Abstract
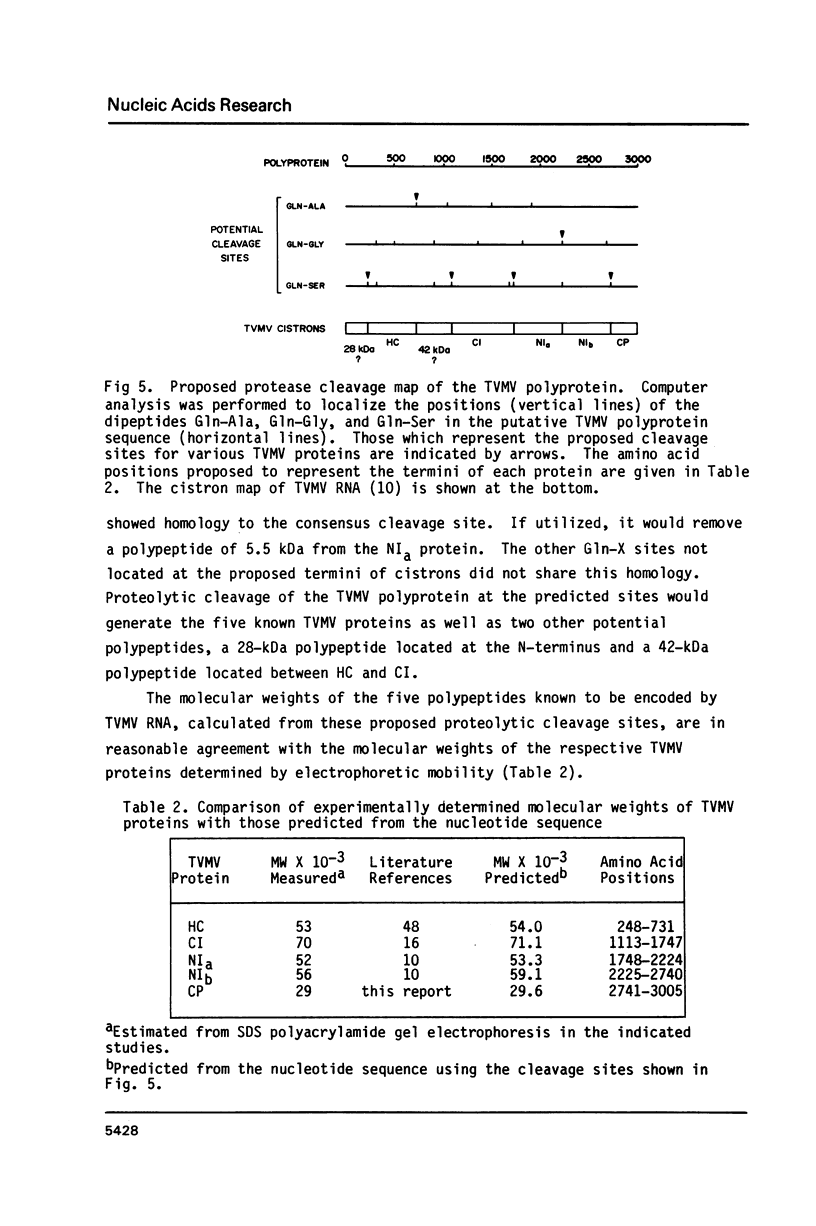
The nucleotide sequence of the RNA of tobacco vein mottling virus, a member of the potyvirus group, was determined. The RNA was found to be 9471 residues in length, excluding a 3'-terminal poly(A) tail. The first three AUG codons from the 5'-terminus were followed by in-frame termination codons. The fourth, at position 206, was the beginning of an open reading frame of 9015 residues which could encode a polyprotein of 340 kDa. No other long open reading frames were present in the sequence or its complement. This AUG was present in the sequence AGGCCAUG, which is similar to the consensus initiation sequence shared by most eukaryotic mRNAs. The chemically-determined amino acid compositions of the helper component and coat proteins were similar to those predicted from the nucleotide sequence. Amino acid sequencing of coat protein from which an amino-terminal peptide had been removed allowed exact location of the coat protein cistron. A consensus sequence of V-(R or K)-F-Q was found on the N-terminal sides of proposed cleavage sites for proteolytic processing of the polyprotein.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Allison R. F., Sorenson J. C., Kelly M. E., Armstrong F. B., Dougherty W. G. Sequence determination of the capsid protein gene and flanking regions of tobacco etch virus: Evidence for synthesis and processing of a polyprotein in potyvirus genome expression. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3969–3972. doi: 10.1073/pnas.82.12.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C., Grosschedl R., Birnstiel M. L. Generation of authentic 3' termini of an H2A mRNA in vivo is dependent on a short inverted DNA repeat and on spacer sequences. Cell. 1982 Apr;28(4):739–745. doi: 10.1016/0092-8674(82)90053-8. [DOI] [PubMed] [Google Scholar]
- Callahan P. L., Mizutani S., Colonno R. J. Molecular cloning and complete sequence determination of RNA genome of human rhinovirus type 14. Proc Natl Acad Sci U S A. 1985 Feb;82(3):732–736. doi: 10.1073/pnas.82.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Forss S., Schaller H. A tandem repeat gene in a picornavirus. Nucleic Acids Res. 1982 Oct 25;10(20):6441–6450. doi: 10.1093/nar/10.20.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., Symons R. H. Cucumber mosaic virus RNA 3. Determination of the nucleotide sequence provides the amino acid sequences of protein 3A and viral coat protein. Eur J Biochem. 1982 Aug;126(2):217–226. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., STEIN W. H., MOORE S. The amino acid composition of ribonuclease. J Biol Chem. 1954 Dec;211(2):941–950. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hobom G., Grosschedl R., Lusky M., Scherer G., Schwarz E., Kössel H. Functional analysis of the replicator structure of lambdoid bacteriophage DNAs. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):165–178. doi: 10.1101/sqb.1979.043.01.023. [DOI] [PubMed] [Google Scholar]
- Howarth A. J., Caton J., Bossert M., Goodman R. M. Nucleotide sequence of bean golden mosaic virus and a model for gene regulation in geminiviruses. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3572–3576. doi: 10.1073/pnas.82.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen G. R., Semler B. L., Wimmer E. Stable hairpin structure within the 5'-terminal 85 nucleotides of poliovirus RNA. J Virol. 1981 Jan;37(1):328–335. doi: 10.1128/jvi.37.1.328-335.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J., Hiebert E. Complementary DNA cloning and expression of the papaya ringspot potyvirus sequences encoding capsid protein and a nuclear inclusion-like protein in Escherichia coli. Virology. 1985 Jun;143(2):435–441. doi: 10.1016/0042-6822(85)90383-6. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Zabel P., Moerman M., Lomonossoff G., Shanks M., Beyreuther K. Cowpea mosaic virus VPg: sequencing of radiochemically modified protein allows mapping of the gene on B RNA. EMBO J. 1984 Jul;3(7):1629–1634. doi: 10.1002/j.1460-2075.1984.tb02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]