Abstract
Tritiated opioid radioligands have proven valuable in exploring opioid binding sites. However, tritium has many limitations. Its low specific activity and limited counting efficiency makes it difficult to examine low abundant, high affinity sites and its disposal is problematic due to the need to use organic scintillants and its relatively long half-life. To overcome these issues, we have synthesized both unlabeled and carrier-free radioiodinated iodobenzoyl derivatives of 6β-naltrexamine (125I-BNtxA, 18), 6β-naloxamine (125I-BNalA, 19) and 6β-oxymorphamine (125I-BOxyA, 20) with specific activities of 2100 Ci/mmol. To optimize the utility of the radioligand, we designed a synthesis in which the radiolabel is incorporated in the last synthetic step, which required the selective iodination of the benzoyl moiety without incorporation into the phenolic A ring. Competition studies demonstrated high affinity of the unlabelled compounds for opioid receptors in transfected cell lines, as did the direct binding of the 125I-ligands to the opioid receptors. The radioligand displayed very high sensitivity, enabling a marked reduction in tissue, as well as excellent signal/noise characteristics. These new 125I-radioligands should prove valuable in future studies of opioid binding sites.
The opioid receptors are G-protein coupled receptors which mediate pain relief through both the central and peripheral nervous systems.[1,2] The opiate antagonists naltrexone (Ntx, 3) and naloxone (Nal, 4) and the agonist oxymorphone (Oxy, 5) are potent and widely used opiates. Three families of opiate receptors have been identified and cloned: mu (MOR-1), kappa1 (KOR-1) and delta (DOR-1). Radioligands were critical in the initial demonstration of the opioid receptors. Early attempts to detect binding sites using 14C-labeled opioids[3] were not successful due to the low specific activity of the radioligands combined with the high affinity and low abundance of the sites. A number of selective tritiated ligands are currently used to label mu ([-DAla2,MePhe4,Gly(ol)5]enkephalin; DAMGO), kappa1 (U50,488H) and delta ([D-Pen2,D-Pen5]enkephalin; DPDPE) receptors. However, tritium presents a number of limitations. While their specific activity, typically around 50 Ci/mmol, is sufficient to identify the receptors in brain tissue or cell lines expressing them, examination of binding sites with very low abundance and high affinity is limited and may impact the ability to identify proposed subtypes of mu,[4-6] delta,[7,8] and kappa receptors[9-11] proposed from both biochemical and cloning studies. The use of tritium also poses practical issues. These range from detection issues, such as the need to utilize scintillation fluor for counting which destroys the labeled sample and special films and long exposures for autoradiography, to its disposal, which is problematic due to the long half-life and the need to use organic scintillation fluors. To avoid these issues, we developed high affinity 125I-labeled opiate ligands that obviate many of these problems. They have high specific activity (2100 Ci/mmol), which permits the examination of binding at very low concentrations and can detect sites of low abundance. They can be counted without the need for scintillation fluor and their disposal is facilitated by their short half-life.
Traditionally incorporating a radioactive 125I into a molecule requires the direct iodination of a phenolic or an aromatic amine. Problems can arise if a molecule has more than one potential site of incorporation. This is particularly difficult if iodination of one of the sites adversely impacts binding affinity or functional activity, as is the case with the A ring of opiates. Our goal to develop 125I-labeled opioids suitable for general use required an approach to incorporate the iodine as the final synthetic step into a specific location of the molecule. Prior studies suggested benzoic acid substitutions at the 6-position of the opiate scaffold can maintain activity.[12,13] We now report the design and synthesis of three novel, high affinity 125I-labeled opiates based upon 6-substituted amines of naltrexone (6), naloxone (7), and oxymorphone (8).
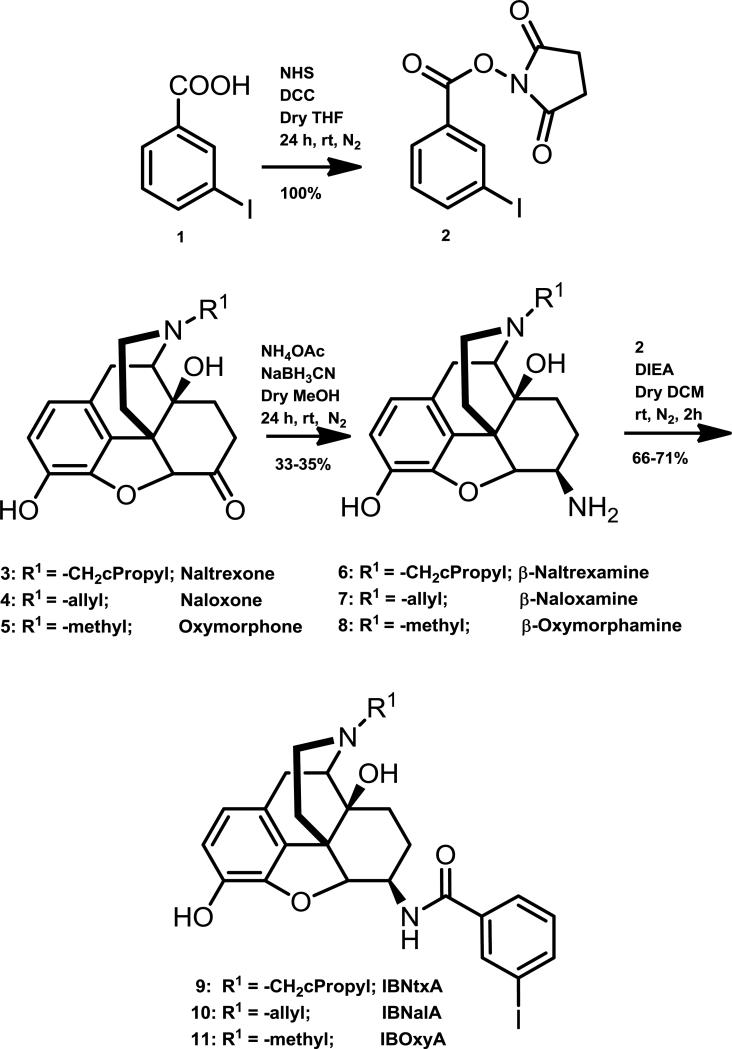
The synthesis of the unlabeled compounds is presented in Scheme 1 (Fig 1). The ketone at the 6-position of the three opiates (3-5) was transformed to an amine (6-8) by reductive amination using NaBH3CN and NH4OAc to yield a mixture of beta and alpha isomers [14]. The beta isomer was purified by column chromatography. In a parallel synthesis, 3-iodobenzoic acid (1) was converted to its N-succinimidyl ester (2) by reacting it with N-hydroxysuccinimide in presence of DCC and THF. The corresponding activated ester (2) was then reacted with the beta isomer of the opiate (6-8) in presence of DIEA and DCM. The 3-Iodo-benzoyl amido derivatives of opiates (9-11) were then purified by column chromatography.
Figure 1.
Synthesis of 3-Iodobenzoyl derivatives of β-naltrexamine, β-naloxamine and β-oxymorphamine
The synthesis of the radiolabelled derivatives of the opiates is presented in Scheme 2 (Fig. 2). 3-Bromobenzoic acid (12) was converted to 3-tributylstannanyl-benzoic acid (13) using BuLi and tributylstannayl chloride [15]. 3-Tributylstannnanyl-benzoic acid (13) was converted to the corresponding N-succinimidyl derivative (14) by reacting it with N-hydroxysuccinimide, DCC and THF. The β-isomers of naltrexamine (6), naloxamine (7) and oxymorphamine (8) were then reacted with the N-hydroxysuccinimidyl ester of 3-tributylstanannayl-benzoic acid (14) in presence of DIEA and DCM. The purified opiate-stannyl analog (15-17) was then used as a precursor for incorporating radioactive iodine. The opiate-stannyl analog precursor (15-17) is stable at 4°C for extended times. Incorporation of the radioiodine was performed in the final step using Na125I and chloramine-T in methanol by incubating the contents at room temperature for 2 min, after which the reaction was terminated by adding sodium metabisulfate. The radioactive derivative (18-20) was purified by RP-HPLC using a gradient of 0.1% TFA/water (A) and 0.1% TFA/ACN (B) as the solvents, with the product eluting at 50% 0.1%TFA/ACN (B).
Figure 2.
Synthesis of radiolabelled compounds
To determine whether the compounds (9-11) retained high affinity for the opioid receptors, we assessed their affinity (Ki) in CHO cell lines stably transfected with MOR-1, DOR-1 or KOR-1 [16,17] using in competition studies which utilized 3H-radioligands specific to the transfected receptor (Table 1). All three compounds (9-11) retained high affinity for mu (MOR-1) receptors and for kappa1 (KOR-1) receptors. Although both IBNtxA (9) and IBNalA (10) displayed high affinity for delta (DOR-1) receptors, IBOxyA (11) did not.
Table 1.
Competition by IBNtxA, IBNalA and IBOxyA against 3H-opioids in transfected cell lines
| Ki (nM) | |||
|---|---|---|---|
| Drug | MOR-1 | KOR-1 | DOR-1 |
| IBNtxA (9) | 2.50 ± 0.82 | 0.23 ± 0.16 | 0.58 ± 0.16 |
| IBNalA (10) | 0.70 ± 0.18 | 0.08 ± 0.006 | 2.55 ± 0.74 |
| IBOxyA (11) | 1.75 ± 0.33 | 9.0 ± 4.0 | 70.76 ± 15.40 |
Competition studies in membranes from CHO cells stably transfected with the indicated opioid receptors were performed with 3H-DAMGO (MOR-1), 3H-U69,593 (KOR-1) 3H-DPDPE (DOR-1) (1 nM) and Ki values determined from the IC50 values obtained by nonlinear regression analysis (Prism, Carlsbad, CA).[20] Results are the means ± SEM of at least three independent replications.
In view of their high affinity for the opioid receptors, we proceeded to synthesize and examine the binding of 125I-labeled versions (18-20) of the compounds directly. Association at 25°C in MOR-1/CHO cells was rapid for all three radioiodinated ligands, reaching steady-state within 1 h and remaining stable for at least 90 min (data not shown). The ratio of total binding to non specific binding typically was 6:1 or greater, providing excellent signal/noise differentiation. With the far higher specific activity and counting efficiency of 125I-ligands, we were able to use only 1/10th the protein in the binding assay that was required with the 3H-labeled ligands and still have more than 5-fold more counts of specific binding.
We next carried out saturation studies with 125IBNtxA (18), 125IBNalA (19) and 125IBOxyA (20) in stably transfected CHO cell lines (Fig 3; Table 2). As anticipated, all three radioligands displayed very high affinity in the MOR-1/CHO cells (Fig 3a). Both 125IBNtxA (18) and 125IBNalA (19) labeled the KOR-1/CHO cells even more potently (Fig 3b). No specific 125I-IBOxyA (20) binding could be detected in either the KOR-1/CHO or the DOR-1/CHO cells at the highest concentrations of radioligands used, presumably due to its very poor affinity. 125IBNtxA (18) displayed high affinity for delta sites in the DOR-1/CHO cells, consistent with its affinity in competition studies while 125I-BNalA (19) labeled sites with a 10-fold lower affinity (Fig. 3c). When assessing the affinity of a drug, measuring its binding directly with the 125I-radioligand is preferable and gives a better estimate of the KD values as compared to competition studies.
Figure 3. 125I-opioid saturation studies in stably transfected CHO cells.
Saturation studies were performed with indicated radioligand in CHO cells stably transfected with the designated opioid receptor. Each figure is a representative experiment that has been independently replicated at least three times. Only specific binding is presented. Differences in Bmax values reflect the use of different tissue preparations with varying levels of expression and do not impact the measure of affinity (KD). Error bars represent the SEM of triplicate samples. Error bars that cannot be seen are smaller than the size of the symbol. All radioligands were used carrier-free, with the exception of the 125I-BNalA saturation in the DOR-1 transfected cells where the specific activity was diluted 10-fold due to the need to examine higher ligand concentrations.
Table 2.
Saturation studies of the 125I-labeled compounds in cell lines.
| Radioligand | KD (nM) | ||
|---|---|---|---|
| MOR-1 | KOR-1 | DOR-1 | |
| 1 125IBNtxA (18) | 0.11 ± 0.02 | 0.027± 0.001 | 0.24 ± 0.05 |
| 1 125IBNalA (19) | 0.22 ± 0.03 | 0.05 ± 0.01 | 2.5 ± 0.22 |
| 125IBOxyA (20) | 0.08 ± 0.03 | ||
Saturation studies were performed with the indicated radioligand in CHO cells stably transfected with the designated opioid receptor. The saturation curves were fit a single site using nonlinear regression analysis (Prism, Carlsberg, CA). KD values are the means ± SEM of at least 3 independent determinations. Bmax values were dependent upon the tissue used and therefore varied among the studies. No specific binding could be detected for 125I-BOxyA in either the DOR-1/CHO or KOR-1/CHO cells at the highest concentrations. Bmax values for MOR-1 were 3.81 ± 0.42, 1.18 ± 0.2 and 1.49 ± 0.5 pmol/mg protein for 125I-labeled IBNtxA (18), IBNalA (19) and IBOxyA (20), respectively. Bmax values for KOR-1 were 0.57 ±0.02 and 1.15 ± 0.01 pmol/mg protein for 125I-labeled IBNtxA (18) and IBNalA (19), respectively, and for DOR-1 it was 0.96 ± 0.2 fmol/mg and 2.7±1.04 fmol/mg protein for IBNtxA (18) and IBNalA (19), respectively.
Finally, we determined whether the binding of the 125I-radioligands to the various transfected cells showed the same sensitivity towards traditional ligands as determined previously in traditional 3H-radioligand binding assays. Overall, the various selective drugs all competed 125I-radioligand binding to various receptors with high affinity and Ki values similar to those seen with 3H-ligands (Table 3).
Table 3.
Competitions of radioiodinated ligand binding in cell lines
| 125I- IBNtxA (18) | 125I- IBNalA (19) | 125I- IBOxyA (20) | |
|---|---|---|---|
| Drug | Ki (nM) | ||
| MOR-1 | |||
| CTAP | 2.33 ± 0.48 | 2.96 ± 1.07 | 1.06 ± 0.56 |
| Naloxone | 4.23 ± 0.42 | 2.27 ± 0.60 | 0.57 ± 0.12 |
| Levallorphan | 1.29 ± 0.14 | 1.69 ± 0.60 | 0.48 ± 0.11 |
| Naltrexone | 1.15 ± 0.10 | 2.71 ± 1.0 | 0.07 ± 0.00 |
| DAMGO | 3.34 ± 0.43 | 0.45 ± 0.12 | 1.55 ± 0.84 |
| Morphine | 4.60 ±1.81 | 2.52 ± 0.28 | 1.92 ± 0.86 |
| DOR-1 | |||
| DPDPE | 1.39 ± 0.67 | 1.77 ± 0.80 | - |
| Naltrindole | 0.46 ±0.32 | 0.50 ± 0.20 | - |
| KOR-1 | |||
| norBNI | 0.23 ± 0.03 | 0.19 ± 0.08 | - |
| 5’GNTI | 0.15 ± 0.07 | 0.19 ± 0.05 | - |
| (-)U50,488H | 0.73 ± 0.32 | 0.95 ± 0.37 | - |
Competition studies were performed with the indicated compounds against the indicated 125I-ligands (0.1 nM) in membranes from CHO cells stably expressing opioid receptors. Ki values were calculated from the IC50.values[20] and represent the means ± SEM of at least three independent replications.
Our initial goal in these studies was to develop an 125I-labeled opioid radioligand for routine use in receptor binding assays. 125I-labeled compounds have a number of important advantages over analogous 3H-labeled radioligands. 125I-ligand binding is far more sensitive due to its far greater specific activity (2100 Ci/mmol) compared to 3H-drugs (~50 Ci/mmol). This enables the use of far less tissue in the assays, a major help when dealing with limited sources, such as with tissue culture samples. Equally important, counting 125I-drugs can be done without the need for scintillation fluors, eliminating a major problem with disposal seen with the 3H-agents. Its far shorter half-life also helps with the eventual disposal of the materials. Finally, since scintillation fluor is not necessary, samples can be counted without sacrificing them, an important consideration when having to follow multi-step processes. Thus, the use of 125I-ligands has scientific, environmental and financial incentives.
Direct iodination of opiates presents a problem since incorporation of iodine into the phenolic A ring of the opiate scaffold markedly decreases activity. We addressed this by introducing a second iodination site in the molecule. However, the presence of two potential iodination sites required a method to selectively iodinate only the site of interest, in this case the site on the benzoylamide. To achieve this, we generated the tributyl stannate precursor. This route permits the iodination of the molecule at the last step in the synthesis, minimizing manipulation of radioactive species. Iodine substitutes for the aromatic tributyl stannate far more rapidly than it incorporates into phenol rings [18,19]. By using an excess of the opioid relative to Na125I, we were able to ensure that we selectively placed the 125I into the desired benzyolamide.
In conclusion, we have synthesized a series of 125I-labeled opioids of high affinity that should be of value in the study of opioid receptors. The approach required the development of a method to selectively radiolabel a specific site within the molecule which was achieved using tributyl tin. These 125I-radioligands have a number of advantages over 3H-radiolabels. They are more sensitive, enabling the exploration of very high affinity sites and sites of very low abundance. The also offer practical and environmental advantages, including the need for far less tissue, the ability to detect binding without organic scintillation fluors and a less problematic disposal.
Supplementary Material
Acknowledgements
We would like to thank the Analytical Core Facility at Memorial Sloan-Kettering Cancer Center for the use of their facilities. This work was supported, in part, by research grants from the National Institute on Drug Abuse (DA02165, DA06241), a Senior Scientist Award (DA00220) and a grant from the National Genetics Foundation to G.W.P.; and a Core Grant from the National Cancer Institute to MSKCC (CA08748).
Abbreviations
- NHS
N-hydroxy succinimide
- THF
Tetrahydrofuran
- DCC
Dicyclohexyl carbodiimide
- NH4OAc
Ammonium acetate
- NaBH3CN
Sodium cyanoborohydride
- MeOH
Methanol
- rt
room temperature
- DCM
Methylene chloride
- DIEA
N,N-Diisopropyl ethyl amine
- n-Bu3SnCl
Tributyl tin chloride
- BuLi
Butyl lithium
- Na2S2O5
Sodium metabisulfite
- AcOH
Acetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found in the online version, at doi:xxxxxxxxx
Reference List
- 1.Pasternak GW. Clin.Neuropharmacol. 1993;16:1. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Pasternak GW. Neuroscientist. 2001;7:220. doi: 10.1177/107385840100700307. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein A, Lowney LI, Pal BK. Proc.Natl.Acad.Sci.USA. 1971;68:1742. doi: 10.1073/pnas.68.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolozin BL, Pasternak GW. Proc.Natl.Acad.Sci.USA. 1981;78:6181. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasternak GW. Clin.J.Pain. 2010;26(Suppl 10):S3–S9. doi: 10.1097/AJP.0b013e3181c49d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasternak GW. Neuropharmacology. 2004;47(Suppl 1):312. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Takemori AE, Portoghese PS. Eur.J.Pharmacol. 1993;242:145. doi: 10.1016/0014-2999(93)90074-r. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Q, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI, Porreca F. J.Pharmacol.Exp.Ther. 1991;257:1069. [PubMed] [Google Scholar]
- 9.Zukin RS, Eghbali M, Olive D, Unterwald E, Tempel A. Proc.Natl.Acad.Sci.USA. 1988;85:4061. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. J.Pharmacol.Exp.Ther. 1989;251:461. [PubMed] [Google Scholar]
- 11.Rothman RB, Bykov V, DeCosta BR, Jacobson AE, Rice KC, Brady LS. Peptides. 1990;11:311. doi: 10.1016/0196-9781(90)90088-m. [DOI] [PubMed] [Google Scholar]
- 12.Ciszewska GR, Ginos JA, Charton M, Standifer KM, Brooks AI, Ryan-Moro J, Berzetei-Gurske I, Toll L, Pasternak GW. Synapse. 1996;24:193. doi: 10.1002/(SICI)1098-2396(199610)24:2<193::AID-SYN11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Yang K, Zuckerman A, Pasternak GW. Cell Mol.Neurobiol. 2005;25:759. doi: 10.1007/s10571-005-3973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang JB, Hanson RN, Portoghese PS. J.Med.Chem. 1977;20:1100. doi: 10.1021/jm00218a023. [DOI] [PubMed] [Google Scholar]
- 15.Vaidyanathan G, Zalutsky MR. Nat.Protoc. 2006;1:707. doi: 10.1038/nprot.2006.99. [DOI] [PubMed] [Google Scholar]
- 16.Bolan EA, Pasternak GW, Pan Y-X. Synapse. 2004;51:11. doi: 10.1002/syn.10277. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y-X, Bolan E, Pasternak GW. Biochem.Biophys.Res.Commun. 2002;297:659. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]
- 18.Musachio JL, Lever JR. Bioconjug.Chem. 1992;3:167. doi: 10.1021/bc00014a012. [DOI] [PubMed] [Google Scholar]
- 19.Ali H, van Lier JE. Synthesis. 1996;1996:423. [Google Scholar]
- 20.Cheng Y-C, Prusoff WH. Biochem.Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.