Abstract
Background
Individuals with a family history of colorectal cancer (CRC) are at increased risk for CRC. Current screening recommendations for these individuals are based on expert opinion. We investigated optimal screening strategies for individuals with a varying degree of family history of CRC based on a cost-effectiveness analysis.
Methods
We used the MISCAN-Colon micro-simulation model to estimate costs and effects of CRC screening strategies, varying by age to start and stop screening and screening interval. We defined four risk groups, characterized by the number of affected first degree relatives (FDR) and their age at CRC diagnosis. For all risk groups, the optimal screening strategy had an incremental cost effectiveness ratio (ICER) of approximately $50,000 per life-year gained.
Results
The optimal screening strategy for individuals with 1 FDR diagnosed after age 50 was 6 colonoscopies every 5 years starting at age 50, compared to 4 colonoscopies every 7 years starting at age 50 for average risk individuals. The optimal strategy had 10 colonoscopies every 4 years for individuals with 1 FDR diagnosed before age 50, 13 colonoscopies every 3 years for individuals with 2 or more FDRs diagnosed after age 50, and 15 colonoscopies every 3 years for individuals with two or more FDRs of which at least 1 is diagnosed before age 50.
Conclusions
The optimal screening strategy varies considerably with the number of affected FDRs and their age of diagnosis. Shorter screening intervals than the currently recommended 5 years may be appropriate for the highest risk individuals.
Keywords: Early Detection of Cancer, Colonoscopy, Colorectal Neoplasm, Familial Risk, Cost-Effectiveness Analysis
Introduction
Individuals with a family history of sporadic colorectal cancer (CRC) are at increased risk for the disease 1–3. Approximately 11% of the population aged 30–70 has at least one first-degree relative (FDR) diagnosed with CRC 4, 5. Of all CRC cases, 2–5% occur in individuals with known genetic disorders like Familial Adenomatous Polyposis (FAP) and Hereditary NonPolyposis Colorectal Cancer (HNPCC) 6. Without treatment, the lifetime CRC risk is over 95% in individuals with FAP, and 50–80% in individuals with HNPCC 6. Our focus however is on individuals with at least one affected FDR and no known genetic disorders, accounting for another 25% of all CRC cases. These individuals have approximately a twofold increased risk compared to the average risk population. The individual risk level increases with an increasing number of affected FDRs and a younger age of diagnosis of the affected relatives 2. We consider four risk groups of individuals with a CRC family history (excluding known genetic disorders), with a varying relative risk (RR): a) 1.6 for individuals with one FDR diagnosed after age 50; b) 2.6 for individuals with one FDR diagnosed before age 50, c) 3.5 for individuals with two FDRS both diagnosed after age 50, and d) 5.6 for individuals with two FDRs with at least one diagnosed before age 50.
Colonoscopy with removal of adenomas, the non-invasive pre-cursor of CRC, decreases CRC incidence in both average risk individuals and in subjects with hereditary CRC syndromes 7, 8. Although it is generally advised that individuals with a family history of CRC have more intensive screening than the average risk individuals, the optimal strategy remains unclear. Expert opinion based recommendations include a start of colonoscopy screening ten years earlier in individuals with a family history than in the average risk population, or ten years before the youngest age of diagnosis of the affected relatives 9–11. Another recommendation is to use a 5-year interval for individuals with one FDR diagnosed before age 60 or two or more FDRs, instead of the 10-year interval for the average risk population 12–14. The differentiation in current guidelines for the four risk groups defined above is minor, while the risk levels increase considerably, suggesting that more differentiation is needed.
We will identify the optimal screening strategies per risk group based on a cost-effectiveness analysis. We hereto used the MISCAN-Colon simulation model and the results from a recent meta-analysis 2.
Methods
MISCAN-Colon
The MISCAN-Colon micro-simulation model was developed at the Department of Public Health at ErasmusMC, The Netherlands, in collaboration with the U.S. National Cancer Institute to assess the effect of different interventions on the occurrence of CRC in a population. A detailed description of the model and the data sources that informed the quantification of the model can be found in previous publications 15, 16 and also in a standardized model profiler17. In brief, the model simulates individuals from birth to death, first without screening and subsequently with the changes that would occur under the implementation of a screening program. In every individual, adenomas can arise and some of them will develop into cancer. A schematic representation of the natural history as used in the model is given in Figure 1. Adenomas are initially small (1–5mm) and progress to medium (6–9mm) and large (10+mm) adenomas. The majority of adenomas is assumed to be non-progressive and will never develop into cancer. The progressive adenomas have the ability to become cancer but not all of them will make it to cancer in an individual’s lifetime. The adenomas that do become malignant, transform into stage I cancers and will progress into stages II, III and IV, unless diagnosed earlier. The survival after clinical diagnosis depends on age and the cancer stage at diagnosis. Screening can result in a gain in life-years when cancers are detected and treated at earlier stages or when adenomas are detected and consequently removed before they became cancer.
Figure 1.
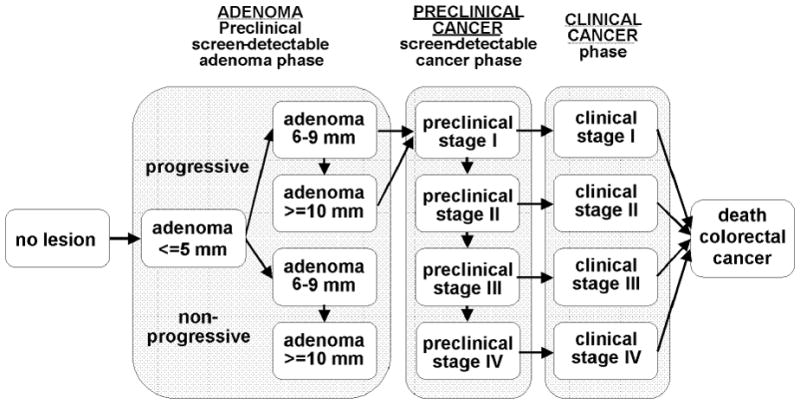
Adenoma and cancer stages in the MISCAN-Colon model. Cancer stages correspond to the American Joint Committee on Cancer / International Union Against Cancer staging system for CRC. Adenomas are categorized by size.
The validity of the model has been tested on the results of large (randomized) screening and surveillance studies. In particular, we were able to simulate the same number of screendetected and interval cancers as observed in the Minnesota Colon Cancer Control Study, the Funen trial and the Nottingham trial 18, the CoCap sigmoidoscopy study 19 and the National Polyp Study 20. The model was able to explain observed incidence and mortality trends in the US when accounting for risk factor trends, screening practice and chemotherapy treatment 21.
For the average risk population of this analysis, disease parameters were adjusted to reproduce adenoma prevalence data from autopsy studies 22–31 and SEER incidence data from 1975–1979 32, when they were not influenced yet by screening. Survival after CRC diagnosis by stage was based on SEER 1996–1999 data 32.
Population and risk levels
We modeled a cohort of 30-year olds in the United States in 2005. We categorized the individuals with a family history in four risk groups, depending on the number of FDRs or age at diagnosis. The relative risk estimates per group were based on a recent meta-analysis 2. The groups consisted of a) individuals with one FDR diagnosed after age 50, with a relative risk (RR) of 1.6, b) one FDR diagnosed at or before age 50 (RR 2.6), c) two or more FDRs diagnosed after age 50 (RR 3.5) and d) two or more FDRs with at least one of them diagnosed at or before age 50 (RR 5.6). These four groups are shortly referred to as “1 FDR > 50”, “1 FDR ≤ 50”, “2+ FDRs > 50” and “2+ FDRs ≤ 50”. Individuals with a family history were simulated by adjusting the relative risk for CRC compared to the average risk population. We modeled the increased risk for CRC by multiplying the age specific adenoma onset rate for both progressive and non-progressive adenomas by the same relative risk for all ages.
Screening strategies
For every risk group we simulated colonoscopy screening strategies, which differed with respect to:
The age to start screening that varied over all years between 30 and 60
The screening interval that varied over all years between 2 and 10
The age to stop screening that was never after the age of 90
The number of colonoscopies followed from the previous 3 parameters, and had to be at least 2.
Individuals with adenomas were referred to surveillance following the guidelines of the US Multi-Society Task Force on Colorectal Cancer 33. If the screening interval was shorter than the recommended surveillance interval, the latter was shortened to the screening interval.
Per polyp sensitivity was assumed to be the same for screening and surveillance colonoscopy: 80% for small adenomas, 85% for medium adenomas and 95% for large adenomas and cancers 34–36. We assumed complications like perforations and bleedings to occur at a rate of 2.4 per 1,000 colonoscopies 37–40. Colonoscopy with polypectomy resulted in mortality once in every 10,000 colonoscopies 41. We assumed a 100% compliance for both screening and surveillance colonoscopies. In this way our analyses focus on optimal strategies for individuals who comply with the guidelines.
Costs
Costs included costs for colonoscopy, complications of colonoscopy and treatment of CRC (Table 1). The costs of colonoscopy screening and surveillance were assumed to be equal but to depend on whether polypectomy was performed. The costs associated with colonoscopy were based on 2007 Medicare average payments 42. Costs for complications of colonoscopy were based on the relevant Diagnosis Related Group codes 42. Treatment costs were derived from a comparison of costs for CRC cases relative to matched controls in the SEER-Medicare files 43. All costs were updated to 2007 dollars using the medical care component of the Consumer Price Index. The final cost inputs used in the model are summarized in Table 1.
Table 1.
Assumptions for costs (2007$) and complications associated with colonoscopy and colorectal cancer treatment43
| Costs colonoscopy | ||||
| Without polypectomy | $662 | |||
| With polypectomy | $846 | |||
| Complications colonoscopy* (with and without polypectomy) | ||||
| Rate per 1,000 colonoscopies | Costs | |||
| Perforations | 0.7 | $12,446 | ||
| Serosal burn | 0.3 | $5,208 | ||
| Bleed with transfusion | 0.4 | $5,208 | ||
| Bleed without transfusion | 1.0 | $320 | ||
| Treatment costs | ||||
| Phase of care** | ||||
| Stage at diagnosis | Initial | Continuous (per year) | Terminal care (death crc) | Terminal care (death other cause) |
| Stage I | $28,668 | $2,395 | $51,935 | $12,703 |
| Stage II | $39,700 | $2,237 | $51,712 | $11,035 |
| Stage III | $48,951 | $3,249 | $54,776 | $14,708 |
| Stage IV | $64,801 | $10,419 | $73,522 | $39,679 |
Once in every 10,000 colonoscopies with polypectomy the complication is assumed to be fatal
Costs for cancer care were divided into three clinically relevant phases of care – initial, continuing and terminal care. The initial phase was defined as the first 12 months following diagnosis, the terminal phase was defined as the final 12 months of life, and the continuing phase was defined as all months between the initial and terminal phase. For patients surviving less than 24 months, the final 12 months were allocated to the terminal phase. The remaining months of observation were allocated to the initial phase.
Analysis
We used the MISCAN-Colon model to estimate costs and number of life-years gained compared to the situation without screening for all screening strategies. For each risk group, we identified the efficient screening strategies, i.e. strategies which did not have an alternative or combination of alternatives which gains more life-years at the same or less costs. This resulted in a set of efficient strategies for each risk group. For every efficient strategy, we determined the incremental cost-effectiveness ratio (ICER), which is calculated as the incremental costs per incremental life-year gained compared to the next less costly efficient strategy. For all risk groups, the optimal strategy was considered the strategy with an ICER-value closest to a threshold of $50,000 per life-year gained 44. Costs and life-years gained were discounted with 3% per year.
Sensitivity analysis
There is uncertainty on the dwell time of adenomas. Recent data from a randomized controlled sigmoidoscopy study have indicated a probably longer dwell time than the 20 years we assumed, at least for distal lesions45. Therefore, we repeated the analysis with an increased mean dwell time of 30 years for the average population. The adenoma incidence by age was concurrently adjusted in order to keep the CRC incidence unchanged. To account for the influence of screening and CRC treatment on quality of life, we used quality adjusted life-years as a sensitivity analysis. We assumed one day loss per colonoscopy, and a loss of 0.26, 0.3, 0.4, or 0.75 per year in stage I, II, III, or IV initial care, a 0.15 loss per year in continuous care, a 0.75 loss per year in terminal care before dying of CRC, and a 0.35 loss per year in terminal care in case of dying of another cause 46, 47. We assessed the influence of discounting by repeating the analysis with a discount percentage of 0% and 5%.
For the highest risk group, we performed a sensitivity analysis on the way the disease develops by modeling the increased risk with a shorter adenoma dwell time of 10 instead of 20 years.
We decided not to perform a probabilistic sensitivity analysis after having weighed the computational effort required against the limited added value due to the lack of data on the probability distributions of most of the parameter values.
Results
The efficient strategies per risk group are shown in Figure 2. The higher the risk among those screened, the more life-years were gained at the same costs. Because of the greater health gain in higher risk groups, more intensive strategies, with shorter screening intervals and wider age ranges, met the criteria of an ICER close to $50,000 per life-year gained (Table 2). For individuals with 1 FDR > 50, the optimal strategy had an interval of 5 years. The optimal age to start screening in this group was 50, and the optimal age to stop screening was 75. For individuals with 1 FDR ≤ 50, the optimal screening interval was 4 years and the optimal ages to start and stop screening were 45 and 81 years. The optimal screening interval for individuals with 2+ FDRs > 50 was 3 years, with the same age range (45–81). For individuals with 2+ FDRs ≤ 50 the optimal screening interval was also 3 years, with a further widened age range of 42 to 84 years. The mortality reduction that resulted from the optimal strategies varied between 72 and 84%, and increased with risk level. The number needed to scope to prevent 1 death decreased from 220 for individuals with 1 FDR > 50, to 130 for individuals with 2+ FDRs ≤ 50.
Figure 2.
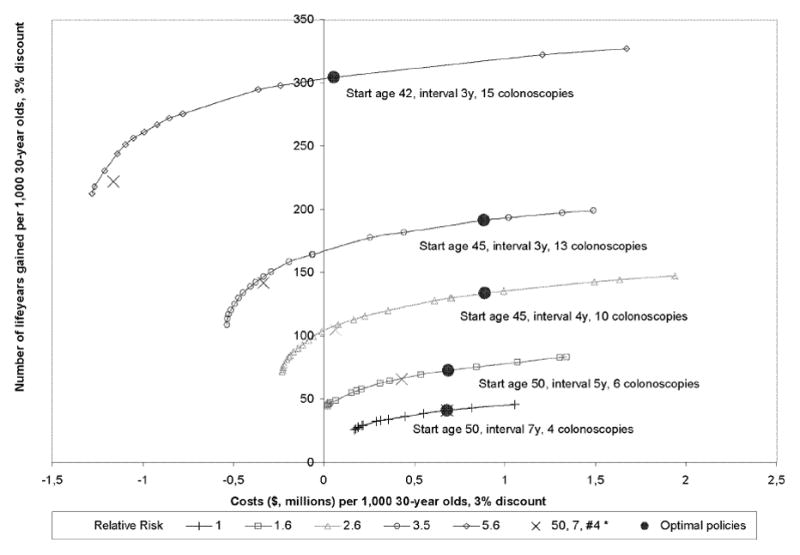
Discounted costs (millions of dollars) and life-years gained per 1,000 30-year olds of the efficient strategies with Relative Risk levels corresponding to the category of family history.
* 50, 7, #4 represents the optimal strategy for the average risk population, with start age 50, interval 7 years, and 4 colonoscopies
Table 2.
Optimal screening strategies at varying relative risk levels associated with a family history of colorectal cancer, and their associated costs ($) and life-years gained per 1,000 30-year olds. Discount of costs and effects is 3% yearly.
| Category of family history | Relative Risk 2 | Optimal strategy | Compared to no screening (per 1,000 30-year olds) | Compared to next less expensive efficient strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| # screens | Screening interval | Age range | Mortality Reduction (%) | # needed to scope to prevent 1 death | Total costs | # life-years gained | ICER | ||
| 1 FDR diagnosed after age 50 | 1.6 | 6 | 5 | 50–75 | 72 | 220 | $690,000 | 72 | $48,000 |
| 1 FDR diagnosed before age 50 | 2.6 | 10 | 4 | 45–81 | 79 | 190 | $890,000 | 134 | $50,000 |
| 2 or more FDRs diagnosed after age 50 | 3.5 | 13 | 3 | 45–81 | 84 | 180 | $880,000 | 191 | $47,000 |
| 2 or more FDRs, at least 1 diagnosed before age 50 | 5.6 | 15 | 3 | 42–84 | 84 | 130 | $50,000 | 304 | $46,000 |
FDR: first-degree relative
ICER: incremental cost-effectiveness ratio
For the average risk population, the optimal screening strategy had an interval of 7 years, starting at age 50 and stopping at age 71 (4 colonoscopies). So according to our model, if one would have the resources to screen the general population 4 times, it would be more (cost-) effective to screen from 50 to 71 every 7 years than to screen e.g. from 50 to 80 every 10 years. Only if one would have no more resources than for screening with two colonoscopies in a life time, one would screen every 10 years.
Sensitivity analysis
With an adenoma dwell time of 30 instead of 20 years, the strategy with 3 instead of 4 colonoscopies every 7 years starting at age 50 had an ICER closest to $50,000 per life-year gained for the average risk population, due to an increased ICER value of both strategies. Adjusting for quality of life resulted in more quality adjusted life-years than non-adjusted life-years due to the prevention of CRC by colonoscopy screening and a consequently lower number of life-years in treatment. The incremental effectiveness on the other hand, decreased with an increasing number of colonoscopies due to the one day loss per colonoscopy. At a threshold of $50,000 per quality adjusted life-year, the optimal strategy for individuals with 1 FDR > 50 changed to screening every 4 instead of every 5 years between ages 50–74. The strategies for the higher risk groups remained the same. Discounting had a more substantial impact on the effects of screening and savings from treatment than on colonoscopy costs, since the latter occur earlier in time. As a consequence, not discounting was favorable for screening, with shorter intervals and more colonoscopies as a result (Table 3). With a 5% discount on the contrary, we found longer screening intervals and fewer colonoscopies. With a shorter adenoma dwell time for individuals in the highest risk group, the optimal screening interval was 2 instead of 3 years, with screening ages between 44–88.
Table 3.
Optimal strategies per relative risk level for the sensitivity analysis on discounting, 0% and 5%.
| Category of family history | Relative Risk2 | Optimal strategy, 0% discount | Optimal strategy, 5% discount | ||||
|---|---|---|---|---|---|---|---|
| # screens | Screening interval | Age range | # screens | Screening interval | Age range | ||
| 1 FDR diagnosed after age 50 | 1.6 | 9 | 4 | 45–77 | 6 | 6 | 49–79 |
| 1 FDR diagnosed before age 50 | 2.6 | 14 | 3 | 42–81 | 10 | 4 | 47–83 |
| 2 or more FDRs diagnosed after age 50 | 3.5 | 15 | 3 | 41–83 | 11 | 4 | 44–84 |
| 2 or more FDRs, at least 1 diagnosed before age 50 | 5.6 | 23 | 2 | 41–85 | 15 | 3 | 43–85 |
FDR: first-degree relative
Discussion
The optimal colonoscopy screening strategy for individuals with a CRC family history had a screening interval of 3 to 5 years, depending on the number of affected relatives and their age at diagnosis. The age ranges of the optimal strategies varied from 50–75 to 42–84.
Sometimes higher thresholds than $50.000 per life-year gained are considered acceptable 44, allowing more frequent colonoscopy screening. Increasing the threshold up to $75,000 resulted in screening intervals of 2 to 4 years and age ranges that varied from 46–78 to 43–89 (results not shown). Further increasing the threshold to $100,000 did not shorten the intervals further, but only resulted in 1 or 2 additional colonoscopies in the highest 2 risk groups.
Screening becomes somewhat less cost-effective in individuals without affected relatives since they have a lower risk than the total population (RR 0.9),. To adjust to the used threshold ICER, theoretically screening intensity needs to be decreased slightly.
There are several US guidelines for CRC screening in individuals with a family history 11, 12, 14. In some guidelines screening starts at age 40 if someone has at least one affected FDR. In case of one FDR diagnosed after age 60, the recommended screening interval is 10 years, as in the general population. For individuals with one FDR diagnosed before age 60 and for individuals with two or more FDRs, 5-yearly colonoscopy is recommended. Others suggested, based on prospective observational studies, that screening should start at age 45–50, and that colonoscopy every 5 years would be sufficient 48, 49. Controlled studies to analyze the effect of these strategies on incidence or mortality are not available. Our results are in line with a recommended starting age of 45, but with shorter intervals. However, note that the shorter intervals for individuals with 1FDR ≤ 50 or 2+ FDRs were approximately halve the interval for the average risk population (3–4 versus 7 years according to our results), which corresponds nicely with the 50% difference in interval as recommended in the guidelines (5 versus 10 years). The 10-yearly recommendation for the average risk population was based on expert opinion, and chosen for simplicity. This strategy was suboptimal in our analysis, because it was as effective as 3 colonoscopies every 7 years starting at age 54 but more expensive ($0.50 instead of $0.45 million). This strategy with a 7-yearly interval had an ICER of $43.000 per life-year gained, which is close to the threshold of $50.000 per life-year gained.
Lengthening the model assumption of the average dwell time for an adenoma to become cancer from 20 to 30 years did not lengthen our optimal screening interval for the average risk population. However, as expected, the incremental cost-effectiveness of 4 colonoscopies relative to 3 colonoscopies became worse due to the lower incremental effectiveness of the last colonoscopy. By lengthening the dwell time even further, the ICER of 3 colonoscopies every 7 years would eventually increase to over $50,000 per life-year gained as well and longer intervals would become optimal in combination with fewer screening rounds. Besides a longer adenoma dwell time, higher colonoscopy costs relative to the treatment costs would also challenge our conclusion that shorter screening intervals may be appropriate than currently recommended. However, this is unlikely in view of the increasing costs of chemotherapy drugs involved in CRC treatment. We looked at the influence of trends in survival and treatment costs in an earlier analysis, where more recent survival data, taking the effects of greater use of adjuvant treatment into account, had a minimal effect on the number of life-years gained 50. This will therefore have a small impact on our results. Another important assumption is that increased cancer risk is due to an equally increased adenoma incidence across all ages. We assessed this assumption in an earlier analysis based on several colonoscopy studies 51. Alternatively, a faster progressive development of adenomas could cause higher risk in these individuals. We found a shorter interval of 2 instead of 3 years for the highest risk group when we assumed the increased risk to be caused by a combination of a higher adenoma incidence and faster progression of the adenomas. So this would imply even more diversification in screening intensity between risk groups.
A limitation of this study is that we did not account for the number of FDRs an individual has, which affects the risk for CRC. For example, an individual with two FDRs both diagnosed with CRC is at higher risk than someone with ten FDRs, two of whom are diagnosed with CRC. Also family history, and thus the estimated risk of an individual changes over time, because relatives are being diagnosed or not with CRC. Ideally, the screening strategy is adjusted accordingly.
Our results show that individualizing screening guidelines based on family history could improve the effectiveness substantially. Individualized guidelines are more complex than the current guidelines and could confuse both physicians and screenees, resulting in lower adherence rates. Individuals could also hesitate to adhere to more frequent invasive colonoscopies, especially if their insurance company does not cover earlier or more frequent colonoscopies. Adherence generally does not influence the cost-effectiveness of screening since in influences both costs and effects, and was therefore assumed 100% in our analysis. However, lower adherence rates would obviously decrease the effectiveness of screening. On the other hand, individualized guidelines could also increase the adherence because of a better awareness of the individuals risk for CRC. Besides, it fits in the trend towards more personalized medical care, and individuals might appreciate the fact that the recommendation is based on their personal risk profile. Implementation studies should look into these issues.
Risk for CRC also depends on lifestyle. Recently, a risk prediction tool has become available that estimates an individual’s CRC risk based on a self-administered questionnaire 52, 53. Both family history and lifestyle factors are included. Results of cost-effectiveness analyses, as those presented in this paper can be used to translate the risk estimates resulting from this prediction model to screening recommendations.
In conclusion, the optimal colonoscopy screening strategy varies considerably with the number of affected relatives and their age of diagnosis. For the high risk individuals, shorter intervals than the currently recommended 5 years may be appropriate.
Acknowledgments
Funding
Funding was received from the National Cancer Institute (U01 CA97426 and U01 CA115935) and from The Netherlands Organization for Health Research and Development (ZonMw 120720011)
Footnotes
Financial disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Baglietto L, Jenkins MA, Severi G, et al. Measures of familial aggregation depend on definition of family history: meta-analysis for colorectal cancer. J Clin Epidemiol. 2006;59:114–124. doi: 10.1016/j.jclinepi.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42:216–227. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 4.de Jong AE, Vasen HF. The frequency of a positive family history for colorectal cancer: a population-based study in the Netherlands. Neth J Med. 2006;64:367–370. [PubMed] [Google Scholar]
- 5.Mitchell RJ, Campbell H, Farrington SM, Brewster DH, Porteous ME, Dunlop MG. Prevalence of family history of colorectal cancer in the general population. Br J Surg. 2005;92:1161–1164. doi: 10.1002/bjs.5084. [DOI] [PubMed] [Google Scholar]
- 6.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 8.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 9.Brenner H, Hoffmeister M, Haug U. Family history and age at initiation of colorectal cancer screening. Am J Gastroenterol. 2008;103:2326–2331. doi: 10.1111/j.1572-0241.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 10.Burt RW. Colon cancer screening. Gastroenterology. 2000;119:837–853. doi: 10.1053/gast.2000.16508. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 12.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–877. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 14.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 15.Loeve F, Boer R, Van Oortmarssen GJ, Van Ballegooijen M, Habbema JDF. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32:13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 16.Loeve F, Brown ML, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92:557–563. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 17.Vogelaar I, van Ballegooijen M, Zauber AG, et al. Model Profiler of the MISCAN-Colon microsimulation model for colorectal cancer. Department of Public Health; Erasmus MC: 2004. [Google Scholar]
- 18.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, Zauber A, Habbema JD. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115:2410–2419. doi: 10.1002/cncr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeve F, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JDF. Final report MISCAN-COLON microsimulation model for colorectal cancer: report to the National Cancer Institute Project No. NO1-CN55186. Department of Public Health, Erasmus University; 1998. [Google Scholar]
- 20.Loeve F, Boer R, Zauber AG, et al. National Polyp Study data: evidence for regression of adenomas. Int J Cancer. 2004;111:633–639. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 21.Vogelaar I, van Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–1633. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 22.Arminski TC, McLean DW. Incidence and Distribution of Adenomatous Polyps of the Colon and Rectum Based on 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;7:249–261. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 23.Blatt L. Polyps of the Colon and Rectum: Incidence and Distribution. Dis Colon Rectum. 1961;4:277–282. [Google Scholar]
- 24.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61:1472–1476. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223–226. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36:179–186. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 27.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508–1514. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24:799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 29.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847–1857. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49:819–825. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Cancer Institute. Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Software, version 5.3.1. 2003. [Google Scholar]
- 33.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Hixson LJ, Fennerty MB, Sampliner RE, Garewal HS. Prospective blinded trial of the colonoscopic miss-rate of large colorectal polyps. Gastrointest Endosc. 1991;37:125–127. doi: 10.1016/s0016-5107(91)70668-8. [DOI] [PubMed] [Google Scholar]
- 35.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 36.Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17–23. doi: 10.1016/s0016-5085(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 37.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 39.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–173. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 40.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 41.Jentschura D, Raute M, Winter J, Henkel T, Kraus M, Manegold BC. Complications in endoscopy of the lower gastrointestinal tract. Therapy and prognosis. Surg Endosc. 1994;8:672–676. doi: 10.1007/BF00678564. [DOI] [PubMed] [Google Scholar]
- 42.Zauber AG, Lansdorp-Vogelaar I, Wilschut JA, Knudsen AB, Ballegooijen Mv, Kuntz KM. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 44.Hunt TL, Luce BR, Page MJ, Pokrzywinski R. Willingness to pay for cancer prevention. Pharmacoeconomics. 2009;27:299–312. doi: 10.2165/00019053-200927040-00003. [DOI] [PubMed] [Google Scholar]
- 45.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 46.Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94:1650–1657. doi: 10.1111/j.1572-0241.1999.01157.x. [DOI] [PubMed] [Google Scholar]
- 47.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–1303. [PubMed] [Google Scholar]
- 48.Dove-Edwin I, de Jong AE, Adams J, et al. Prospective results of surveillance colonoscopy in dominant familial colorectal cancer with and without Lynch syndrome. Gastroenterology. 2006;130:1995–2000. doi: 10.1053/j.gastro.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Dove-Edwin I, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. Bmj. 2005;331:1047. doi: 10.1136/bmj.38606.794560.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JD, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst. 2009;101:1412–1422. doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilschut JA, Habbema JD, Ramsey SD, Boer R, Looman CW, van Ballegooijen M. Increased risk of adenomas in individuals with a family history of colorectal cancer: results of a meta-analysis. Cancer Causes Control. 2010 doi: 10.1007/s10552-010-9654-y. [DOI] [PubMed] [Google Scholar]
- 52.Freedman AN, Slattery ML, Ballard-Barbash R, et al. Colorectal Cancer Risk Prediction Tool for White Men and Women Without Known Susceptibility. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Cancer Institute. Colorectal Cancer Risk Assessment Tool. 2008. [Google Scholar]


