Abstract
Objective
Preterm infants are frequently transfused with red blood cells based on standardized guidelines or clinical concern that anemia taxes infants’ physiological compensatory mechanisms and thereby threatens their health and well-being. The impact of various transfusion guidelines on long-term neurocognitive outcome is not known. The purpose of this study is to evaluate long-term neurocognitive outcome on children born prematurely and treated at birth with different transfusion guidelines.
Methods
Neurocognitive outcomes were examined at school age for 56 preterm infants randomly assigned to a liberal (n = 33) or restrictive (n = 23) transfusion strategy. Tests of intelligence, achievement, language, visual-spatial/motor, and memory skills were administered. Between-group differences were assessed.
Results
Those in the liberal transfusion group performed more poorly than those in the restrictive group on measures of associative verbal fluency, visual memory, and reading.
Conclusions
Findings highlight possible long-term neurodevelopmental consequences of maintaining higher hematocrit levels.
Keywords: Preterm, Neuropsychology, Red Blood Cell Transfusion, Hematocrit, Longitudinal
The high incidence of anemia in preterm, very low birth weight (VLBW; <1500 grams) infants mandates frequent red blood cell (RBC) transfusions during the first few weeks after birth. Though all infants experience decreased hemoglobin (i.e., physiologic anemia) during the first months after birth, anemia of prematurity is common in infants weighing less than 1 kg (< 32 weeks gestation), and is essentially low endogenous erythropoietin (EPO; a hormone that stimulates RBC production in response to low oxygen saturation) production and exaggeration of physiologic anemia in which hemoglobin levels fall 7–10 g/dl more than in term infants and remain lower for longer periods of time (Bain & Blackburn, 2004). Concern regarding the preterm infant’s capacity to tolerate anemia as well as the potential consequences (e.g., increased risk of death), have resulted in routine RBC transfusions among preterm infants (Hebert et al., 1999; Straus, 1995). Views on the risks of anemia and the effectiveness of RBC transfusion in addressing these risks, however, are varied, while the clinical signs of anemia are nonspecific and require significant physician interpretation (Maier et al., 2000; Strauss, 1995). Information has begun to emerge about the impact of transfusion strategy on the outcome of anemic preterm infants, but the outcomes analyzed to date have been limited to the neonatal period and later infancy (i.e., up to 18 to 21 months of corrected age) (Whyte et al., 2009). Evaluating long-term neurodevelopmental outcomes associated with different transfusion thresholds is critical for assessing the safety of current transfusion practice especially in terms of potential cognitive and behavioral morbidity.
The Impact of Preterm Birth on Neurocognitive Outcomes
The survival rate of preterm infants has improved in recent decades as a result of advances in neonatal intensive care. Although rates of mortality and severe morbidity have decreased, children and adolescents who were born prematurely are nevertheless at risk for problems in psychosocial, behavioral, and cognitive functioning (Hoff, Hansen, Munck, & Mortensen, 2004; Litt, Taylor, Klein, & Hack, 2005; Miceli, Goeke-Morey, Whitman, Kolberg, Loncar, & White, 2000; Nadeau, Tessier, Boivin, Lefebvre, & Robaey, 2003; Taylor, Hack, & Klein, 1998, 2000). It has been estimated that preterm children, as a group, perform an average of 10 points lower than full-term children on standardized intellectual assessments (Goyen, Lui, & Woods, 1998). Preterm birth is also associated with increased risk for high-prevalence, low-severity deficits in many functions, including memory, language/vocabulary, concept formation, executive functioning, perceptual, visuomotor, and gross motor (e.g., balance, gait, and coordination) abilities. (Aylward, 1997, 2002, 2005; Breslau, 1995; Hack, Taylor, Klein, Eiben, Schatschneider, & Mercuri-Minich, 1994; Taylor et al., 1998). These deficits are considered “high-prevalence” because of the elevated rates at which they have been found to occur in children born preterm as compared to the general population. In addition, studies suggest that these deficits result in learning, attention, and behavior problems in approximately 50–70% of children born with very low birth weight (VLBW) (Aylward, 1997, 2002; Taylor et al., 1998). These deficits have been termed “low-severity” because they are not necessarily associated with global deficits in intellectual or psychomotor functioning (e.g., intellectual disability, cerebral palsy) and often go unrecognized in early development.
Preterm birth is associated with increased risk of problems in executive functioning (or the processes of planning, initiating, and sustaining goal-directed behavior and self-directing behavior and emotion), including attention, working memory, and inhibitory control in school-age children and adolescents (Anderson, Doyle, & the Victorian Infant Collaborative Study Group, 2004; Aylward, 2005; Bhutta, Cleeves, Casey, Cradock, & Anand, 2002; Harvey, O’Callaghan, & Mohay, 1999; Luciana, Lindeke, Mills, & Nelson, 1999; Stephens & Vohr, 2009; Taylor et al., 1998). A global dysexecutive pattern has been found among preterm groups with average intellectual abilities (Waber & McCormick, 1995), while others have found that differences between preterm and full term groups remain after controlling for general cognitive abilities (IQ) (Taylor, Minich, Klein, & Hack, 2004) and after excluding children with neurosensory impairment (Anderson et al., 2004).
Overall, research on achievement outcomes of premature and LBW infants at school age indicates poor general academic functioning in addition to more specific areas of weakness in reading, arithmetic, and spelling (Hack et al., 1994; Hagen et al., 2006; Litt et al., 2005; O’Callaghan, Burns, Gray, & Harvey, 1996). Some research suggests that deficits in the academic skills of decoding, problem solving, and calculation not only persist, but also become more pronounced over the course of preterm children’s development (Espy, Fang, Charak, Minich, & Taylor, 2009). Within preterm groups, males perform more poorly in school than females (Saigal, Hoult, Streiner, Stroskopf, & Rosenbaum, 2000) and are more likely to than females to be identified as learning disabled (Whitfield, Eckstein Grunau, & Holsti, 1997). Evidence suggests that specific neurocognitive weaknesses can interfere with learning, academic performance, and social-emotional/behavioral functioning of former preterm children and adolescents. For example, Mulder, Pitchford, and Marlow (2010) recently found deficits in processing speed and working memory predicted overall academic achievement in very preterm (i.e., < 31 weeks) children at 9–10 years of age. The level of LBW has been associated with math, but not reading, performances (i.e., < 1500 g infants perform poorer than heavier LBW infants in math) in a longitudinal study of a stratified sample of LBW and non-low birth weight (NBW; Breslau, Johnson, & Lucia, 2001) infants from diverse family and environmental backgrounds (i.e., inner city/low income and suburban/middle class). Breslau et al. (2001) further demonstrated that while approximately 80% of the difference in reading and math between LBW and NBW groups at age 11 was accounted for by intellectual ability at age six, a subset of VLBW infants had significantly poorer math achievement independent of IQ. In the Breslau et al. (2001) study, phonological awareness and visual-motor integration measures predicted reading performances at 11 independent of IQ. Furthermore, social-environmental factors were more highly predictive of achievement than LBW, with low maternal education and single mother status predicted reading performance at age 11. An interaction between academic area assessed and inner city versus the suburbs was observed, whereby inner city placement continued to adversely affect math performance above and beyond the initial effects on cognitive ability affecting performance at school entry.
Red Blood Cell Transfusion
It is estimated that 80% (out of approximately 38,000) of preterm infants with birth weights less than 1500 grams who are born annually receive multiple red blood cell (RBC) transfusions during the first few weeks of life due to phlebotomy blood losses secondary to severe respiratory disease and to physiologic factors resulting in decreased circulating RBC mass (Levy et al., 1993; Strauss, 1991, 1995). Traditionally, transfusions have been small to modest in volume (5 to 15 mL of RBC per kg bodyweight per does). Transfusions are often repeated, with mean donors per neonate estimated between 8 to 10 (Strauss, 1991; Strauss, Sacher, & Blazina, 1990).
Decisions about when and how much to transfuse preterm neonates are made based upon a clinical approach in which the hematocrit level is maintained according to what is thought to be most advantageous for each infant’s clinical status (Strauss, 1995). Though definitions of liberal and restrictive transfusion strategies vary by study, they generally refer to RBC transfusion criteria in which hemoglobin concentration or hematocrit are maintained at higher (for liberal) and lower (for restrictive) levels in order to better understand the way in which different transfusion practices may affect the developing brain. In restrictive approaches, the timing of RBC transfusions is intentionally delayed (i.e., the hemoglobin or hematocrit threshold at which transfusions were performed was allowed to fall to lower levels) in order to reduce transfusion numbers, donor exposures, and total blood volume transfused (Beeram, Krauss, & Riggs, 2001; Brooks, Marcus, Gillis, Pirie, Johnson, & Bhatia, 1999; Franz & Pohlandt, 2001; Maier et al., 2000; Widness et al., 1996). Little is known about what, if any, role medical interventions such as antenatal corticosteroid treatment, surfactant replacement therapy, and RBC transfusion play in reducing the risk of neurocognitive, academic, and behavioral problems in preterm infants. However, it is clear that such practices have significantly decreased rates of mortality in the postnatal period. Faster weight gain, less severe apnea, and increased oxygenation of the blood as a result of transfusion (Hudson, Cooke, & Holland et al., 1990; Murray & Roberts, 2003; Ross et al., 1989) are thought to play important roles in the immediate survival of small, critically ill neonates. Transfusion is also thought to protect against brain damage that may occur as a result of oxygen deprivation or brain hemorrhage that results from the body’s natural attempts to increase blood supply to the brain (Bell et al., 2005).
Comparing Liberal and Restrictive Transfusion Strategies
As with any medical procedure, the benefits and protective factors associated with RBC transfusion must be carefully weighed in light of potential risks. In addition to being costly (Murray & Roberts, 2004), transfusions are associated with a number of well-documented risks associated with blood incompatibility, immunosuppression or immune activation (Bordin, Heddle, & Blajchman, 1994; Jeschke, Chinkes, Finnerty, Przkora, Pereira, & Herndon, 2007; Marik, 2009; van de Watering et al., 1998), and transfusion reactions or infections such as viral hepatitis and cytomegalovirus (Szekely et al., 2009). Studies have also shown that the risks of transfusion complications may be more pronounced for the most critically ill infants and as the amount of blood transfused increases (Jeschke et al., 2007).
No consensus exists regarding the optimal point at which to transfuse preterm infants. Recent investigations have begun to address whether restrictive approaches to transfusion would result in poorer outcomes compared to more liberal approaches. The primary concern with performing transfusions in a more restrictive manner is greater risk for brain injury, as higher hemoglobin levels assure higher cerebral oxygen delivery and, in addition, are thought to help prevent apnea episodes that may both result from and contribute to hypoxia-ischemia in critically ill preterm infants (Bell, 2006).
A number of studies have compared liberal transfusion strategies with more restrictive approaches (Beeram et al., 2001; Brooks et al., 1999; Franz & Pohlandt, 2001; Maier et al., 2000; Widness et al., 1996). These studies have failed to document increased risks of morbidity and mortality in restrictive transfusion groups compared to liberal strategies. In comparison to liberal transfusion groups, restrictive groups did not show increased risk for primary outcomes (e.g., death or presence of a major developmental defect such as cerebral palsy; cognitive delay; severe visual or hearing impairment; retinopathy of prematurity [ROP]; intraventricular hemorrhage [IVH]; necrotizing enterocolitis; chronic lung disease; or sepsis) or secondary outcomes (e.g., poorer weight gain, increased length of hospital stay, or increased need for respiratory support and oxygen therapy) (Beeram et al., 2001; Brooks et al., 1999; Franz & Pohlandt, 2001; Kirpalani et al., 2006; Lacroix et al., 2007; Maier et al., 2000; Whyte et al., 2009; Widness et al., 1996). Moreover, there has even been some indication that restrictive strategies may be superior. For example, Venancio et al. (2006) found that stricter guidelines reduced the need for RBC transfusions in VLBW preterm infants and reduced the volume of blood transfused without adversely affecting clinical outcomes. These authors also found that infants in the liberal group were more likely to have retinopathy, and hypothesized that increased transfusions may increase the risk of diseases related to the release of free radicals (Venancio et al., 2006).
Limitations to Existing Research
Despite emerging evidence suggesting that restrictive strategies may be equivalent or superior to liberal strategies, transfusion practices are inconsistent (Hume, 1997; Levy et al., 1993; Murray & Roberts, 2004). In addition, there is limited understanding of the effects of different RBC transfusion strategies on neurocognitive functioning. Even less is known with regard to the potential benefits and risks of transfusions at the upper and lower hemoglobin limits (Bell, 2008). The Iowa trial set a higher hemoglobin threshold than previous studies in order to better compare the effects of significant differences in mean hemoglobin levels (Bell, 2008; Bell et al., 2005). In the Iowa trial, one hundred preterm infants were stratified by birth weight (500–750g, 751–1000g, 1001–1300g) and randomly assigned to either restrictive or liberal transfusion groups. Infants received 15 mg/kg RBC when hematocrit fell below prescribed thresholds. For mechanically ventilated infants, the thresholds were hematocrit 46% for the liberal group and 34% for the restrictive group. Liberal infants received more RBC transfusions (mean = 4.2; standard deviation = 4.5) than restrictive infants (mean = 3.3; standard deviation = 2.9). The number of donors per infant (median = 2 for both groups) was not statistically significant between groups. The median age at first transfusion was 8 days in the restrictive group and 3 days in the liberal group, though this difference was not statistically significant. No differences in survival or risk of PDA, ROP, or bronchopulmonary dysplasia were found between transfusion groups.
Liberal and restrictive groups did not differ in length of hospitalization, time on assisted ventilation, time on supplemental oxygen, time to regain birth weight, or time to double birth weight. Initial results of the Iowa trial (Bell et al., 2005) also indicated a higher incidence of adverse neurological events such as intraparenchymal brain hemorrhage, periventricular leukomalacia, or both, and more frequent episodes of apnea, in the restrictive group (Bell et al., 2005). Given these findings and the worse neurocognitive outcomes at 18–21 months of the restrictive group in the PINT trial (Whyte et al., 2009), further examination of the neurocognitive outcomes of these preterm infants in late childhood and adolescence is warranted.
Aims of the Current Study
The current study was designed to fill an important void in the literature by evaluating the long-term neurocognitive outcomes of differing transfusion practices in anemic preterm infants. The infants from the Iowa trial were evaluated at school age (8–15 years old) to compare the neurocognitive impact of liberal and restrictive transfusion strategies.
Methods
Participants
This study was approved by the university’s institutional review board. Participants were recruited from the 100 infants who participated in the Iowa transfusion trial (Bell et al., 2005). For the original study, eligible participants had birth weights between 500 and 1300 g and received neonatal care at the University of Iowa Children’s Hospital between December 1992 and June 1997. Infants were excluded if they had alloimmune hemolytic disease, congenital heart disease, other major birth defects requiring surgery, or a chromosomal abnormality; if their parents had strong philosophical or religious objections to transfusion; if they were thought to face imminent death; if they had received more than two transfusions prior to enrollment in the study; or if they were already enrolled in a clinical study that might interfere with the conduct or outcome of this study.
The follow-up study began in 2005—13 years after the initial study began. The parents were contacted by research nurses and asked if they would be interested in having their child participate in a study on the effects of RBC transfusion on brain structure and function in children born prematurely, as a follow-up to the original transfusion study. (Given the large nature of this study, the neuroimaging data have not been been analyzed and will be analyzed separately.) Parents who expressed interest were asked screening questions. None of the children who could be located met any of the exclusion criteria: significant hearing loss or history of epilepsy, brain tumor, or head injury resulting in unconsciousness or concussion. A total of 44 preterm subjects from the original 100 did not participate in the follow-up study: 3 were deceased, 12 declined to participate, and 29 were unable to be contacted. A death index search was conducted on those children who were lost to follow-up. These children did not match any death records through 2007. A complete flow chart of participants and non-participants by transfusion group and gender is presented in Table 1. Because the original study found differences with rates of PVL between groups two Chi-Square analyses run to determine if incidence of IVH and PVL were different between those tested, both were non-significant for differences between participating Restrictive and Liberal children (IVH: χ2 (100) = 0.097, p = .333 and PVL: χ2 (100) = −0.025, p = .805).
Table 1.
Flow Chart of Participants and Non-participants by Transfusion Group and Gender, with Frequency of IVH and PLV and Death
| Participant | N = 100 | |||||||
|---|---|---|---|---|---|---|---|---|
| Liberal n = 51 | Restrictive n = 49 | |||||||
| Male n = 21 | Female n = 30 | Male n = 30 | Female n = 19 | |||||
| Yes = 12 | No = 9 | Yes = 21 | No = 9 | Yes = 19 | No = 11 | Yes = 4 | No = 15 | |
| IVH = 1–4 | 50% | 44% | 29% | 11% | 32% | 36% | 25% | 13% |
| IVH = 4 | - | - | - | - | 5% | 9% | - | 7% |
| PVL | - | - | - | - | 5% | 9% | 25% | 7% |
| Deceased | - | - | - | 11% | - | - | - | 13% |
Note. Intraventricular Hemorrhage (IVH), Periventricular Leukomalacia (PVL).
The Score for Neonatal Acute Physiology (SNAP; Richardson et al., 1993) was recorded on the day of birth and once daily through the first week of life. Multivariate ANOVAs were conducted to examine differences in neonatal characteristics of participants (n = 56) and nonparticipants (n = 44). After correction for multiple comparisons, results indicate no significant difference in birth weight, gestational age, grade of IVH,, days on ventilator, number of apnea episodes requiring stimulation, or SNAP on the day of birth or averaged over the first week of life (Table 2; SNAP data were not available for one infant). Although the multivariate analysis demonstrated no overall effect between groups (F (6, 93) = 1.358, p = .240) mean Hematocrit level (HCT) during hospitalization did reach a significant group difference (F (1, 98) = 5.139, p = .022), where non-participants had a lower mean HCT.
Table 2.
Means, Standard Deviations, and the Results of MANOVA Analyses on Neonatal and Demographic Characteristics of Participants and Non-participants.
| Participant (n = 56) | Non-Participant (n = 44) | F | d.f. | p-value | Effect Size | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| BW | 944.57 | 198.12 | 970.55 | 185.79 | 0.447 | 98 | .505 | .005 |
| GA | 27.79 | 2.03 | 27.73 | 1.83 | 0.025 | 98 | .876 | <.001 |
| IVH Grade | 0.71 | 1.14 | 0.61 | 1.20 | 0.183 | 98 | .670 | .002 |
| HCT Mean | 41.26 | 5.22 | 38.94 | 4.55 | 5.439 | 98 | .022 | .053 |
| Vent | 22.32 | 23.70 | 23.45 | 28.61 | 0.047 | 98 | .829 | <.001 |
| Apnea | 30.18 | 31.64 | 26.25 | 27.37 | 0.427 | 98 | .515 | .004 |
| SNAP1 | 14.33 | 8.32 | 13.89 | 7.42 | 0.076 | 97 | .784 | .001 |
| SNAP2 | 10.20 | 6.61 | 10.23 | 6.70 | <.001 | 97 | .984 | <.001 |
Note. Birth weight (BW), Gestational Age (GA), Intraventricular Hemorrhage (IVH), Vent (Days on Ventilator), Apnea (Number of Apnea episodes requiring stimulation), Score for Neonatal Acute Physiology Day of Life 0 (SNAP1), Score for Neonatal Acute Physiology Week 1 Average (SNAP2). Effect sizes are generated Partial Eta Squared values.
Procedure
Guardians of participants were reimbursed for travel, lodging, and meal expenses, and child participants were compensated monetarily. Guardians were asked to accompany their children to the hospital, and informed consent was obtained in writing from one or both guardians prior to their child’s participation. Guardians completed a demographic questionnaire designed for this study that included questions regarding academic performance and socioeconomic status. Children completed a battery of cognitive, neurologic, behavioral, and social-emotional tests (administration lasted approximately 160–180 minutes). All assessments were conducted by licensed psychologists and psychology graduate assistants who were blind to the transfusion group of the children.
Measures
Cognitive Functioning
Cognitive functioning was assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003a, 2003b). The General Ability Index (GAI) is a composite of verbal and perceptual domains that was used as an estimate of global cognitive ability. In addition, prorated index scores were obtained for the Verbal Comprehension Index (VCI) using Similarities and Vocabulary subtests; Perceptual Reasoning Index (PRI) using Block Design and Matrix Reasoning subtests; and Processing Speed Index (PSI) using Digit Symbol-Coding and Symbol Search subtests. Internal reliability (r = .79 to .90) and test-retest reliability (r = .76 to .92) for these subtests are excellent, as are internal reliability estimates for the GAI (r = .96) (Raiford et al., 2005). Standard scores for GAI, VCI, PRI, and PSI were used in the analyses. The time necessary to complete these subtests is approximately 90 minutes.
Memory
Memory was assessed using the Color Span Test (Richman & Lindgren, 1978), which has established norms for ages 6 to 13 and differentiates various learning disability subtypes (Lindgren & Richman, 1984; Lindgren et al., 1986). This test assesses short-term sequential memory for information presented visually (trials 1 and 2) and verbally (trials 3 and 4). Trials 1 and 4 were used in the current study in order to detect differences in memory for visual and verbal material. In the first trial (visual-visual), information is presented visually and the participant is required to respond in a visual-motor (i.e., pointing) manner. In the fourth trial (verbal-verbal), information is presented verbally and the participant is required to respond in a verbal manner. The examiner must ensure that the child recognizes each color prior to administration. There are seven spans (increasing from 2 to 8 colors) for each subtest. Each correct response is given 1 point, and the maximum score is 14 for each subtest. Raw scores were converted to z-scores for each of the four trials. Scores for the first trial provide a measure of memory for visually presented colors and the fourth trial provides a measure of verbally presented color names. The time necessary to complete this test is approximately 20 minutes.
Associative Verbal Fluency
Associative verbal fluency was assessed using a test of Controlled Oral Word Association (COWA) (using the letters “CFL”) from the Multilingual Aphasia Examination (Benton & Hamsher, 1983). Participants were asked to list as many words as possible in one minute for each of three common letters. The score is the total number of words provided for all three letters. Raw scores were converted to z-scores, which were used in the analyses. The time necessary to complete this test is approximately 5 minutes.
Rapid Automatized Naming
Rapid naming was assessed using the Color Naming subtest of the Rapid Automatized Naming (RAN) test (Denkla & Rudel, 1976). This test requires rapid serial naming of colors that appear multiple times throughout the page. The score is the total time needed to name all colors. Raw scores were converted to z-scores, which were used in the analyses. The time necessary to complete this subtest is less than 5 minutes.
Fine Motor Coordination
Fine motor coordination was assessed by the Grooved Pegboard Test (GPB). This test was developed by Kløve (1963) and is designed to assess fine-motor coordination. It requires participants to quickly place pegs into small holes using their dominant and nondominant hands. The score used in the analyses is the total completion time for the dominant hand; these times were converted to z-scores for the analyses. The time necessary to complete this test is approximately 5 minutes.
Visual-Motor Integration
Visual-motor integration was assessed using the Bender Visual-Motor Gestalt Test, Second Edition (Bender-II) (Grannigan & Decker, 2003). For this test, participants were asked to copy increasingly complex geometric designs from cards. Scores for each design are based on the degree of accuracy with which the figure was drawn. Raw scores were converted to z-scores for the purpose of analyses. Administration time is typically under 15 minutes.
Visual-Spatial Reasoning
The Benton Judgment of Line Orientation Test (JOL) (Benton et al., 1994) assesses visual-spatial reasoning. Participants were asked to judge the relative orientation of lines on cards. The score is the total number correct out of 30 items. Raw scores were converted to z-scores, which were used in the analyses. The test takes approximately 15 minutes to complete.
Reading Ability
The Reading subtest of the Wide Range Achievement Test, Third Edition (WRAT-3) (Wilkinson, 1993) was administered to assess decoding or word recognition abilities. The raw score—or total number of correctly identified words—was converted to a standard score for each participant in order to perform the statistical analyses. The time necessary to complete this test is approximately 10 minutes.
Statistical Analysis
All analyses were performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, Illinois). Standard scores and z-scores were computed for participants using population norms. For participants outside the age ranges of the available norms were computed based upon the highest age norms available (i.e., for RAN, COWA, Color Span, JOL, and GPB). To evaluate the differences between restrictive and liberal transfusion groups, we conducted five sets of comparisons using independent samples t-tests. Analyses were performed with transfusion group as the independent variable and with no covariates. Each set comprised several comparisons, and the alpha levels were adjusted with the Bonferroni correction. The following comparisons were performed:
neonatal and demographic characteristics (6 variables), values below p = .008 are significant;
cognitive ability and achievement (i.e., GAI, VCI, PRI, PSI, WRAT Reading), values below p = .01 are significant;
language functioning (i.e., COWA, RAN), values below p = .025 are significant;
visual-spatial/motor functioning (JOL, GPB, Bender-2), values below p = .017 are significant; and
memory (Visual and Verbal Memory - Color Span), values below p = .025 are significant.
Results
Demographic Characteristics
Follow-up assessments occurred when the children were between 8 and 15 years of age. Of the 100 infants enrolled in the Iowa trial, 56 participated in the follow-up assessment. Twenty-three subjects (19 males, 4 females) were from the restrictive group (median age = 12.42 years, mean = 12.53, SD = 1.55). There were 33 subjects (12 males, 21 females) in the liberal group (median age = 12.42, mean = 11.94, SD = 1.70). There were too few females in the restrictive group to allow gender comparisons.
Independent samples t-tests were conducted to compare liberal and restrictive groups with respect to neonatal and demographic characteristics (Table 3). Liberal and restrictive groups did not differ in birth weight, gestational age, grade of IVH, days on ventilator, number of apnea episodes requiring stimulation, SNAP on the day of birth, SNAP averaged over the first week of life, age at follow-up, or parental ratings of SES (based on Hollingshead scale; Hollingshead & Redlich, 1958). As expected, the mean HCT for those in the Restrictive group (mean = 36.65, SD = 2.41) was significantly lower than the mean for those in the Liberal group (mean = 44.47, SD = 1.10; t (54) = −8.203, p < .001). Both participating transfusion groups were predominantly Caucasian (90% in the Restrictive group and 81% in the Liberal). The Restrictive group was comprised of one Hispanic and one multiracial participant while the Liberal group was comprised of four African American and two multiracial participants.
Table 3.
Means, Standard Deviations, and the Results of Independent Samples t-Test Analysis on Neonatal and Demographic Characteristics of Participating Liberal and Restrictive Groups.
| Liberal (n = 33) | Restrictive (n = 23) | t | d.f. | p-value | Effect Size | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| BW | 936.52 | 210.13 | 956.13 | 183.5 | 0.362 | 54 | .719 | .049 |
| GA | 27.88 | 2.28 | 27.67 | 1.65 | −0.371 | 54 | .712 | .050 |
| IVH Grade | 0.82 | 1.18 | 0.57 | 1.08 | −0.815 | 54 | .419 | .110 |
| HCT Mean | 44.47 | 4.10 | 36.65 | 2.41 | −8.203 | 54 | <.001 | .745 |
| Vent | 24.33 | 26.96 | 19.43 | 18.24 | −0.758 | 54 | .452 | .103 |
| Apnea | 24.00 | 24.16 | 39.04 | 38.92 | 1.785 | 54 | .080 | .236 |
| SNAP1 | 15.06 | 7.57 | 13.30 | 9.33 | −0.771 | 53 | .444 | .105 |
| SNAP2 | 10.34 | 6.69 | 10.00 | 6.64 | −0.188 | 53 | .851 | .026 |
| Age | 11.94 | 1.70 | 12.53 | 1.55 | 1.323 | 54 | .191 | .177 |
| SES | 2.76 | 0.64 | 2.74 | 0.58 | −0.110 | 54 | .913 | .015 |
| Mom Ed | 13.94 | 2.50 | 13.71 | 2.95 | −0.301 | 52 | .765 | .042 |
| Dad Ed | 12.79 | 3.06 | 13.55 | 2.78 | 0.886 | 46 | .380 | .130 |
Note. Birth weight (BW), Gestational Age (GA), Intraventricular Hemorrhage (IVH), Vent (Days on Ventilator), Apnea (Number of Apnea episodes requiring stimulation), Score for Neonatal Acute Physiology Day of Life 0 (SNAP1), Score for Neonatal Acute Physiology Week 1 Average (SNAP2), Socioeconomic Status (SES). Mother and Father Education are reported in years or school. Effect sizes (r) were calculated by r = √(t2/(t2+df)).
Intelligence and Achievement
Differences between liberal and restrictive groups in cognitive and achievement measures were analyzed with independent samples t-tests (Table 4). Liberal group children obtained lower scores than restrictive children on all intelligence and achievement outcome variables. This reached statistical significance for reading (t (54) = 3.300, p = .002), with a large effect size of r =.41. No significant differences between transfusion groups were found on measures of GAI, VCI, PRI, or PSI.
Table 4.
Means, Standard Deviations, and the Results of Independent Samples t-Test Analysis on Cognitive and Achievement Scores for Liberal and Restrictive Groups.
| Liberal (n = 33) | Restrictive (n = 23) | t | d.f. | p-value | Effect Size | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| GAI | 93.21 | 20.7 | 103.61 | 15.7 | 2.036 | 54 | .047 | .267 |
| VCI | 93.85 | 26.0 | 104.78 | 15.7 | 1.798 | 54 | .078 | .238 |
| PRI | 91.67 | 18.1 | 99.70 | 15.5 | 1.729 | 54 | .089 | .229 |
| PSI | 88.82 | 14.4 | 95.5 | 14.8 | 1.694 | 54 | .096 | .225 |
| WRAT-III | 93.94 | 15.0 | 105.83 | 10.2 | 3.300 | 54 | .002 | .410 |
Note. General Ability Index (GAI), Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Processing Speed Index (PSI), Wide Range Achievement Test, Third Edition (WRAT-III). Effect sizes (r) were calculated by r = √(t2/(t2+df)).
Neuropsychological Functioning
In the same pattern seen for intelligence and achievement assessments, liberal group children scored lower than restrictive children on all neuropsychological tests. This reached statistical significance for associative verbal fluency (t (54) = 3.072, p = .003) and visual memory (t (54) = 2.516, p = .015; Table 5), with effect sizes ranging from medium for visual memory (r = .32) to large for verbal fluency (r = .39). No significant differences between liberal and restrictive groups were found for verbal memory, rapid naming, visual-spatial reasoning, fine motor dexterity, or visual-motor integration.
Table 5.
Means, Standard Deviations, and the Results of Independent Samples t-Test Analysis on Language, Visual-Spatial/Motor, and Memory Scores for Liberal and Restrictive Groups.
| Liberal (n = 33) | Restrictive (n = 23) | t | d.f. | p-value | Effect Size | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| COWA | −1.30 | 1.24 | −0.31 | 1.10 | 3.072 | 54 | .003 | .386 |
| RAN | 0.08 | 1.70 | 0.59 | 1.02 | 1.400 | 53 | .167 | .189 |
| JOL | −1.06 | 1.54 | −0.81 | 1.23 | 0.540 | 35 | .593 | .091 |
| GPB | −0.75 | 2.00 | −0.24 | 0.97 | 1.130 | 54 | .264 | .152 |
| Bender-II | 0.12 | 1.19 | 0.75 | 0.90 | 2.138 | 54 | .037 | .279 |
| Vis Mem | −3.05 | 1.75 | −1.95 | 1.38 | 2.516 | 54 | .015 | .324 |
| Ver Mem | −1.41 | 1.42 | −0.92 | 0.96 | 1.435 | 54 | .157 | .192 |
Note. Controlled Oral Word Association (COWA), Rapid Automatized Naming (RAN), Judgment of Line (JOL), Grooved Pegboard (GPB), Visual Memory (Vis Mem), Verbal Memory (Ver Mem). Levene’s Test of Inequality of Variances was significant for the RAN (F = 6.610, p = .013); t-test for unequal variances is used. Only 37 participants (20 Liberal and 17 Restrictive) were administered the JOL. Effect sizes (r) were calculated by r = √(t2/(t2+df)).
Discussion
The primary purpose of the current study was to examine the long-term neurocognitive impact of different transfusion strategies for preterm infants. We examined neurocognitive outcomes of preterm children randomly assigned to either a liberal or a restrictive transfusion protocol. This analysis is important because of evidence from the neonatal period and later infancy suggesting that liberal transfusion may confer neuroprotection (Bell et al, 2005; Whyte et al, 2009).
On the contrary, the findings of the present study indicate that liberally transfused preterm subjects perform as poorly as or worse than restrictive subjects on measures of neurocognitive and academic functioning. Although liberal group mean scores were consistently lower than restrictive scores on all measures (see Figures 1 and 2), the differences between transfusion groups were significant only for associative verbal fluency, visual memory, and reading. These results provide evidence that liberal blood transfusion practices can have a significant negative impact on neurocognitive functioning and academic achievement above and beyond the impact that is associated with preterm status alone.
Figure 1.
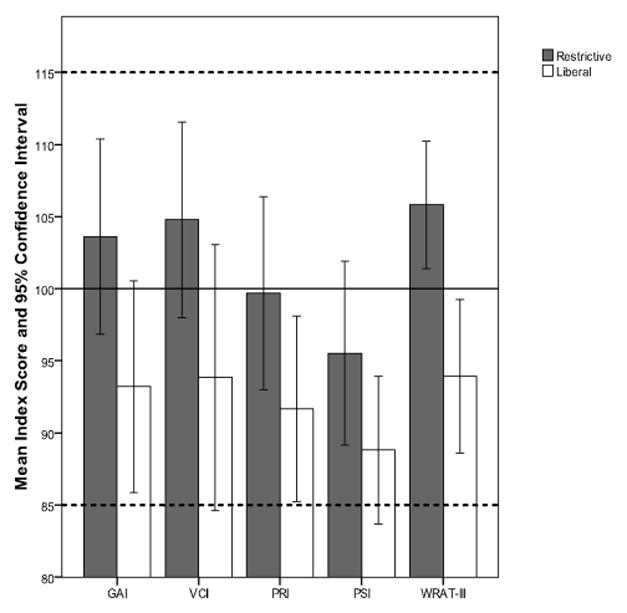
Mean Intelligence and Reading Achievement Scores and Confidence Intervals for each Transfusion Group.
Figure 2.
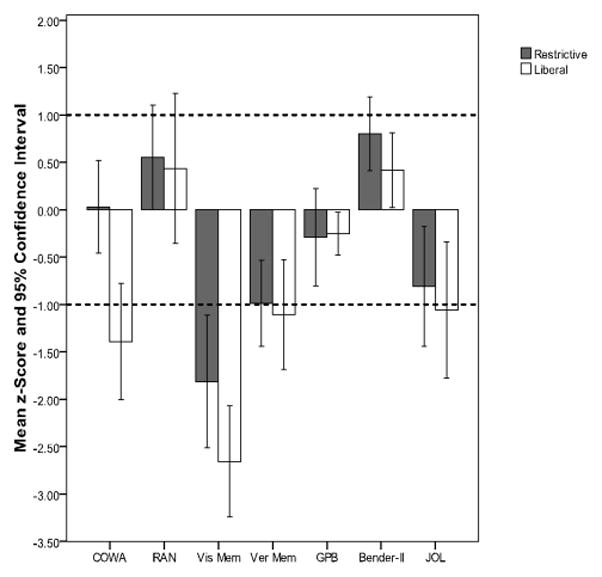
Mean z-Scores and Confidence Intervals on Neurocognitive Tests for each Transfusion Group.
The current findings did not support the results of the initial comparison conducted in the Bell et al. (2005) trial and the PINT study (Whyte et al., 2009), both of which suggested more favorable outcomes for the liberal group – at least at younger ages. Our findings indicating better outcomes for the restrictive group are particularly noteworthy given the significantly higher proportion of males - who are typically at greater risk than females for developmental learning disabilities - in the restrictive group compared to the liberal group. Post-hoc comparisons were run on neonatal characteristics (i.e., BW, GA, SNAP1 and SNAP2) to ensure that the children in the liberal group who participated were not sicker than those who did not participate and that those in the restricted group who did not participate were not sicker than those who did. Multivariate analysis of BW, GA, SNAP1 and SNAP2 with independent variables of transfusion group and participation status did not yield any significant interaction effect (F (4, 92) = 0.402, p = .807) or main effects for transfusion group (F (4, 92) = 0.189, p = .943) or participation status (F (4, 92) = 0.181, p = .948). This ensures that it was not the sickest from the liberal group and the healthiest from the restricted group being tested.
The Possible Role of EPO-Suppression
Although the mechanisms by which liberal transfusion criteria might lead to worse outcomes in the preterm infant are unknown, recent studies using animal models point to the phenomenon of erythropoietin-suppression as a possible explanation. Erythropoietin (EPO) is a central nervous system protein that has been shown to play a significant role in protecting the brain against short- and long-term damage from inadequate blood supply and/or oxygen deprivation (Siren et al., 2000). Both endogenous EPO production and treatment with recombinant erythropoietin (rEPO; an exogenous form of the hormone) may protect the brain by reducing inflammation secondary to reduced blood flow effects (Agnello et al., 2002; Jun, JiangTao, YuanGui, YongBin, Jun, XiaoJun et al., 2009; Liu, Xie, Liu, Duan, Jia, Li, & Xu, 2006; Siren et al., 2001). Findings from a retrospective cohort study of 82 preterm infants evaluated at 12 months corrected age indicated a dose-response relationship between total rEPO dose within the first 6 weeks of life and Bayley Mental Developmental Index (MDI) scores. This finding lends further support to the possible protective effect of cumulative rEPO on developmental outcome (Brown, Eichorst, LaLa-Black, & Gonzalez, 2009). Thus, the phenomenon of EPO-suppression provides a possible mechanism for the poorer neurocognitive outcomes of the liberal transfusion group in the current study. Of note, the plasma EPO levels were significantly higher in the restrictive group in the Iowa trial (Bell et al., 2005).
Studies have also shown that RBC transfusion may temporarily inhibit or delay the body’s EPO response, resulting in decreased protection against infection and inflammation (Frey, 2002; Keyes, Donohue, Spivak, Jones, & Oski, 1989). In other words, RBC transfusion may reduce the body’s ability to effectively respond to the threat of brain injury in a protective fashion. The protective action of EPO may have the most pronounced impact on brain areas such as the hippocampus that are known to be especially vulnerable to cell death and cerebral damage (Jun et al., 2009).
Neurocognitive Outcomes: Verbal Fluency, Visual Memory, and Reading
The liberal transfusion group performed significantly more poorly on a test of associative verbal fluency than the restrictive group. Impairments in verbal fluency, even in the absence of global cognitive impairment or structural MRI abnormalities, have been found in other preterm groups (Rushe et al., 2001; Steward et al., 1999). Wolke and Meyer (1999) also found evidence of impairment in language and pre-reading skills (speech, articulation, and number naming) in a group of German preschoolers who were born preterm.
Deficits in verbal fluency, in conjunction with impaired performances on tests of memory for visual material and reading, are consistent with neurocognitive profiles frequently seen in children with developmental language and reading disability (Lindgren & Richman, 1984; Lindgren et al., 1986). Children who do not automatically apply verbal labels to visual information in order to facilitate recall also frequently demonstrate difficulty rapidly generating verbal information and calling to mind the names of well-rehearsed information (i.e., the names of colors) (Kail & Leonard, 1986; Korhonen, 1995). The finding that there were significant differences for colors presented visually but not for colors presented verbally may indicate that the problem is more one of efficient verbal labeling of visual information (Dysnomia) rather than an overall verbal memory deficit (Dysmnesia). Word-finding or word retrieval difficulties, which are often exacerbated under pressure or timed conditions, have been strongly associated with developmental language disorders (Kail & Leonard, 1986; Kail, Hale, Leonard, & Nippold, 1984; Kirchner & Klatzky, 1985). Verbal deficits of this nature are also strong predictors for later academic difficulties in reading, arithmetic, and spelling, in addition to problems associated with decoding and/or reading comprehension (i.e., developmental reading disability; dyslexia). Although the magnitude of difference in rapid naming performance between liberal and restrictive groups was not statistically significant, the liberal group nevertheless demonstrated lower group mean raw differences in color naming speed (liberals m = 41.56, SD = 11.38; restrictive, m = 37.92, SD = 8.55).
Potential distinctions between rapid naming and verbal fluency are of particular interest given our finding that the liberal group performed worse than the restrictive group on a test of associative verbal fluency but that scores were not as discrepant on a test of rapid naming. Studies suggest that associative verbal fluency is related to but separate from other common language functions, and that deficits in verbal fluency may be associated primarily with damage or dysfunction in prefrontal/premotor and hippocampal regions (Boone, Pontón, Gorsuch, Gonzalez, & Miller, 1998; Stuss, Alexander, Hamer, Palumbo, Dempster, Binns, Levine et al., 1998; Whitney, Weis, Krings, Huber, Grossman, & Kircher, 2008). Research also suggests that associative verbal fluency is strongly associated with if not dependent upon processing speed (Boone et al., 1998) and executive functions, as it requires both “efficient and restricted search of the lexicon” (Narhi, Ahonen, Aro, Leppasaari, Korhonen, Tolvanen, & Lyytinen, 2004, p. 51).
One possible explanation for the more impaired associative verbal fluency in the liberal group is that liberal transfusion may increase the risk for selective damage or dysfunction to brain regions involved in verbal fluency. Findings from neuroimaging studies of adults with brain lesions (Damasio, Anderson, & Tranel, In Press), patients with schizophrenia (Kircher, Whitney, Krings, Huber, & Weis, 2008; Spence et al., 2000), patients with temporal lobe epilepsy (Alessio et al., 2006; Gleissner & Elger, 2001), and normal healthy controls (Whitney et al., 2008) indicate significant involvement of both prefrontal and hippocampal regions in tasks of verbal fluency (although differences in brain region involvement may present as a function of whether the verbal fluency task is phonological or semantic in nature).
Though of potential interest, the current findings are preliminary and should be interpreted with caution given the current study’s limitations. It is our objective to use the current information regarding relative neurocognitive strengths and weaknesses in liberal and restrictive transfusion groups to generate a targeted follow-up evaluation that will further contribute to our understanding of the highly specific aspects of language, working memory, processing speed, inhibitory control, attention, and executive functioning potentially affected by preterm status and RBC transfusion. Evaluation of specific and subtle neuropsychological functions will likely also be fruitful in more clearly identifying vulnerable brain regions and elucidating specific patterns of neurocognitive weakness in older elementary and adolescent individuals who as neonates received differential treatment for critical illness associated with preterm status. Caution in interpreting these preliminary findings is warranted given the fact that the liberal group performed more poorly than the restrictive group on all measures, though these differences were significant only for a subset of functions assessed. It will be important to corroborate the current findings with larger samples, and to investigate neurocognitive outcomes by gender.
Limitations and Future Directions
The results of this study would be strengthened if corroborated by neuroimaging data. Neuroimaging documenting abnormalities associated with the right parietal, hippocampal, prefrontal, and cerebellar regions would broaden our understanding of the impact of transfusion and preterm status on specific brain areas as well as further validate the present findings. The absence of such corroboration should be interpreted with caution, however, given research indicating that structural abnormalities are inconsistent predictors of functional impairment (Vicari et al., 2004; Stewart et al., 1999).
Secondly, despite rigorous attempts to locate all of the original subjects for follow-up examination, a disproportionate number of females were in the restrictive transfusion group. As a result, analysis of the outcomes by sex was not conducted, limiting our understanding of potential interactions between sex and transfusion group. Given established sex differences in brain development, it will be crucial to further explore the way in which sex may alter or moderate the effects of transfusion on the brain and associated neurocognitive functions. Furthermore, we concur with Vicari et al. (2004) and Espy et al. (2009) that cross-sectional evaluation of preterm groups is insufficient and that longitudinal follow-up of preterm cohorts will be crucial in identifying the extent to which specific neurocognitive deficits identified early in development persist, resolve, or worsen over the course of adolescence and adulthood. Additional limitations of the current study include the poor retention rate (56%), small sample and power limitations, and wide age range of the participants at follow-up. It is crucial to recognize the potential impact of age, gender, and environmental factors (e.g., SES, geographic location) on preterm outcomes. These potential contributing factors will need to be better addressed in future research with larger samples.
Conclusion
The results of the current study suggest the possibility of long-term neurodevelopmental risks associated with maintaining the hematocrit at higher levels by liberally transfusing preterm neonates. The results also highlight the importance of interpreting early outcomes with caution. Research in the area of EPO-suppression provides a plausible explanation for the findings of the present study. Further research with animal models and critically ill human subjects may help to elucidate whether EPO-suppression is a valid and comprehensive explanation for the poorer outcomes observed in the liberal transfusion group. Future studies might also provide insight into other brain regions likely to be affected by EPO-suppression as well as the exact neural mechanisms implicated in endogenous EPO reactions. It is clear that further exploration into the role of the hippocampus and related subcortical structures and neural pathways is also warranted.
The reduction in serious brain abnormalities seen on ultrasound with liberal transfusion in the Iowa trial (Bell et al, 2005) and the positive 18-month neurocognitive outcomes in the liberally transfused group from the PINT trial (Whyte et al., 2009) suggested that there might be neuroprotective effects from maintaining higher hemoglobin and hematocrit levels through more liberal transfusion. However, the findings of the current study appear to contradict this possible neuroprotective effect and suggest instead that maintaining higher hemoglobin and hematocrit levels may have long-term adverse effects on brain function. More information is needed before definitive recommendations can be made regarding the target hemoglobin or hematocrit levels that are optimal for preterm infants.
Acknowledgments
This publication was made possible by Grant Number UL1RR024979 from the National Center for Research Resources (NCRR), a part of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CTSA or NIH”) and our PPG (NIH Program Project Grant P01 HL046925). We would also like to acknowledge the ICTS/CTSA CRU for their assistance with and support of this project.
Abbreviations
- RBC
Red Blood Cell
- SNAP
Score for Neonatal Acute Physiology
- SES
Socioeconomic Status
- GAI
Global Abilities Index
- VCI
Verbal Comprehension Index
- PRI
Perceptual Reasoning Index
- PSI
Processing Speed Index
- WRAT
Wide Range Achievement Test – III
- COWA
Controlled Oral Word Association
- RAN
Rapid Automatized Naming
- Vis Mem
Color Span Visual Memory
- Ver Mem
Color Span Verbal Memory
- GPB
Grooved Pegboard
- JOL
Judgment of Line Orientation
References
- Agnello D, Bigini P, Villa P, MEnnini T, Cerami A, Brines ML, Ghezzi P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoinmune encephalomyelitis. Brain Research. 2002;952:128–134. doi: 10.1016/S0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- Alessio A, Bonilha L, Rorden C, Kobayashi E, Min LL, Damasceno BP, Cendes F. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy & Behavior. 2006;8:593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW the Victorian Infant Collaborative Study Group. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. 10.1542peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Developmental and Behavioral Pediatrics. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. 0196-206X/05/2606-0427. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Cognitive and neuropsychological outcomes: More than IQ scores. Mental Retardation and Developmental Disabilities. 2002;8:234–240. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Infant and Early Childhood Neuropsychology. New York and London: Plenum Press; 1997. [Google Scholar]
- Bain A, Blackburn S. Issues in transfusing preterm infants in the NICU. Journal of Perinatal and Neonatal Nursing. 2004;18:170–182. doi: 10.1097/00005237-200404000-00011. [DOI] [PubMed] [Google Scholar]
- Beeram MR, Krauss DR, Riggs MW. Red blood cell transfusion practices in very low birth weight infants in 1990s postsurfactant era. Journal of the National Medical Association. 2001;93:405–409. [PMC free article] [PubMed] [Google Scholar]
- Bell EF. When to transfuse preterm babies. Arch Dis Child Fetal Neonatal. 2008;93:469–473. doi: 10.1136/adc.2007.128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EF. Transfusion thresholds for preterm infants: How low should we go? Journal of Pediatrics. 2006;149:287–289. doi: 10.1016/j.jpeds.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, Cress GA, Johnson KJ, Kromer IJ, Zimmerman MB. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–1691. doi: 10.1542/peds.2004–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City: Department of Neurology, University of Iowa Hospitals and Clinics; 1983. [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. 2. New York: Oxford University Press; 1994. [Google Scholar]
- Bhutta A, Cleves M, Casey P, Cradock M, Anand K. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Boone KB, Pontón MO, Gorsch RL, González JJ, Millar BL. Factor analysis of four measures of prefrontal lobe functioning. Archives of Clinical Neuropsychology. 1998;13:585–595. doi: 10.1016/S0887-6177(97)00074-7. [DOI] [PubMed] [Google Scholar]
- Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–1721. 0006-4971/94/8406-0013. [PubMed] [Google Scholar]
- Breslau N. Psychiatric Sequelae of Low Birth Weight. Epidemiology Review. 1995;17:96–106. doi: 10.1093/oxfordjournals.epirev.a036191. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO, Lucia VC. Academic achievement of low birthweight children at age 11: The role of cognitive abilities at school entry. Journal of Abnormal Child Psychology. 2001;29:273–279. doi: 10.1023/A:1010396027299. [DOI] [PubMed] [Google Scholar]
- Brooks SE, Marcus DM, Gillis D, Pirie E, Johnson C, Bhatia J. The effect of blood transfusion protocol on retinopathy of prematurity: A prospective, randomized study. Pediatrics. 1999;104:514–518. doi: 10.1542/peds.104.3.514. [DOI] [PubMed] [Google Scholar]
- Brown MS, Eichorst D, Lala-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2008;124:681–687. doi: 10.1542/peds.2008-2701. [DOI] [PubMed] [Google Scholar]
- Brown NC, Inder TE, Bear MJ, Hunt RW, Anderson PJ, Doyle LW. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. Journal of Pediatrics. 2009;155:32–38. doi: 10.1016/j.jpeds.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Anderson SW, Tranel D. The frontal lobes. In: Heilman K, Valenstein E, editors. Clinical Neuropsychology. 5. New York: Oxford University Press; (In Press) [Google Scholar]
- Denkla M, Rudel R. Rapid automatized naming (RAN): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Saigal S. Long-term outcomes of very preterm or tiny infants. NeoReviews. 2009;10:e130–137. doi: 10.1542/neo.10-3-e130. [DOI] [Google Scholar]
- Espy KA, Fang H, Charak D, Minich N, Taylor HG. Growth mixture modeling of academic achievement in children of varying birth weight risk. Neuropsychology. 2009;23:460–474. doi: 10.1037/a0015676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Shear PK, Cahn-Weiner DA, Marsh L, Lim KO, Yesavage JA, et al. Structural brain correlates of verbal and nonverbal fluency measures in Alzheimer’s disease. Neuropsychology. 2000;14:29–40. doi: 10.1037/0894-4105.14.1.29. [DOI] [PubMed] [Google Scholar]
- Franz AR, Pohlandt F. Red blood cell transfusions in very and extremely low birthweight infants under restrictive transfusion guidelines : Is exogenous erythropoietin necessary? Arch Dis Child Fetal Neonatal Ed. 2001;84:F96–F100. doi: 10.1136/fn.84.2.F96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B. Transfusion in premature infants impairs production and/or release of red blood cells and platelets. Journal of Paediatric Child Health. 2002;38:265–267. doi: 10.1046/j.1440-1754.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- Gleissner U, Elger CE. The hippocampal contribution to verbal fluency in patients with temporal lobe epilepsy. Cortex. 2001;37:55–63. doi: 10.1016/S0010-9452(08)70557-4. [DOI] [PubMed] [Google Scholar]
- Goyen T, Lui K, Woods R. Long-term follow-up of infants and later neurobehavioral function. Journal of the American Medical Association. 1998;261:1767–1772. [Google Scholar]
- Grannigan GG, Decker SL. Bender visual-motor gestalt test. 2. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 740 g. The New England Journal of Medicine. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- Hagen EW, Palta M, Albanese A, Sadek-Badawi M. School achievement in a regional cohort of children born very low birthweight. Journal of Developmental & Behavioral Pediatrics. 2006;27:112–121. doi: 10.1097/00004703-200604000-00005. 0196-206X/06/2702-0112. [DOI] [PubMed] [Google Scholar]
- Harvey J, O’Callaghan M, Mohay H. Executive Function of children with extremely low birthweight: A case control study. Developmental Medicine and Child Neurology. 1999;41:292–297. doi: 10.1111/j.1469-8749.1999.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Hoff B, Hansen BM, Munck H, Mortensen EL. Behavioral and social development of children born extremely premature: 5-year follow-up. Scandinavian Journal of Psychology. 2004;45:285–292. doi: 10.1111/j.1467-9450.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: A community study. New York, NY: John Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson I, Cooke A, Holland B, et al. Red cell volume and cardiac output in anaemic preterm infants. Critical Care Medicine. 1990;18:1360–1362. doi: 10.1136/adc.65.7_spec_no.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume H. Red blood cell transfusions for preterm infants: The role of evidence-based medicine. Seminars in Perinatology. 1997;21:8–19. doi: 10.1016/s0146-0005(97)80015-8. [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Chinkes DL, Finnerty CC, Przkora R, Pereira CT, Herndon DN. Blood transfusions are associated with increased risk for development of sepsis in severely burned pediatric patients. Critical Care Medicine. 2007;35:579–583. doi: 10.1097/01.CCM.0000253812.09236.98. [DOI] [PubMed] [Google Scholar]
- Jongmans M, Mercuri E, de Vries L, Dubowitz L, Henderson SE. Minor neurological signs and perceptual-motor difficulties in prematurely born children. Archives of Disease in Childhood. 1996;76:F9–F14. doi: 10.1136/fn.76.1.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun Y, Tao XJ, YuanGui H, YongBin S, Jun Z, XiaoJun M, JianChun X, Heng X, XiaoXin Z, XinXiang X. Erythropoietin pre-treatment prevents cognitive impairments following status epilepticus in rats. Brain Research. 2009;1282:57–66. doi: 10.1016/j.brainres.2009.05.062. [DOI] [PubMed] [Google Scholar]
- Kail R, Hale CA, Leonard LB, Nippold MA. Lexical storage and retrieval in language-impaired children. Applied Psycholinguistics. 1984;5:37–49. doi: 10.1017/S0142716400004823. [DOI] [Google Scholar]
- Keyes WG, Donohue PK, Spivak JL, Jones D, Jr, Oski FA. Assessing the need for transfusion of premature infants and role of hematocrit, clinical signs, and erythropoietin level. Pediatrics. 1989;84:412–417. [PubMed] [Google Scholar]
- Kircher T, Whitney C, Krings T, Huber W, Weis S. Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophrenia Research. 2008;101:242–255. doi: 10.1016/j.schres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Kirchner DM, Klatzky RL. Verbal rehearsal and memory in language-disordered children. Journal of Speech and Hearing Research. 1985;28:556–565. doi: 10.1044/jshr.2804.556. [DOI] [PubMed] [Google Scholar]
- Kirpalani HK, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, Peliowski A, et al. The premature infants in need of transfusion (PINT) study: A randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. Journal of Pediatrics. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Kløve H. Clinical neuropsychology. In: Forester FM, editor. Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- Korhonen TT. The persistence of rapid naming problems in children with reading disabilities: A nine-year follow-up. Journal of Learning Disabilities. 1995;28:232–239. doi: 10.1177/002221949502800405. [DOI] [PubMed] [Google Scholar]
- Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, et al. Transfusion strategies for patients in pediatric intensive care units. New England Journal of Medicine. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- Levy GJ, Strauss RG, Hume H, Schloz L, Albanese MA, Blazina J, Werner A, et al. National survey of neonatal transfusion practices: I. Red blood cell therapy. Pediatrics. 1993;91:523–529. [PubMed] [Google Scholar]
- Lindgren SD, Richman LC. Immediate memory function of verbally deficit reading- disabled children. Journal of Learning Disabilities. 1984;17:222–225. doi: 10.1177/002221948401700407. [DOI] [PubMed] [Google Scholar]
- Lindgren SD, Richman LC, Eliason MJ. Memory processes in reading disability subtypes. Developmental Neuropsychology. 1986;2:173–181. [Google Scholar]
- Litt J, Taylor HG, Klein N, Hack M. Learning disabilities in children with very low birth weight: Prevalence, neuropsychological correlates, and educational interventions. Journal of Learning Disabilities. 2005;38:130–141. doi: 10.1177/00222194050380020301. [DOI] [PubMed] [Google Scholar]
- Liu X, Xie W, Liu P, Duan M, Jia Z, Li W, Xu J. Mechanism of the cardioprotection of rhEPO pretreatment on suppressing the inflammatory response in ischemia-reperfusion. Life Sciences. 78:2255–2264. doi: 10.1016/j.lfs.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Luciana M, Lindeke L, Mills M, Nelson C. Neurobehavioral evidence for working memory deficits in school-aged children with histories of prematurity. Developmental Medicine and Child Neurology. 1999;41:521–533. doi: 10.1111/j.1469-8749.1999.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Maier RF, Sonntag J, Walka MM, Liu G, Metze BC, Obladen M. Changing practices of red blood cell transfusions in infants with birth weights less than 1000 g. Journal of Pediatrics. 2000;136:220–224. doi: 10.1016/S0022-3476(00)70105-3. [DOI] [PubMed] [Google Scholar]
- Marik P. The hazards of blood transfusion. British Journal of Hospital Medicine. 2009;70:12–15. doi: 10.12968/hmed.2009.70.1.37688. [DOI] [PubMed] [Google Scholar]
- Miceli PJ, Goeke-Morey MC, Whitman TL, Kolberg KS, Miller-Loncar C, White RD. Brief report: Birth status, medical complications, and social environment: Individual differences in development of preterm, very low birth weight infants. Journal of Pediatric Psychology. 2000;25:353–358. doi: 10.1093/jpepsy/25.5.353. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Marlow N. Processing speed and working memory underlie academic attainment in very preterm children. Archives of Disease in Childhood – Fetal and Neonatal Edition. 2010;95:F267–F272. doi: 10.1136/adc.2009.167965. [DOI] [PubMed] [Google Scholar]
- Murray NA, Roberts IAG. Neonatal transfusion practices. Arch Dis Child Fetal Neonatal Ed. 2004;89:101–107. doi: 10.1136/adc.2002.019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau L, Tessier R, Boivin M, Lefebvre F, Robaey P. Extremely premature and very low birthweight infants: A double hazard population? Social Development. 2003;12:2–15. doi: 10.1111/1467-9507.00231. [DOI] [Google Scholar]
- Narhi V, Ahonen T, Aro M, Leppasaari T, Korhonen TT, Tolvanen A, Lyytinen H. Rapid serial naming: Relations between different stimuli and neuropsychological factors. Brain and Language. 2005;92:45–57. doi: 10.1016/j.bandl.2004.05.004. [DOI] [PubMed] [Google Scholar]
- O’Callaghan MJ, Burns YR, Gray PH, Harvey JM. School performance of ELBW children: A controlled study. Developmental Medicine & Child Neurology. 1996;38:917–926. doi: 10.1111/j.1469-8749.1996.tb15048.x. [DOI] [PubMed] [Google Scholar]
- Raiford SE, Weiss LG, Rolfhus EL, Coalson D. Clinical validity (WISC-IV Technical Report No. 4) 2005 Retrieved August 19, 2008, from http://harcourtassessment.com/hai/Images/pdf/wisciv/WISCIVTechReport4.pdf.
- Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for neonatal acute physiology: A physiological severity index for neonatal intensive care. Pediatrics. 1993;91:617–623. doi: 10.1111/j.1651-2227.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- Richman LC, Lindgren SD. Color Span Test Manual. Iowa City, IA: Educational Resources; 1978. [Google Scholar]
- Ringer SA, Richardson DK, Sacher RA, Keszler M, Churchhill WH. Variations in transfusion practice in neonatal intensive care. Pediatrics. 1998;101:194–200. doi: 10.1542/peds.101.2.194. [DOI] [PubMed] [Google Scholar]
- Ross MP, Christensen RD, Rothstein G, et al. A randomized trial to develop criteria for administering erythrocyte transfusions to anemic preterm infants 1 to 3 months of age. Journal of Perinatology. 1989;9:246–253. [PubMed] [Google Scholar]
- Rushe TM, Rifkin L, Stewart AL, Townsend JP, Roth SC, Wyatt JS, Murray RM. Neuropsychological outcome at adolescence of very preterm birth and its relation to brain structure. Developmental Medicine & Child Neurology. 2001;43:226–233. doi: 10.1111/j.1469-8749.2001.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Saigal H, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Saigal H, Hoult LA, Streiner DL, Stroskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- Siren A, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. PNAS. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SA, Liddle PF, Stefan MD, Hellewell JSE, Sharma T, Friston KJ, et al. Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. British Journal of Psychiatry. 2000;176:52–60. doi: 10.1192/bjp.176.1.52. [DOI] [PubMed] [Google Scholar]
- Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatric Clinics of North America. 2009;56:631–646. doi: 10.1016/j.pcl.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Rifkin L, Amess PN, Kirkbride V, Townsend JP, Miller DH, Lewis SW, Kingsley DPE, Moseley IF, Foster O, Murray RM. Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet. 1999;353:1653–1657. doi: 10.1016/S0140-6736(98)07130-X. [DOI] [PubMed] [Google Scholar]
- Strauss RG. Red blood cell transfusion practices in the neonate. Perinatal Hematology. 1995;22:641–655. [PubMed] [Google Scholar]
- Strauss RG. Transfusion therapy in neonates. American Journal of the Disabled Child. 1991;145:904–911. doi: 10.1001/archpedi.1991.02160080082025. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, Levine B, Izukawa D. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4:265–278. [PubMed] [Google Scholar]
- Szekely A, Cserep Z, Sapi E, Breuer T, Nagy CA, Vargha P, Hartyanskzy I, Szatmari A, Treszl A. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Annals of Thoracic Surgery. 2009;87:187–197. doi: 10.1016/j.athoracsur.2008.09.079. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Hack M, Klein NK. School-age consequences of birthweights less than 750 g: A review and update. Developmental Neuropsychology. 2000;17:289–321. doi: 10.1207/S15326942DN1703_2. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Hack M, Klein NK. Attention deficits in children with < 750 gram birth weight. Child Neuropsychology. 1998;4:21–34. doi: 10.1076/chin.4.1.21.3188. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: Neuropsychological findings. Journal of the International Neuropsychological Society. 2004;10:149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- Van de Watering LMG, Hermans J, Houbiers JGA, van den Broek PJ, Bouter H, Boer F, Harvey MS, Huysmans HA, Brand A. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: A randomized clinical trial. Circulation. 1998;97:562–568. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- Venancio JP, dos Santos AMN, Guinsburg R, Peres C, Shinzato AR, Lora MI. Strict guideline reduces the need for RBC transfusions in premature infants. Journal of Tropical Pediatrics. 2006;53:78–82. doi: 10.1093/tropej/fml062. [DOI] [PubMed] [Google Scholar]
- Vicari S, Caravale B, Carlesimo GA, Casadei AM, Allemand F. Spatial working memory deficits in children ages 3-4 who were low birth weight, preterm infants. Neuropsychology. 2004;18:673–678. doi: 10.1037/0894-4105.18.4.673. [DOI] [PubMed] [Google Scholar]
- Waber D, McCormick M. Late neuropsychological outcomes in preterm infants of normal IQ: Selective vulnerability of the visual system. Journal of Pediatric Psychology. 1995;20:721–735. doi: 10.1093/jpepsy/20.6.721. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Administration and Scoring Manual. 4. San Antonio: The Psychological Corporation; 2003a. Wechsler Intelligence Scale for Children. [Google Scholar]
- Wechsler D. Technical and Interpretive Manual. 4. San Antonio: The Psychological Corporation; 2003b. Wechsler Intelligence Scale for Children. [Google Scholar]
- Whitfield MF, Eckstein Grunau RV, Holsti L. Extremely premature (?800 g) schoolchildren: Multiple areas of hidden disability. Archives of Disease in Childhood – Fetal Neonatal Edition. 1997;77:F8–F90. doi: 10.1136/fn.77.2.F85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Weis S, Drings T, Huber W, Grossman M, Kircher T. Taskdependent modulations of prefrontal and hippocampal activity during intrinsic word production. Journal of Cognitive Neuroscience. 2008;21:697–712. doi: 10.1162/jocn.2009.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, LaCorte M, Robertson CMT, Clarke MC, Vincer MJ, Doyle LW, Roberts RS. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–213. doi: 10.1542/peds.2008-0338. [DOI] [PubMed] [Google Scholar]
- Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. Journal of Pediatrics. 1996;129:680–687. doi: 10.1016/S0022-3476(96)70150-6. [DOI] [PubMed] [Google Scholar]
- Wiig EH, Nielsen NP, Minthon L, McPeek D, Said K, Warkentin S. Parietal lobe activation in rapid automatized naming by adults. Perceptual and Motor Skills. 2002;94:1230–1244. doi: 10.2466/PMS.94.3.1230–1244. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test: Administration Manual. Wilmington, Delaware: Wide Range, Inc; 1993. [Google Scholar]
- Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: The Bavarian longitudinal study. Developmental Medicine & Child Neurology. 1999;41:94–109. doi: 10.1111/j.1469-8749.1999.tb00561.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Third Report of the Expert Committee on Maternal Care and Child Care. Geneva: WHO; 1961. Public Health Aspects of Low Birth Weight. [Google Scholar]


