar]
- Stiegelis HE, Ranchor AV, Sanderman R. Psychological functioning in cancer patients treated with radiotherapy. Patient Education and Counseling. 2004;52:131–141. doi: 10.1016/s0738-3991(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Swore Fletcher BA, Dodd MJ, Schumacher KL, Miaskowski C. Symptom experience of family caregivers of patients with cancer. Oncology Nursing Forum. 2008;35:E23–44. doi: 10.1188/08.ONF.E23-E44. [DOI] [PubMed] [Google Scholar]
- Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. In: Hancock GR, Samuelsen KM, editors. Advances in Latent Variable Mixture Models. Information Age Publishing; Charlotte, NC: 2008. pp. 317–341. [Google Scholar]
- Weisberg RB. Overview of generalized anxiety disorder: epidemiology, presentation, and course. Journal of Clinical Psychiatry. 2009;70(Suppl 2):4–9. [PubMed] [Google Scholar]
- Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]