Abstract
Our aim is to present a working model that may serve as a valuable heuristic to predict enduring effects of drugs when administered during development. Our primary tenet is that a greater understanding of neurodevelopment can lead to improved treatment that intervenes early in the progression of a given disorder and prevents symptoms from manifesting. The immature brain undergoes significant changes during the transitions between childhood, adolescence, and adulthood. Such changes in innervation, neurotransmitter levels, and their respective signaling mechanisms have profound and observable changes on typical behavior, but also increase vulnerability to psychiatric disorders when the maturational process goes awry. Given the remarkable plasticity of the immature brain to adapt to its external milieu, preventive interventions may be possible. We intend for this review to initiate a discussion of how currently used psychotropic agents can influence brain development. Drug exposure during sensitive periods may have beneficial long-term effects, but harmful delayed consequences may be possible as well. Regardless of the outcome, this information needs to be used to improve or develop alternative approaches for the treatment of childhood disorders. With this framework in mind, we present what is known about the effects of stimulants, antidepressants, and antipsychotics on brain maturation (including animal studies that use more clinically-relevant dosing paradigms or relevant animal models). We endeavor to provocatively set the stage for altering treatment approaches for improving mental health in non-adult populations.
Introduction
Numerous psychiatric disorders can be traced to developmental processes gone awry, and many of these disorders have a genetic link. However, no single psychiatric disorder is 100% genetically determined, suggesting that environmental factors are necessary for disease manifestations. Exposure to environmental information/stimulation can positively or negatively shape brain development as well as have maximal impact during certain periods of maturation (Sonuga-Barke, 2010, Sanchez et al., 2001, Andersen and Navalta, 2004, Andersen et al., 2008). For example, early behavioral interventions can have a positive effect in ameliorating behavioral problems and improving psychosocial functioning of children with autism (Dawson, 2008). In contrast, child maltreatment and other severe childhood adversities have a negative impact on mental health (Andersen and Teicher, 2008, 2009). While these examples are readily accepted, we must now critically examine whether pharmacological interventions during childhood are also associated with positive or negative effects long-term. This review keenly recognizes the fact that prescribing clinicians are faced with the daunting task of deciding the best drug treatment options for pediatric populations with relatively little information to guide their decisions. However, failure to appropriately treat disorders in childhood may increase the likelihood of psychological problems later in life (Edwards et al., 2003, Felitti et al., 1998, Beesdo et al., 2007) – a shortcoming that also deserves equal consideration. Our aim is to provide the reader with information that suggests novel therapeutic treatments may ultimately reduce or prevent symptoms in adolescence or adulthood by impacting brain development.
We initially discuss the role that animal studies play in increasing our understanding of both brain development, generally, and enduring medication effects, specifically. We then present a basic background on developmental neuroscience and the processes that define an individual at different stages of life. Finally, this information is used to provide a framework for a predictive model that may serve as a valuable heuristic to understanding enduring drug action.
Psychotropic medications have been used in pediatric populations to produce short-term palliative care
The increasing use of psychotropic medications
The increased acknowledgement and awareness that children and adolescents experience psychiatric problems has led to the increased use of medication for acute symptom management. For example, diagnostic prevalence of ADHD has risen from 6.3% (Szatmari et al., 1989) to as high as 27% (Vasconcelos et al., 2003), with some correction lately (Polanczyk and Rohde, 2007). Similarly, and maybe more controversial, is the increased recognition of pediatric bipolar disorder (Blader and Carlson, 2007, Post et al., 2008, Soutullo et al., 2005). In parallel with increased rates of diagnoses in children and adolescents, prescription rates of medications that are FDA-approved for adult use are generally rising (Skaer et al., 2009, Hugtenburg et al., 2005). The use of stimulants, antidepressants, and anti-epileptics has increased (Zito et al., 2007, Zito et al., 2006, Hunkeler et al., 2005), although their use varies across countries (Zito et al., 2008). Historically, pharmacological treatment of immature populations has generally been based on the principle that children are merely “little adults” (Vitiello, 2003). Given that psychotropic medications work by the same mechanism in both the immature and mature brain, medicating younger populations provides short-term, palliative effects with little apparent risk of adverse side effects similar to adult populations. More importantly, clinicians treating pediatric populations have been predominantly guided by the potential negative consequences of not treating their patients, including relapse and/or a worsening of symptoms (Ryan, 1992, 2008). However, medication exposure in young populations may produce enduring effects on the immature brain that differ considerably from comparable adult exposures (Andersen, 2003, Andersen and Navalta, 2004) and this assertion also needs to be taken into consideration when deciding to treat. Clinicians unfortunately have relatively little research to guide treatment practices in the context of possible, and potentially serious, consequences.
Only recently has attention been paid to the long-term effects of medication in young populations. Exemplified by studies including the MTA, TADS, PATS, and STAR*D, multi-center trials have been conducted to study whether drug exposure during childhood or adolescence produces enduring effects relative to other interventions, such as cognitive behavioral therapy or community clinical care (Molina et al., 2009, Reinecke et al., 2009, Vaughan et al., 2008). These studies have a number of limitations (reviewed extensively elsewhere; Pelham, 1999); notwithstanding, these studies have relatively short endpoints that are within a few years of drug exposure – not the years required to study drug effects that manifest in adulthood. In addition, these studies are focused on whether reductions in the original target symptoms are observed (e.g., impulsivity in ADHD), not whether other seemingly unrelated symptoms arise (e.g., depression in ADHD patients) in the long-term. Extrapolation of adult practices generally has worked well for guiding clinical care in younger populations. However, animal studies indicate that certain factors may need to be considered when treating immature populations. To date, studies that have examined the factors discussed below in more detail, including age of treatment, age of assessment, and sex, have usually been underpowered to detect significant differences. Clearly, parsing out whether therapeutic interventions produce enduring effects from a number of other issues is difficult, but awareness needs to be raised to this possibility. In this regard, animal studies can play a critical role by informing and guiding clinical practice in ways not possible in human studies.
The role of preclinical studies to inform clinical practice
Animal studies can experimentally determine cause and effect under controlled conditions to elucidate whether medication exposure in immature populations produces long-term effects. Such experimental manipulations are not ethically possible in the clinical setting, but may be readily addressed in preclinical studies. Four main advantages of preclinical studies are:
Animal studies can examine drug effects independent of sequential drug exposures or drug-drug interactions typically found in clinical trials. Robust clinical studies use random assignment to study drug effects. While the ethics of not providing optimal treatment to ill children is of grave concern, this approach is technically necessary within the principles of internally valid study design. However, individuals who receive an inferior treatment in a study often seek secondary, and often improved, treatment later (Van der Oord et al., 2008). This understandable reality further limits long-term conclusions about medication effects.
Animal studies can parse the effects of drug exposure independent of the underlying disease state. Disease state has been and remains very difficult to tease apart in clinical trials. Two issues arise here. First, chronically administering psychoactive agents to healthy, typical children is objectionable. Second, matching symptoms and their progression in humans is difficult. Children with more severe symptoms and impairment are often treated earlier than cases that are less severe or emerge later in life. The possibility exists that these earlier treatments contribute further to the poorer outcome in this population. However, clinical studies show that the risks of not treating are worse than the risk of treatment in the short-term. For example, depression relapse is higher in placebo treated children and adolescents than those treated with fluoxetine within a 6 month period (Emslie et al., 2008; Ryan 2008). Clearly, reduced relapse is of immediate concern for the clinician and is not going to be observed in animal studies. It does stand to reason that any observed changes following the initial treatment in animals may be directly attributable to drug exposure.
Studying the long-term effects of medication exposure in animals is more efficient. Practically speaking, animals have significantly shortened life cycles whereby adulthood is reached within months for rodents and a few years in non-human primates. As long-term drug effects do not manifest completely until adulthood, a wait of a decade or more for results in humans would be necessary. Few clinical prospective studies exist with such a long-term endpoint (e.g., Mannuzza et al., 2008). Complicating this issue further is that long-term, prospective studies are difficult (e.g., subject attrition, non-adherence to protocol, etc.); experimenters have little control over intervening variables; and funding is inconsistent.
Animal studies can be more invasive, especially when aimed at understanding mechanisms. Neuroimaging in humans has been helpful in elucidating some brain changes, but only to a limited extent as magnetic resonance imaging (MRI) is an indirect measure of pharmacological effects on blood flow, energy utilization, and other processes. Positron emission tomography (PET) scanning, which permits pharmacological assessment, is rarely used in children and adolescents. Neuroimaging findings can be replicated in animals and extended to the molecular level for a more thorough assessment. Thus, the limitations of clinical studies may be readily addressed with animal studies that can directly examine drug effects and can subsequently provide a platform to interpret findings from clinical studies.
However, animal “models” have their own limitations that should be acknowledged and addressed. Inter-species differences in the complexity of brain development and aligning comparable developmental periods for comparison raise the greatest concern. Phylogenetic differences do exist among species; yet among mammals, both genes and basic developmental processes are incredibly more similar than different. General principles of brain development (discussed below) allow extrapolation across species. Despite these limitations, animal research can and does serve a vital function by identifying potential targets and metrics of interest to guide future studies or offering experimental data to support the normalization of abnormal behavior.
Studies in developing animals suggest that exposure to therapeutic drugs produces long-term effects on brain structure and function
Drug exposure in typically-developing animals
As a general rule, short-term developmental exposure to psychotropic agents produces long-term effects that would not be predictable based on findings from similar exposure models in adult animals (Andersen and Navalta, 2004). Enduring drug effects should ideally normalize the aberrant behavior (a long-term effect), which may be the case if researchers could recapitulate the disorder in animals. In this vein, we believe that information from normal animals may be misleading in terms of guiding clinical practice directly (Rapoport and Gogtay, 2008). Rather, studies in normal animals may provide an important foundation for a potentially and more radical treatment model – one that is preventive and curative rather than symptom ameliorative. In contrast, drug exposure studies in normal animals may also highlight adverse effects in ways not noticed or reported in clinical trials. While a single drug’s potential to eliminate or prevent all symptoms is unlikely, focusing on a cluster of inter-related symptoms will also facilitate detection of enduring effects. The next section briefly gives examples of the potentially beneficial or deleterious effects of drug exposure on clusters of behavior in developing animals. Together, the studies suggest that both ADHD and depression (our selected examples) are sufficiently malleable to treatment during a sensitive period; with an appropriate agent, we may be able to identify drugs that can reprogram an abnormal trajectory back on course.
Developmental exposure to stimulants
The targets of drug action have changed little since the serendipitous use of Benzedrine for the treatment of minimal brain damage, as ADHD was initially called. Three main classes of pharmacotherapy are used for the treatment of ADHD: methylphenidate (MPH), amphetamines, and atomoxetine (Heal et al., 2009). The FDA has recently approved guanfacine. MPH is a mainstay of pharmacological treatment for ADHD and works by blocking catecholamine uptake. Amphetamine works by a different mechanism that increases monoamine levels. Both MPH and amphetamines have cortical and subcortical actions, but affect dopamine and norepinephrine in different ways (Heal et al., 2009). Agents that target dopamine activity are effective, but untoward side effects include sleeplessness, irritability, cardiac effects, and abuse liability. Similarly, drugs that target the prefrontal cortex (PFC) noradrenergic system are also effective in reducing ADHD symptoms, with untoward side effects of sedation and cardiovascular issues.
Some evidence exists for a relatively permanent reduction in certain behaviors following childhood drug treatment. While ADHD has been conceptualized primarily as an executive dysfunction disorder (Barkley, 1997), our recent understanding of ADHD pathology has broadened to include dysfunction in reward-related domains (Sonuga-Barke, 2003, Castellanos et al., 2006). For example, ADHD subjects are less sensitive to changes in reinforcement contingencies (Murray and Kollins, 2000), prefer smaller rewards sooner over larger rewards later (a measure of impulsivity known as delay aversion; Sonuga-Barke, 2003), and are insensitive to punishment and partial reinforcement (Quay, 1997). Novelty-seeking is another important characteristic of ADHD that may reflect a greater need for rewarding stimulation (Ernst et al., 2006). These behavioral issues that occur in ADHD are also risk factors for substance use disorder (SUD) (Luman et al., 2005). In fact, drug use begins earlier in the ADHD population and is more severe than drug use of typical, same-age peers (Wilens et al., 2003). Individuals with ADHD have a two-fold higher risk of developing a SUD than individuals without ADHD (Mannuzza et al., 2003, Mannuzza et al., 2008, Wilens et al., 2003). In this context, the use of stimulants for the treatment of ADHD seems highly counter-intuitive. However, a number of studies show the opposite effect. Pre-pubertal treatment of ADHD with stimulants reduces the risk for SUD in some instances – a finding that is supported by both retrospective and prospective studies (Mannuzza et al., 2003, Mannuzza et al., 2008, Wilens et al., 2003).
The clinical observation of reduced SUD risk following pre-pubertal stimulant exposure can be attributable to a number of psychosocial variables, including improved social support systems for the patient and the premise that a treated individual would not need to self-medicate with illicit substances. However, a number of preclinical studies have been conducted that expose animals to stimulants within clinical dose ranges to reveal a different and physiologically-based explanation for reduced SUD. Our discussion here will be limited to animal studies that used 3 mg/kg of MPH or less (Kuczenski and Segal, 2002) and not those studies designed to test hypotheses related to off-label recreational use. These doses are close to clinical levels (~0.5 mg/kg), but take into account the higher metabolism in rats (Wargin et al., 1983).
Behavioral evidence in animals shows that exposure to stimulants during the juvenile period produces lasting changes on drug-seeking behavior that endure into adulthood. Juvenile MPH reduces sensitivity and produces an aversion to cocaine-associated cues (Andersen et al., 2002b), reduces the sensitizing effects of stimulants on locomotor activity (Dafny and Yang, 2006, Torres-Reveron and Dow-Edwards, 2005), and attenuates novel object recognition in adult rats (LeBlanc-Duchin and Taukulis, 2007, Bethancourt et al., 2009). While this latter observation has been suggested to reflect some degree of memory impairment, an alternative explanation is that MPH reduces novelty-seeking in general. The above example illustrates how a subset of ADHD symptoms can be reduced and possibly prevented by early intervention.
Stimulants may also impact reward systems in an adverse way by increasing anhedonia. Using the same treatment paradigms as described above, juvenile exposure to MPH produces depressive-like effects as well as reduces preference for sucrose water and sex (Bolanos et al., 2003, Mague et al., 2005). Stimulant exposure increases the threshold for brain reward stimulation, suggesting that the reward system of the brain is attenuated by MPH (Mague et al., 2005). Unlike the reduction in SUD-related behaviors observed in the clinic (Mannuzza et al., 2008), these anhedonic properties are not observed in adults with a childhood history of stimulant exposure (Biederman et al., 2009). We believe that the anhedonia findings illustrate the importance of a clinical baseline when interpreting preclinical findings, and discuss this issue more below.
Developmental exposure to antidepressants
Antidepressants typically elevate 5-HT levels, either by direct re-uptake inhibition of the 5-HT transporter (the selective serotonin reuptake inhibitors, SSRIs), blockade of the noradrenergic transporter (the selective noradrenergic reuptake inhibitors, SNRIs), less specific monoamine blockade, or through other more direct signaling mechanisms. Generally, the SSRIs are most frequently used to treat depression. Elevation of 5-HT reduces depressive symptoms in adults, while exposure early in development may have the opposite consequence when examined long-term. Behaviorally, acute fluoxetine seems to attenuate depressive-like symptoms in immature rats (Bylund and Reed, 2007). In contrast, the long-term effects of early life exposure are quite different. Postnatal exposure to fluoxetine or the tricyclic antidepressant clomipramine increases depressive- and anxiety-like behaviors in adulthood (Mirmiran et al., 1981, Vogel et al., 1990, Ansorge et al., 2004, Karpova et al., 2009, Oh et al., 2009), with these behaviors emerging during late adolescence (Ansorge et al., 2008). The behavioral evidence is specific to enhanced 5-HT neurotransmission during a sensitive period of development and not alterations in norepinephrine levels (Hyttel, 1994, Ansorge et al., 2008, Popa et al., 2008). Postnatal exposure to other antidepressant agents, specifically citalopram and clomipramine, produces similar depressive effects in adulthood (Ansorge et al., 2008). Early clomipramine exposure also increases OCD-like behaviors in adulthood (Andersen et al., 2010). These results illustrate the long-term, untoward effects of elevated monoamine levels during early development. From an optimistic point of view, these results suggest that manipulating developmental processes to minimize depressive symptoms later in life is posible.
Taken together, preclinical studies provide experimental and causal relationships of drug exposure that are not ethically possible in clinical studies. To this end, the field would greatly benefit if clinical and preclinical researchers were to collaborate with one another. The goal of such synergistic efforts would be to develop novel strategies aimed not only at symptom reduction, but also improvement in functional status and ultimately prevention of psychopathology. By harnessing the powers of development, achieving such a goal is a genuine possibility.
Drug exposure will have its greatest impact during a sensitive period of development when the brain may be the most vulnerable
The role of critical and sensitive periods
As brain systems develop, critical periods exist in which the brain needs to receive certain stimulation to develop normally. Sensitive periods are also present when brain regions or systems are most strongly influenced by experience. The existence of critical periods implies that children need to have necessary formative experiences – at the ages they need them to – develop specific skills or abilities. Similarly, awareness of sensitive periods reveals windows of opportunity when children can best take advantage of formative experiences to learn or acquire skills in the easiest and most natural manner. For example, early social interaction is necessary for the initial development of emotional brain regions (critical period; Sullivan, 2003, Moriceau et al., 2006), whereas social interactions during childhood and adolescence optimize emotional regulation (sensitive period; Leussis and Andersen, 2008, Chronis-Tuscano et al., 2009).
From a mental health viewpoint, an increased understanding of critical and sensitive periods will help to identify discontinuities between normality and pathology, their possible causal processes, and potential windows of vulnerability and points for intervention (Andersen and Teicher, 2008, Teicher et al., 2009, Rutter, 2000). Critical periods have typically been investigated in sensory systems, especially the visual cortex, and delineated by changes in neuronal activity and their relationship to growth factors such as brain-derived neurotrophic factor (BDNF) (Webster et al., 2002, Hensch, 2005, Lein et al., 2000). Studies in adults show that SSRIs increase BDNF levels, neurogenesis, and dendritic branching (Duman and Monteggia, 2006, Wang et al., 2008, Malberg et al., 2000, Lee et al., 2001, Gould, 1999) and therefore are likely to influence critical periods. The closing of a critical period following SSRI exposure during development is likely to occur during the acute phases of drug exposure, as early fluoxetine exposure does not produce epigenetic changes in BDNF levels in the hippocampus as might be expected (Karpova et al., 2009).
Neuronal activity is largely dictated by the balance between excitation and inhibition, and developmental changes in glutamate and GABA aid in the formation of neuronal circuitry (Hensch, 2005). For example, the critical window for modulating visual circuitry can be closed by excess glutamatergic activity or prolonged by increased GABA activity primarily through its actions via the GABAA receptor subtype (Fagiolini and Hensch, 2000, Fagiolini et al., 2004). GABAergic neurons that express the calcium-buffering protein parvalbumin (PVB) may be the most relevant to manipulating critical/sensitive periods in relationship to psychiatric disorders. PVB GABA neurons are fast-spiking interneurons that modulate neuronal activity (Bartos et al., 2007), and changes (typically reductions) have been found in post-mortem studies of schizophrenia, bipolar disorder, and depression (Benes, 2009, Lewis et al., 1999). We discuss recent advances in developing animals that further highlight the importance of sensitive periods in the individual drug sections.
Sensitive periods are associated with the maturational events of neurogenesis, differentiation, and survival in a manner that maximizes adaptation to the environment (Bottjer and Arnold, 1997, Koehl et al., 2002, Nowakowski and Hayes, 1999, Sanchez et al., 2001, Andersen, 2003, Heim and Nemeroff, 2001). While the processes that actually define a sensitive period are unknown, plausible mechanisms of change include, but are not limited to, the modification of brain repair mechanisms, altered expression of neurotrophic factors, and the development of signaling mechanisms. Alterations in any of these factors during a sensitive period produce an enduring effect on structure and function (Andersen, 2003, Nair et al., 2006).
A need for a trajectory approach at multiple levels of analysis
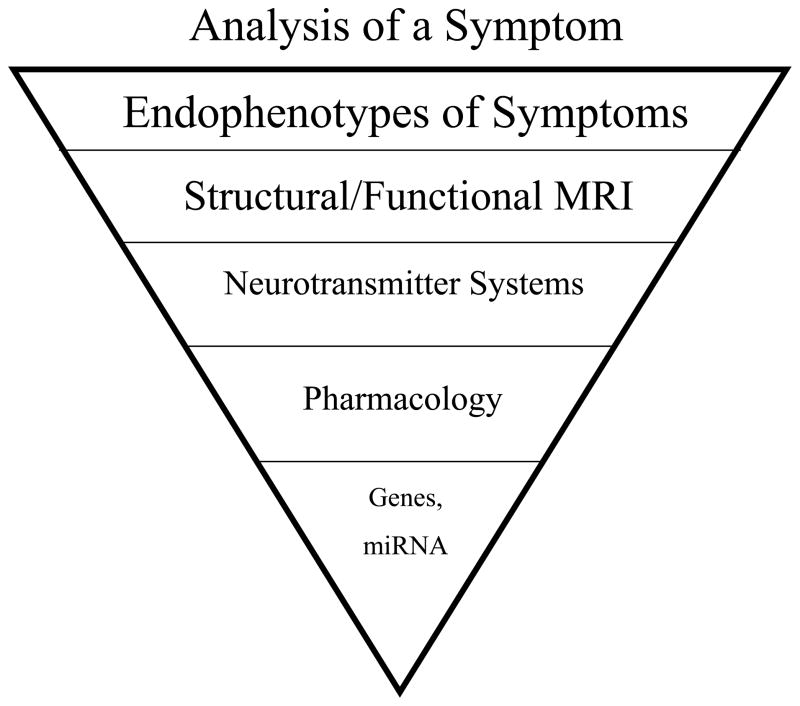
From the perspective that childhood pathology stems from abnormal development, one must understand normal developmental processes first to determine the point of divergence leading to abnormality (Cicchetti, 1984, Cicchetti and Toth, 2009). Normal development is not a linear process, but follows a trajectory with a path that rises and falls. The course of this path is determined by the interaction of a number of factors that provide a temporal, positional, and molecular framework necessary to achieve normal system development. Within this framework, complex neural networks exist that are sculpted by both spontaneous and experience-driven activity with changes that are unique to specific windows of development (Katz and Shatz, 1996, Zhang and Poo, 2001, Ben-Ari, 2002, Francis et al., 2002). Activity guides neurons to their target, aids in synaptogenesis, and stabilizes growth. If one or more of these factors is altered during development, a different trajectory for that system is likely to occur (Andersen, 2003, Andersen and Navalta, 2004, Nair et al., 2006). Such a trajectory approach for understanding the interaction among brain development, pathology, and drug exposure (both acute and chronic) needs to take place at multiple levels of analysis as identified in Figure 1. The following sections discuss developmental changes at each level and how they set the stage for a window of opportunity to intervene and even prevent symptoms later in life. Targeting a specific pathway gone awry during key developmental stages may provide a novel preventive approach for the treatment of many mental health disorders.
Figure 1. Level of analysis for developmental drug effects.
To fully understand the long-term effects of drug exposure, levels of analysis need to be conducted from the most molar to molecular ranges (including microRNAs, post-transcriptional regulators that typically repress gene expression) and placed into a systems-level of understanding.
Synaptogenesis and myelination
Stemming from the original post-mortem human studies by Huttenlocher (Huttenlocher, 1979, Huttenlocher and de Courten, 1987) and Benes (Benes et al., 1987), modern day structural imaging studies further show changes in gray and white matter across key maturational periods of childhood and adolescence (Giedd et al., 1999, Giedd et al., 1996a, Giedd et al., 1996b, Sowell et al., 2001, Sowell et al., 2004, Tau and Peterson, 2010, Paus et al., 2008). Synapses within each brain region are over-produced and then subsequently pruned with maturation (Brenhouse and Andersen, in press).
Structural changes are accompanied by age-related rates of myelination, which allows for smooth conduction of neuronal impulses (Fields, 2005). Glial cells (e.g., astrocytes) serve to regulate neurotransmitter levels, modulate metabolic function, and maintain the blood-brain barrier, although little is known about their postnatal development. We are only beginning to understand how synaptogenesis and pruning interact with myelinating processes and brain function (Paus et al., 2008). However, recognition that sub-optimal myelination alters the timing of neuronal communication (Fields, 2005) represents a new and important facet of drug effects on brain development to keep in the forefront. For example, reduced frontal white matter volumes (Steingard et al., 2002) and increased prevalence and severity of white matter signal hyperintensities are observed in ADHD (Lyoo et al., 1996); this loss of white matter is less in children with ADHD treated with stimulants (Castellanos et al., 2002).
Regional differences and the formation of neuronal networks
Within the orchestration of building a brain, each region has its own developmental timecourse of maturation (Tau and Peterson, 2010, Brenhouse and Andersen, in press). Generally, cortical areas mature later than subcortical areas, which are reviewed in greater detail elsewhere (Rapoport and Gogtay, 2008, Casey et al., 2000).
Developmental delays or precocial development within individual nodes of neuronal network formation are likely to produce a domino-like effect by setting off a chain of developmental events that alter the trajectory of multiple brain regions (Haber and Rauch, 2010, Ernst et al., 2009). From this perspective, longitudinal studies will be helpful in determining the sequence of regional brain changes as different cascades of events unfold (Gogtay et al., 2006, Sowell et al., 2004). For example, Shaw and colleagues (Shaw et al., 2007) have shown that cortical development lags in children with ADHD relative to their peers, but catches up by adulthood. In contrast, childhood-onset schizophrenia is associated with earlier regressive pruning than observed in typical children (Rapoport et al., 1999). Studies such as these are important for tracking the course of the disorder (Mackie et al., 2007) and simultaneously highlight windows of development that may be more or less susceptible to outside influences. Altered cortical development in both ADHD and schizophrenia, however, also highlights the limitations of MRI. Neuroimaging technologies such as MRI do not allow us to peer directly into the brain to determine chemical function. Thus, while the aforementioned studies show a shift in timecourse with a similar anatomical endpoint to control subjects, the function of the affected brain regions by adulthood is not considered “normalized”.
Neurotransmitter system differences
With continued refinement in our level of analysis, synaptic changes occurring at differing rates within individual neurotransmitter systems have been demonstrated, including innervation patterns, neurotransmitter levels, and signaling mechanisms (Andersen, 2003). Developmental neuroscience shows that neurotransmitter systems increase in expression during the postnatal period. One of two patterns emerges: 1) ectopic and transient expression in regions where the transmitter is absent by adulthood; or 2) a trajectory of overproduction and pruning, such that changes in expression typically peak during late childhood/early adolescence and are reduced to reach adult levels. Aside from psychosocial treatments, pharmacological intervention remains the most accessible (only?) option to clinicians treating psychiatric disorders. Because medications modify neurotransmitter levels, this level of analysis is the focus of the majority of the discussion.
Ectopic neurotransmitter expression is associated with directing growth into a given brain region. Ectopic expression is best characterized for the monoamines: dopamine (Gelbard et al., 1990, Todd, 1992, Lankford et al., 1988, Kalsbeek et al., 1988), norepinephrine (Feeney and Westerberg, 1990, Kline et al., 1994), and 5-HT (Lauder and Krebs, 1978, Kuppermann and Kasamatsu, 1984, Whitaker-Azmitia and Azmitia, 1986). For example, 5-HT7 receptors are transiently expressed in the striatum (Vizuete et al., 1997) and the hippocampus, along with transient expression of 5-HT2C and 5-HT5A (Garcia-Alcocer et al., 2006). The density of these receptors rises and falls early in development and then can rise again; expression levels are either absent or relatively lower by adulthood. Another example is that noradrenergic receptors are found in white matter during the time when many brain pathways are myelinating (Sanders et al., 2005). While the monoamine transmitters have received more attention given their long-standing status in the research community, other neurotransmitters and peptides likely serve a similar purpose. Transient neurotransmitters have trophic functions and increase synaptic sprouting, axonal growth, and synapse formation early in development. These effects, however, are concentration-dependent (Mazer et al., 1997), suggesting that baseline levels are integrally important for the nature of effect. Reduced levels of 5-HT during the equivalent of the early childhood period in the rat reduces synaptic markers in adolescence and is associated with learning deficits by adulthood. Similarly, reduced levels of norepinephrine during development markedly change patterns of immediate early gene expression in adulthood (Murrin et al., 2007). Too much 5-HT during this window can also alter development. Elevated levels of 5-HT with the drug fluoxetine during the rodent juvenile period “arrest development” (Norrholm and Ouimet, 2000). Dendritic branching is less in drug-exposed adults compared with non-drug-exposed adults, but comparable to normal juveniles. Thus, manipulations of neurotransmitters during the period of ectopic expression can have lasting effects on the wiring of the immature brain. Although we know relatively little about transient expression in terms of both location and timing for a number of markers, the likelihood is great that drug exposure during periods of ectopic expression has incredibly different effects when compared with adult exposure as well as possibly when compared with exposure mid-childhood to adolescence.
The second pattern of neurotransmitter signaling mechanisms follows a trajectory of overproduction and pruning. Overproduction and regressive elimination are believed to fine-tune the brain for efficiency (Teicher et al., 1995, Paus et al., 2008). Similar to ectopic expression, the overproduction phase represents a second state of vulnerability to exogenous influences. The over-expression of neurotransmitters and their associated pathways is greatest between childhood and adolescence when the synaptic selection process reaches its peak. This specific process is when childhood and adolescent drug exposure may also have a significant effect by selecting synapses or signaling mechanisms to be pruned or retained (i.e., Andersen et al., 2008a). For example, juvenile MPH exposure reduces D3 dopamine expression in the prefrontal cortex (PFC) in rats (Andersen et al., 2008a). This effect is mimicked by juvenile exposure to the D3 agonist ± 7-OHDPAT. An analysis of the time course of the drug-induced changes in D3 expression demonstrates that juvenile D3 agonist exposure reduces the adolescent over-expression of this receptor but does not affect D3 pruning. The end result is an overall reduction in D3 expression in adulthood, which has been previously associated with reduced drug-taking behavior in adult rats (Le Foll et al., 2002). Manipulation of a single receptor subtype localized in a single brain region can also initiate a cascade of developmental effects that affect distal regions. In an elegant study by Kellendonk (Kellendonk et al., 2006), over-expression of the D2 dopamine receptor in the striatum of genetically-modified animals produces subsequent (adolescent-onset) changes within working memory function in the PFC. This highly important finding suggests that correcting elevated levels of D2 receptors early in life may serve as a potent target towards prevention of schizophrenia.
Sex differences in brain development
Gray matter volume peaks earlier in girls than males (11.3 years versus 12.6 years, respectively) and likely corresponds to an earlier – and certainly different – trajectory of behavioral development (Giedd et al., 1997). In this review, we present sex differences in drug effects when possible, although little is known.
Early insult, delayed effects
The manifestation of an early insult, which may also include a genetic polymorphism, is often not immediate (Weinberger, 1987, Andersen and Teicher, 2004). Early life events program a trajectory and time is needed for its complete maturation. Some effects are readily observable in the short-term, whereas other effects emerge later in life. For example, early life stress produces delayed anatomical changes that parallel the adolescent emergence of depression in this population (Andersen and Teicher, 2004, Andersen and Teicher, 2008). Similar effects are observed for drug exposure (Andersen and Teicher, 2009). Likewise, genetic polymorphisms may be relatively dormant before manifesting later in life. The serotonin transporter (5-HTT) genetic polymorphism (5-HTTLPR) increases 5-HT levels during development. In humans, the 5-HTTLPR is associated with greater fearfulness in children (Hayden et al., 2007) and biased attention to emotional pictures in adolescents (Perez-Edgar et al., 2010). These traits seem evident as early as 2 months of age (Auerbach et al., 1999). However, the influence of a dysfunctional 5-HTT on depressive-like symptoms does not emerge until adulthood (at least in mice at 12 weeks of age; Ansorge et al., 2004, Vinkers et al., 2010). In addition, detection of polymorphism-associated changes may transition over time. In a recent clinical study (Harro et al., 2009), associated depressive traits longitudinally assessed were more readily detected during mid-as compared to late-adolescence. Thus, these converging lines of evidence strongly indicate that a single point of assessment along the developmental continuum is too myopic. Rather, a developmental perturbation needs to be studied at multiple ages or in adulthood to appreciate the full impact.
The ‘equal, but opposite’ model may predict the enduring effects of drug exposure during sensitive periods of development
The ‘equal, but opposite’ model of enduring drug action
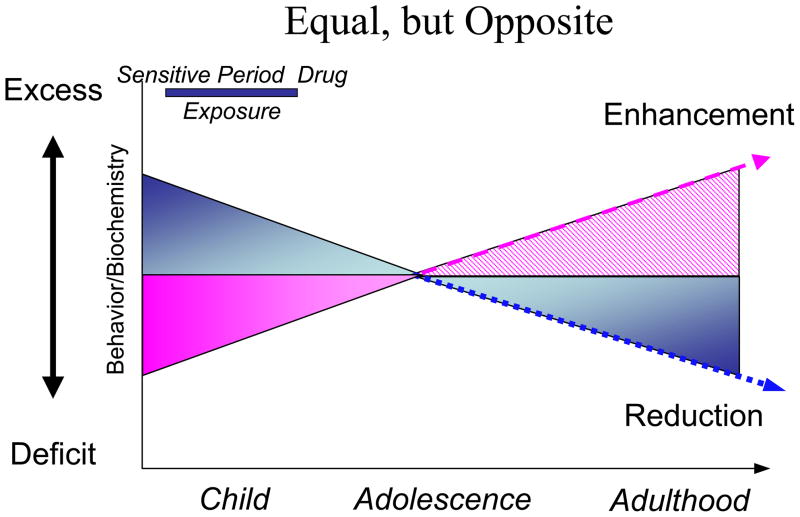
The working hypothesis of this model states that if a drug produces a given long-term effect when administered in adulthood, then a similar effect in the opposite direction will result following administration during development. The “equal, but opposite” model is based on the existing literature to potentially predict the enduring effects of drugs within a development framework (Andersen, 2003, Andersen, 2005). The model captures two important aspects of how drug exposure during development manifest: the short- and long-term (Figure 2). Short-term drug effects are mediated by the same mechanism of action and produce the same acute palliative effect in the mature and immature system. For example, stimulants increase catecholamine levels in both young and old subjects – this effect is illustrated with a positive triangle during both stages as seen in Figure 2. However, because the acute drug effects are identical, this adaptive outcome may have provided a false sense of safety by implying that the long-term effects in children must be comparable to those in adults. In reality, the long-term effects between old and young organisms differ markedly. In adults, chronic drug administration is accompanied by compensatory changes in structure and function (Creese et al., 1977, Nestler and Aghajanian, 1997, Brunello et al., 2002, Cryan et al., 2002). Such changes return to the original state typically within days to weeks upon withdrawal. That is, the adult system accommodates the drug only temporarily. In contrast, withdrawal from chronic drug exposure produces a different reaction in young animals. Here, the drug assimilates into the juvenile brain by producing permanent alterations of the system (Andersen, 2003, Norrholm et al., Lidow et al., 2001). The immature brain re-programs its developmental trajectory as if the drug was part of its local environment: the “use it or lose it” philosophy of brain development. If the drug was present when the brain system was programming its phenotype, the drug was “used” as part of the environment. Upon drug withdrawal during a sensitive period, a deficit state is produced. For example, stimulants increase reward-associated function, but withdrawal during the juvenile period manifests as reduced reward function later in life (as discussed above). For an antipsychotic that reduces dopamine activity in the short-term, the long-term effect could be an increase in dopamine activity in adulthood (Moran-Gates et al., 2006). The former change represents a positive benefit, the latter a negative effect. Taken together, chronic exposure to commonly used therapeutic agents during a sensitive period has the potential to either prevent or exacerbate symptoms later in life. In the latter case, the induction of a new constellation of psychiatric symptoms would be a ‘worst case’ scenario that clinicians need to take into consideration when deciding upon initiating a psychotropic medication trial.
Figure 2. The “equal, but opposite” hypothesis of predicting enduring developmental drug effects.
The diagram illustrates different trajectories of response depending on the baseline level of a behavior/symptom and/or biochemical marker. If a behavior or a biochemical marker is elevated pathologically during development, then exposure to additional stimulation during a sensitive period is predicted to reduce expression by adulthood by programming a new set-point or baseline of activity. In the absence of drug treatment, the behavior or the marker will be expressed to a relatively lower degree. Conversely, deficit behaviors are predicted to benefit from low levels of blockade to challenge the set-point to over-come the deficit. The arrows illustrate the need for continued maturation before the full-benefit of early pharmacotherapy is realized. Modified from (Andersen and Navalta, 2004, Andersen, 2005)
In summary, Figure 2 provides a working model of how sensitive periods interact with brain development to either produce an increase in behaviors or reduce behaviors, depending on the nature of the drug that is used (Andersen and Navalta, 2004, Andersen, 2005). Because psychiatric disorders change with age in concert with the underlying neuronal substrate, understanding how manipulation of neuronal systems at key stages of developmental trajectories is needed to optimize treatment. Yet, we may not know enough about how different systems develop at the present time to create novel treatments. Preclinical research strongly suggests that postnatal drug exposure during childhood and adolescence alters the development of brain regions where the drugs are active or have down-stream influences. As a result, the normal trajectory is altered in such a way that differs from what would be predicted based on exposure during adulthood (Andersen, 2003, Andersen, 2005). Several drugs have delayed effects on anatomy and function that are not apparent until adolescence or later. Such findings will be reviewed individually in the following sections.
Input variables necessary for predicting drug action
Enduring drug action may be predictable within this “equal, but opposite” framework if we can determine the critical input variables. Our work, and the work of others, highlights the importance of understanding two main variables: baseline and timing. The first input value is knowledge about baseline values of the system involved in the target behavior (e.g., D2 dopamine receptors, 5-HT1a receptors). Baseline can be interpreted as a deficit state (e.g., low cortical control) or an excess state (e.g., hyperactivity). Other factors that may contribute to baseline differences are the sex of the subject (although sex may have individual effects of its own), the time course of drug administration, and drug dosages. Age-related pharmacokinetic differences may also influence enduring drug effects. For example, the route of drug administration may be important, although preclinical studies that have directly compared different routes suggest that this factor does not significantly influence enduring effects as long as administration is not stressful (Brenhouse et al., 2009, Ansorge et al., 2008).
The second important input variable is timing: timing of both the age of drug exposure and the age of assessment. Drug exposure needs to occur during a sensitive period of development to have an enduring effect. For at least some drug effects, exposure needs to occur prior to the pruning of the targeted mechanism; such pruning typically occurs during the peripubertal period (e.g., the D3 dopamine effect described above). Much more data is needed, however, to show that the “pre-pruning” period is indeed a sensitive period for intervention. The age of the organism at the time of assessment is also important for predicting when outcomes (short- and long-term) become evident (Brenhouse et al., 2008b). As an illustration, we have shown that juvenile methylphenidate (MPH) exposure produces an aversion to cocaine-associated environments in late adolescence/early adulthood (Andersen et al., 2002b). However, this effect is relatively blunted when tested during adolescence (Brenhouse et al., 2009). Why? During adolescence, rats normally demonstrate a high sensitivity to cocaine environments (Brenhouse et al., 2008b), which competes with MPH effects and reduces treatment impact behaviorally and mechanistically (Andersen et al., 2002b, Andersen et al. submitted).
Understanding how these variables interact with each other may lead to predicting an “opposite” treatment in an immature population. We discuss below what is known about two major classes of drugs, stimulants and anti-depressants, as examples of the need to challenge deficit states rather than treat them. Throughout the text, we provide examples of how the heuristic of “equal, but opposite” can be used to inform both theory and clinical practice as the field pursues novel therapeutic agents. The following sections review information about the specific sensitive period associated with the use of stimulant, anti-depressant, and neuroleptic/antipsychotic drugs.
By understanding developmental processes as well as where and when they go awry, we may be able to apply the “equal, but opposite” hypothesis to identify novel therapeutic agents that can reprogram development, allowing the field to move from a palliative to a curative model
From a theoretical perspective, the stimulant/ADHD/SUD story provides an example of how a novel and paradoxical treatment aimed at developmental processes can produce long-lasting consequences. Consistent with the idea that ADHD and SUD possess underlying, convergent neurobiological mechanisms, exposure to medication before puberty is associated with reduced risk of developing a SUD in approximately 50% of ADHD cases (Mannuzza et al., 2008). Within the “equal, but opposite” framework, the use of stimulants is predictably understandable and targeting reward dysfunction may be what the stimulants do best. In the final section of this review, we provide an overview of what is known about the development of neural circuits and signaling mechanisms associated with ADHD and depression. Within each section, we review the effects of drug exposure of commonly used agents during different points of development and discuss emerging or novel approaches. Finally, we review the use of animal models to get to the issue of disease-relevant baseline differences that are absent in the majority of studies that use typically-developing animals.
Drug Exposure and Novel/Alternative Treatments for Immature Populations
Psychostimulant effects on neurotransmitter systems in animals – a means to identify novel mechanisms of action
Dopamine
Cortical levels of dopamine are reduced in ADHD patients (Ernst et al., 1998). MPH increases levels of dopamine acutely and exposure in juvenile rats produces an enduring increase in extracellular levels of dopamine in the PFC (Jezierski et al., 2007). The enduring increase in dopamine levels may be attributable to a decrease in PFC D3 receptor mRNA (Andersen et al., 2008a), which modulates cortical levels of dopamine during development (Andersen et al., 1997). Reduced autoreceptor activity would allow tonic levels of dopamine to increase within the PFC and offer therapeutic benefit. This hypothesis is consistent with an autoreceptor-like mechanism that has been proposed (Grace, 2000, Seeman and Madras, 1998), but awaits further experimental confirmation. Moreover, juvenile treatment with the D3-preferring agonist ± 7-OHDPAT mimics the behavioral effects of juvenile MPH exposure, suggesting that D3 agonism may provide a novel treatment during development (Andersen et al., 2008a). D1 dopamine receptors on glutamate neurons in the PFC are also implicated in SUD as they enhance the motivational salience of the environment when activated (Kalivas et al., 2005, Brenhouse et al., 2008a). In addition to D3 receptors, juvenile stimulant exposure affects the D1 dopamine receptor within the PFC by reducing D1 coupling to G proteins (Zhao et al., 2008). We have recently shown that juvenile MPH exposure specifically reduces the D1 receptor population that is expressed on glutamate neurons projecting to the nucleus accumbens. In addition, reduction of D1 receptors on glutamate neurons may also improve executive function deficits by reducing “noise” from other inputs (Vijayraghavan et al., 2007, Russell et al., 2005).
Norepinephrine
Few papers have investigated the developmental effects of stimulants on norepinephrine, much less the development of noradrenergic markers relevant to ADHD. Alpha2A receptors are of particular interest as they are involved in maintaining focused activity of glutamate output neurons (Arnsten, 2006). In a study by Gray and colleagues (Gray et al., 2007), 5 mg/kg MPH was administered to rats between P7-35, which transiently increased norepinephrine transmission. Significant increases in innervation, reduced norepinephrine transporters, and neurogenesis in the PFC and hippocampus were evident during adolescence in treated rats, but these markers returned to control levels by adulthood (Gray et al., 2007). The relatively short-lived benefit of this higher stimulant dose may be sufficient to facilitate learning of behavioral skills (such as those provided by cognitive behavior therapy), even though the neurochemical and anatomical effects are no longer grossly observed.
Atomoxetine affects norepinephrine neurotransmission primarily, but also dopamine in the PFC (Bymaster et al., 2002). In the single study in juvenile animals, 1 mg/kg atomoxetine reduced hyperactivity (Robinson et al., 2008). The advantage of atomoxetine, unlike other ADHD treatments, is its low abuse potential because the agent does not significantly increase dopamine levels in the ventral striatum. In fact, this selectivity may have use for the treatment of SUDs by reducing the motivational salience of novelty and other cues (including drug-associated cues). For example, we recently found that atomoxetine decreases the time required for adolescent rats to extinguish cocaine-associated cues (Brenhouse et al., 2010), which typically is almost twice as long as adults (Brenhouse and Andersen, 2008).
The “equal, but opposite” hypothesis would predict that developmental (child) exposure to an agent that reduces norepinephrine (or dopamine?) transmission acutely would be effective long-term. Notably, little long-term research is available on noradrenergic manipulations in immature populations. Alpha2A receptors are pre- and post-synaptically localized (Feuerstein et al., 2000), which implies that developmental manipulations are highly dose-dependent. Clonidine is also an α2 agonist that is under development for clinical use for ADHD (May and Kratochvil, 2010). Early life exposure in rats (day 8; infancy or very early childhood in humans) suggests that low doses of clonidine may facilitate developmental processes, but higher doses produce adverse effects (Kreider et al., 2004). When given between 8–21 days of age (childhood), clonidine (0.1 mg/kg) inhibits norepinephrine activity in the PFC in juvenile rats (Feenstra et al., 1992). However, the long-term effect may be a desensitization of an autoreceptor-like process, leading to a relatively permanent increase in noradrenergic activity. Thus, clonidine exposure, like the stimulants discussed above, alters developmental processes in normal animals in a manner that permanently increases the short-term effects, albeit by a secondary mechanism. Guanfacine is an α2A agonist and its long-term effects are not known. Like atomoxetine, little preclinical information is available about guanfacine’s effectiveness to reduce impulsivity in adults and none in developing animals. Francowicz et al (Franowicz and Arnsten, 2002) found that 0.1–0.7 mg/kg guanfacine improves cognition in normal mice and Bari et al (2009) found 0.3 mg/kg to be effective in the stop signal reaction time task, which is a measure of impulsive action. These behavioral effects are associated with reduced glutamatergic excitation in layer V PFC (Ji et al., 2008), which reduces cortical output to the accumbens and decreases impulsivity. Higher doses of guanfacine, however, produce sedation (Franowicz and Arnsten, 1998), which may prevent reaching an effective therapeutic dose for an enduring effect. How activation of α2A receptors modulates ADHD-associated symptoms long-term is unknown. Clearly, more research is needed.
Serotonin
MPH has a low affinity for the 5-HT transporter (Johnson et al., 1998), although MPH exposure during development can still impact the 5-HT system. The development of the 5-HT system is intimately linked with the development of the dopamine system (Soghomonian et al., 1987, Cunningham et al., 2005). For example, preclinical studies show dopamine may initially facilitate 5-HT innervation into the cortex; but during the course of development, 5-HT suppresses further dopaminergic innervation. Changes in 5-HT are of interest for the treatment of ADHD because of a demonstrated role in impulsivity (Robbins and Arnsten, 2009). In adult normal rats and non-human primates, elegant microinjection studies have determined that changes in the 5-HT1a and 5-HT2c receptors play a role in impulsivity within specific cortical and subcortical regions in adults (Dalley et al., 2008). Moreover, MPH itself has a relatively high affinity to 5-HT1a and 5-HT2b receptors, which may expand its mechanism of action beyond the catecholamines (Markowitz et al., 2006). Thus, the “equal, but opposite” hypothesis would predict that childhood exposure to MPH reduces 5-HT1a and 5-HT2c receptors, leading to decreased impulsivity. Novel therapeutics, however, aimed at 5-HT1a receptors especially during development need to consider sensitive periods of this receptor for anxiety-related behaviors (Gross et al., 2002). Increased striatal 5-HT transporters (5-HTT) have been linked to hyperactivity (Zhang et al., 2002), further supporting a role in ADHD that has been under-examined. Currently, preclinical research on 5-HT and stimulant exposure has been primarily focused on enduring changes in 5-HTT.
The enduring benefit of stimulant exposure on reduced SUD is especially applicable to ADHD individuals with comorbid conduct disorder (Mannuzza et al., 2008, Molina et al., 2007). Reducing conduct disorder may be important for future optimizing ADHD treatments. Conduct disorder is typically associated with excessive aggression – a behavior that has been linked to low levels of 5-HT in both human and non-human primates (Higley et al., 1996). Alterations in 5-HT function occur following prepubertal stimulant effects. In normal animals, juvenile MPH exposure increases depressive-like features consistent with altered 5-HT in general, as well as the above-mentioned dopamine effects. Juvenile MPH exposure increases depression-like behaviors in young adulthood in response to stress (Bolanos et al., 2003, Carlezon et al., 2003) and reduces reactivity to natural rewards of sucrose and sex (Bolanos et al., 2003). These depressive effects are reversible with subsequent treatment with a selective 5-HT reuptake inhibitor (Bolanos et al., 2008). One mechanism through which MPH may exert its effects is increasing 5-HT1B receptor function/expression. Knockout mice that lack 5-HT1B receptors show more impulsivity (a characteristic associated with violent suicide), including impulsive aggression, faster acquisition of cocaine self-administration, and increased ingestion of alcohol relative to wild-type mice (Brunner and Hen, 1997). Similarly, 5-HT1B antagonism decreases drug-induced locomotion (Borycz et al., 2008). Taken together, agonism of the 5-HT1B receptor during development may be an effective target for certain aspects of ADHD.
Gene expression
Gene expression may also be related to plasticity or could at least serve to identify regions that are affected by drug exposure (Yano and Steiner, 2005). Stimulants affect Arc expression in a manner consistent with “arrested development” in that the response patterns of normal juveniles resemble those of drug-exposed juveniles in adulthood, but are opposite of gene activation in normal adults (Banerjee et al., 2009). Other genes that have been identified as unique to the spontaneously hyperactive rat (SHR) include Oprm1, Calcyon, Calmodulin, Lhx1, and Hes6 (DasBanerjee et al., 2008).
Other novel targets
The field of cognitive enhancers in general is increasing with clear applications to the treatment of ADHD. Such enhancers include adenosine antagonists (Pires et al., 2009), caffeine (Prediger et al., 2005), nicotine (Day et al., 2007), and H3 histamine antagonists (Fox et al., 2002), which are under active investigation. Not all of these agents are effective in treating ADHD as a homogeneous group; rather, different subtypes of ADHD may be more responsive to different mechanisms of cognitive enhancement. As with the majority of stimulant research, these agents are being tested in adult animals. Nicotine has an inverted U, dose-dependent effect in the attentional 5-choice serial reaction time test with performance worsening at higher doses (Day et al., 2007). The use of nicotine therapeutically is interesting given that a significant proportion of teens with ADHD smoke, possibly to self-medicate (Milberger et al., 1997). In utero exposure to cigarette smoking has been recently associated with ADHD symptoms (Ernst et al., 2001, Knopik et al., 2005). Similarly, ADHD-like symptoms can be recapitulated in animals exposed to nicotine during development (Heath and Picciotto, 2009). Thus, nicotine may represent an ideal treatment for these individuals as predicted by the “equal, but opposite” model, in which a deficit state (low adenosine levels?) results from early nicotine exposure. Agents that target specific nicotine receptors are in Phase II clinical trials for their use in ADHD (Taly et al., 2009). The H3 histamine antagonist, ciproxifan, is effective when tested in a 5-choice serial reaction test (Pires et al., 2009, Day et al., 2007) or in SHR pups with an avoidance task (Fox et al., 2002). Notably, both MPH and atomoxetine increase histamine release in the PFC of adult rats (Horner et al., 2007, Pires et al., 2009), leading to further interest in this molecule.
At this stage of investigation, these studies focus on the effectiveness of these agents to reduce specific behaviors, not a syndrome of behaviors. A battery to evaluate several interrelated behaviors still needs to be devised to differentiate certain features of classic drugs, such as MPH and amphetamine, from each other as well as newer drugs, such as atomoxetine and the above-mentioned new compounds. Perhaps these novel mechanisms are the key to this needed differentiation and may further lead to promising candidates for the future treatment of ADHD.
Paramount to the questions raised by early drug exposure studies in general is whether stimulants change the brain permanently or reduce plasticity to later challenge. Clinical studies that have examined the trajectory of cortical development of gray matter suggest that stimulants are not associated with gross changes in structure (Shaw et al., 2009). However, the age of drug exposure was not taken into account. Carefully controlled animal studies suggest that juvenile stimulant exposure may alter innervation patterns. Juvenile exposure to amphetamine, for example, increases dendritic branching in the PFC, but has no effect in the accumbens (Diaz Heijtz et al., 2003). Functionally, acute MPH increases cerebral blood metabolism and flow in humans (Kim et al., 2001, Vaidya et al., 1998), and preclinical data suggests that repeated MPH exposure in juvenile rats permanently increases relative cerebral blood flow in response to drug challenge (Andersen et al., 2008a). Notably, these positive effects of drug exposure on function are age-dependent. MPH reduces BDNF levels and neurogenesis in cortical and hippocampal regions when given to juvenile rats, but has no effect following adult administration (Lagace et al., 2006, Banerjee et al., 2009). Reduced neurotrophic factors signal the closing of a sensitive period (as described in the visual system; Hensch, 2005). Although the functional significance of MPH-induced BDNF changes are not well-understood, drug-induced changes within the genome (e.g. c-fos immunoreactivity) differ depending on the age of exposure (Penner et al., 2002, Brandon et al., 2003).
Improved animal models of ADHD with a developmental profile of symptom expression
ADHD animal models need to show a developmental phenotype to provide a more clinically-relevant study of drug-induced changes (Volkow and Insel, 2003). Consistent with the clinical picture, elevated activity in response to novel environments, increased impulsivity, and attentional impairment should be apparent during the juvenile stages of these models relative to a control group. Currently, three approaches of study exist, including two prominent models: the Spontaneously Hypertensive Rat (SHR) and 6-hydroxydopamine (6-OHDA) depletions (Table 1). Both of these models have unique limitations that should be taken into consideration when interpreting data on developmental drug effects. The alternative approach is to behaviorally screen subjects for the desired phenotype and treat thereafter. Table 1 provides a brief synopsis of these animal models and the effects of developmental drug treatments.
Table 1.
Animal models of ADHD
| Animal Model | Characteristics | Manipulation | Investigators | |||
|---|---|---|---|---|---|---|
| Behavioral Phenotype | Neurobiology | Drug | Age of Exposure | Response | ||
| SHR rat | hyperlocomotion hyperactivity | increased striatal DAT density (Watanabe et al., 1997) or decreased glutamate activity (Russell et al., 2005); elevated striatal 5-HTT | MPH (2 mg/kg in drinking water) | P21-39 | normalized DAT expression | Roessner et al. (2010) |
| MPH (2 mg/kg in drinking water) | P21-39 | no reduced hyperactivity | Ferguson et al. (2007) | |||
| MPH (0.6 mg/kg) | adolescence | prevention of locomotor sensitization to cocaine in adolescence | Barron et al. (2009) | |||
| MPH | reduced impulsivity in subset of rats | Adriani et al. (2004) | ||||
| 6-OHDA-lesioned rats | hyperactivity working memory impairment | whole brain DA depletions; elevated striatal 5-HTT | citalopram fluvoxamine nisoxetine desipramine atomoxetine |
decreased hyperactivity decreased hyperactivity decreased hyperactivity decreased hyperactivity decreased hyperactivity decreased DAT decreased D3 increased PFC blood flow |
Davids et al. (2002) Zhang et al. (2004) |
|
| Normal rats | MPH | P21-35 | Moll et al. (2001) | |||
| MPH (2 mg/kg) | P20-35 | Andersen et al. (2008) | ||||
Sex differences
While both boys and girls are diagnosed with ADHD, females show greater symptoms than males in adulthood (Ernst et al., 1994, Teicher et al., 2002). Currently, research on females is limited. Given the potential importance of the timing of medication exposure in producing an enduring effect, a substantial amount of data will be needed to determine sex differences at different intervals of treatment. Boys with ADHD are more likely to receive treatment and receive treatment earlier than girls with ADHD (Barbaresi et al., 2006, Bussing et al., 2003). This earlier intervention may permanently reduce some of the ADHD symptoms in males, which would lead to the apparent greater impairment in ADHD women compared with men in adulthood (Ernst et al., 1994). Nevertheless, girls are less likely to exhibit the hyperactive symptoms of ADHD than boys and more likely to escape diagnostic attention.
Estrogenic effects on catecholamine transporters (Becker and Hu, 2008) should enhance drug action in cycling females, but the effects on α2a signaling are unknown. Other research comparing drug action between juvenile and older female rats supports the prediction that MPH enhances sensitivity to stimulants later in life (Zakharova et al., 2009, Melnick and Dow-Edwards, 2001, Brenhouse et al., 2009), which is opposite to the effects in male rats at the same age of exposure (Andersen et al., 2002b) or clinical outcomes (Wilens et al., 2003). These data suggest that doses for these agents should be reduced as females begin menstruating and even possibly altered with different phases of their cycle. The relatively sparse literature in animals highlights a need for investigation of enduring stimulant effects in females.
Anti-Depressants: developmental effects of SSRIs and the identification of novel targets
Classic antidepressants that work by elevating levels of 5-HT have limited efficacy in children and adolescents and are associated with a significant number of adverse effects – suicidal ideation and suicide in a few cases. In a context where approximately 50% of the cases of depression emerge by 14 years of age (Kessler et al., 2005), the need for better interventions for this mental health disorder is paramount. Drug exposure studies in animals may help to refine current treatments by focusing on specific receptors or provide insight into novel treatments altogether, thus leading to improved treatment for child and adolescent depression.
Targets for the treatment of depression in immature populations
Converging evidence suggests that elevated 5-HT significantly influences the development of anxiety and depressive-related circuits. In the context of the “equal, but opposite” hypothesis, the sensitive period for these circuits seems to occur very early in postnatal life. For example, elevated 5-HT activity during early development (e.g., 9–16 days of age in the rat) increases anxiety and depression in rats and mice later in life (Feng et al., 2001, Gross et al., 2002, Andersen et al., 2002a, Ansorge et al., 2004). These effects may work via the 5-HT1a receptor (Gross et al., 2002), as this period of drug exposure overlaps with a sensitive period for the development of anxiety-like behavior associated with this receptor subtype. Similarly, humans with the s-type 5-HTTLPR polymorphism, which functionally elevates 5-HT levels, have increased amygdala activity to fearful faces that is further associated with reduced PFC volume (Pezawas et al., 2005, Munafo et al., 2008). This same polymorphism increases susceptibility to depression by interacting with the number of adverse experiences to which an individual is exposed (Caspi et al., 2003). Taken together, the anxiety and depressive systems may be the most vulnerable to serotonergic influences early in life. Developmental drug exposure studies that manipulate this system – often with increased depressive effects later in life – may lead to the identification of novel targets for treatment at later ages. The following studies show how malleable these circuits are to early influences. Little work has been done with antidepressant exposure at later ages.
Serotonin
5-HT is involved in sculpting maturing circuitry and this process depends on 5-HT levels (Azmitia and Whitaker-Azmitia, 1991, Verney et al., 2002). Levels of 5-HT that are too high or low early in life reduce synaptic development. Depletion of 5-HT during early postnatal development in the rat produces a permanent loss of synapses in the hippocampus (Mazer et al., 1997), whereas elevated 5-HT decreases metabolic activity in the PFC and a significant loss of 5-HT innervation (Whitaker-Azmitia, 2005). Reductions in 5-HT innervation are consistent with structural MRI findings found in clinical studies of depressed individuals (Drevets, 1999) and animal studies causally show that increased 5-HT levels increase depressive-like symptoms later in life (Mirmiran et al., 1981, Vogel et al., 1990, Ansorge et al., 2004, Karpova et al., 2009, Oh et al., 2009).
The preclinical studies have focused primarily on SSRI exposure during early life, which has the greatest relevance for exposure during pregnancy. SSRIs are used to control maternal mood problems and are one of the most frequently prescribed classes of medications during pregnancy (Velves et al., 2006). SSRIs readily cross the placenta and the blood-brain barrier and maternal SSRI treatment can alter central serotonin signaling of the fetus (de Montigny et al., 1990). A major confounding issue in the clinical realm, however, is the simultaneous prenatal exposure to SSRIs and maternal depression and thus ascertaining to what extent does each differentially contribute to observed mental health outcomes. Earlier studies by Nulman and colleagues (1997, 2002) showed no negative consequences of in utero antidepressant exposure (i.e., TCAs and fluoxetine) on global intelligence, language development, or behavioral development in preschool and early school-age children. However, more recent findings illustrate a range of health and mental health outcomes, especially during the early postnatal period. Infants of depressed mothers treated with SSRIs are at increased risk for low birth weight and respiratory distress (Oberlander et al., 2006); have altered motor activity, startle response, sleep, and heart rate variability (Zeskind and Stephens, 2004); reduced responsiveness to noxious stimuli (Oberlander et al., 2005); and altered HPA stress response patterns and reduced early evening basal cortisol (Oberlander et al., 2008). Preschoolers exposed to SSRIs in utero can exhibit subtle motor development and control deficits (Casper et al., 2003) as well as increased internalizing behaviors – the latter of which appears to be moderated by the serotonin transporter promoter (SLC6A4) genotype (Oberlander et al., 2010). Not surprisingly, the timing of in utero exposure to antidepressants varied significantly across these studies and others or were not specified (Gentile 2010). In concert with a major theme of the present review, the possible adverse sequelae on the immature brain as relevant to the depressive-like (Ansorge et al., 2007) and obsessive-compulsive-like (Andersen et al., 2010) behaviors observed in animal studies certainly warrants a more thorough investigation of these early time points.
While the antidepressant exposure literature indicates that the sensitive period for these effects occurs during early postnatal life, this period may be extended to the juvenile (prepubertal) period as well. Exposure to fluoxetine during juvenile development (21–42 days of age) reduces adult hippocampal spine density to levels equivalent to the original juvenile density state (Norrholm and Ouimet, 2000). In contrast, Wegerer and colleagues (Wegerer et al., 1999) found that chronic treatment with 5 mg/kg fluoxetine in the drinking water between 25–39 days of age (childhood through adolescence) increases 5-HTT binding in the PFC later in life. This effect was not observed when drug exposure was initiated at 50 days of age (later adolescence), suggesting that the sensitive period for permanently altering structure had ended. While these data suggest that child/adolescent exposure may improve 5-HT function by increasing PFC innervation (e.g., increased 5-HTT binding), elevated 5-HTT may further reduce 5-HT levels in the PFC. More work needs to be done to better understand the functional effects of drug exposure during this period.
Manipulations of specific receptor subtypes may provide greater insight for the development of novel treatments for depression. Here, animal studies can be used to identify which of the myriad of 5-HT receptor subtypes are affected by drug exposure paradigms that produce depression. For example, treatments aimed at the 5-HT1a receptor may aid in reducing anxiety that leads to depression. A recent study showed that 5-HT1a receptor antagonism during pre- and postnatal ages (until 21 days of age) in rats increases anxiety behavior and elevated GABAa receptors in the PFC and hippocampus (Vinkers et al., 2010). These results are reminiscent of the 5-HT1a receptor knockout study whereby such mice show an anxious phenotype unless “rescued” by pharmacological replacement (Gross et al., 2002). The effects of elevating 5-HT1a receptors developmentally have yet to be delineated, but may be a potential ameliorative treatment for anxiety and/or depression. The “equal, but opposite” hypothesis would predict that a 5-HT1a agonist could reduce anxiety and possibly produce resiliency towards the emergence of depression later in life. Targeting 5-HT receptors that are ectopically (transiently) expressed may offer another approach to altering misguided developmental processes. 5-HT4 receptors are one such example as 5-HT4 knock-out mice show elevated anxiety and increased excitability to stress (Compan et al., 2004). The effects of replacement therapy (e.g., 5-HT4 agonists) are not known in these animals, but could help promote reduced excitability as described above.
Novel treatments for future development in the treatment of depression
The following section highlights some of the emerging advances in the preclinical literature that may hold promise for the treatment of depression. Some of these approaches are still in a highly theoretical phase of development, but show how manipulation of developmental processes may reduce/prevent later psychopathology. Other approaches are at various stages of pharmaceutical development.
GABA and chondroitin sulfate proteoglycans (CSPGs)
GABA plays a significant role in shaping immature neuronal circuitry (Hensch, 2005). Elevated levels of GABAa receptors close a critical period for development, in part through expression of CSPGs that modulate synaptic maturation (Yamagata and Sanes, 2005). By manipulating CSPGs, Gogolla and colleagues (Gogolla et al., 2009) were able to enhance extinction to fear stimuli that recapitulates an earlier state of development when juvenile animals show rapid extinction. This intriguing finding raises the possibility of reducing an anxious phenotype before the transition to depression. Viewed from a more global perspective, we may soon be able to manipulate brain development to revisit sensitive periods well after they have passed.
Norepinephrine and dopamine
Drugs that work at noradrenergic receptors may serve as effective antidepressants for immature popuations, but little research is available. Most of what we know is based on studies that used noradrenergic drugs as the comparison to 5-HT drugs, and these agents are non-selective and include actions at norepinephrine and dopamine. Juvenile noradrenergic treatments (e.g., desipramine) fail to produce delayed depressive effects (Hyttel, 1994, Reed et al., 2009, Ansorge et al., 2008), which may be due to immature noradrenergic regulatory mechanisms (Deupree et al., 2007). Alternatively, these observations may also suggest that noradrenergic activity might be “protective” against depressive-like behaviors and perhaps these treatments should be revisited for pediatric populations. Adverse cardiac effects, however, are a concern that need to be kept in mind. Early studies of noradrenergic antidepressants utilized very low doses and thus, their purported ineffectiveness may have been premature. Notably, receptor-specific targeting of noradrenergic receptors may provide an important intervention, such as those that are ectopically expressed (Happe et al., 2004). For example, treatment with an α2a receptor agonist may help prevent the loss of white matter volume that is observed in an animal model of stress-related depression during adolescence (Leussis and Andersen, 2008).
Glutamate
Excessive glutamate activity during development may lead to depressive symptoms. Agents that reduce glutamate actions, such as NMDA antagonists or a NR2b receptor antagonist, may provide some benefit under conditions in which depression is related to increased basolateral amygdala activity (Maeng et al., 2008). Furthermore, given that 62% of pediatric cases of generalized anxiety disorder convert to depression in adolescence (Beesdo et al., 2007), reducing glutamate may serve as an effective prevention of symptoms. In a study of adolescent depression produced by exposure to social stressors, we demonstrated a reversal of synaptic loss in the PFC by treatment with the NMDA antagonist MK-801 (Leussis and Andersen, 2008). Intervention approaches in younger animals have yet to be demonstrated. A newer mechanism is the organic cation transporter (hOCT), which takes up glutamate and other catecholamines into glia cells for inactivation.
Neuropeptides – Oxytocin and Dynorphin
Oxytocin is a neuropeptide that plays a role in anxiety and is altered under conditions of abnormal maternal care. Because of its role in bonding and attachment, investigators have shown a steady increased interest in oxytocin as an indicator of the degree of attachment (at all ages). Recently, oxytocin was demonstrated to be strongly associated with low parental attachment, but with a high propensity for anxiety and depressive symptoms in young adults (Gordon et al., 2008). Oxytocin expression is reduced in rats that have pre- and post-natal exposure to 5-HT (Whitaker-Azmitia, 2005), further strengthening interest in this peptide’s role in depression. Dynorphin may represent a second novel neuropeptide treatment given its role in anhedonic behaviors (Pliakas et al., 2001, Knoll and Carlezon, 2010). Manipulation of dynorphin levels reverses drug-induced anhedonia, anxiety, and helplessness assessed by the forced swim test (FST) (Knoll et al., 2007).
Pharmacogenomics
This rapidly evolving field may identify new targets, including four genes affected by antidepressants in adult animals (Crowley et al., 2006, Heurteaux et al., 2006, Svenningsson and Greengard, 2007, Tsankova et al., 2006). The first is TREK1 (KCNK2) whereby TREK1 knock-out animals are depression-resistant and insensitive to SSRIs (Heurteaux et al., 2006). The second gene is the vesicular monoamine transporter VMAT2, which is encoded by SLC18A2; (Crowley et al., 2006). Third, the p11 gene (encoded by S100A10) increases signaling by 5-HT1b receptors (Honore, 2007) with p11 knock-outs showing depressive behaviors (Svenningsson and Greengard, 2007). The fourth gene, a histone deacetylase (HDAC5), modulates antidepressant response by its actions on the BDNF promotor (Tsankova et al., 2006). Of these, only the KCNK2 gene is consistently associated with antidepressant response in human samples (Perlis et al., 2008). The potassium channel encoded by this gene is modulated by 5-HT4, mGLuR1, and mGluR5 receptors (Perlis et al., 2008). Thus, developmental treatments aimed at these receptors specifically might be effective.
Animal models for testing antidepressant exposure
Similar to research on early postnatal exposure to psychostimulants, preclinical research on clinically relevant exposure to antidepressants during development has increased within the last decade. A majority of this research utilizes one of four approaches: 1) drug exposure in normal animals; 2) the study of genetic mutant models to produce a depressive-phenotype, although this strategy may be aimed more at the causal process of depression than treatment; 3) selective breeding of animals for a depressive phenotype; and 4) stress models. Data on specific animal models 2 and 4 are presented in Table 2.
Table 2.
Animal models of depressive behaviors
| Animal Model | Characteristics | Manipulation | Investigators | |||
|---|---|---|---|---|---|---|
| Behavioral Phenotype | Neurobiology | Drug | Age of Exposure | Response | ||
| 5-HTT knock-out mice | Elevated depressive-like behavior; altered sleep patterns and anhedonia | increased 5-HT during development | Ansorge et al. (2004); Popa et al. (2008) | |||
| Selective breeding of animals for a depressive phenotype | Flinder Sensitive line: depressive | escitalopram | P43-73 | reduced immobility in FST | El Khoury et al. (2006) | |
| lithium | P42-49 | increased growth factors in hippocampus, frontal cortex, occipital cortex and striatum | Angelucci et al (2003) | |||
| High sensitivity to tail suspension | low serotonin levels and enhanced 5-HT1a autoreceptor sensitivity | El Yacoubi et al. (2003) | ||||
| Stress-based models: maternal separation | Elevated anxiety | increase in GABA-A receptor | Caldji et al (2004) | |||
| Elevated depressive-like behavior | Increase CRH, corticosterone | Liu et al (1997) | ||||
| Increase in 5-HT2c mRNA | fluoxetine environmental enrichment |
adolescence | reduced 5-HT2c behavioral improvement, no 5-HT2c change | Bhansali et al (2007) | ||
| Increased 5-HT1B in hippocampus | desipramine | normalized 5-HT1B | Vazquez et al (2002) | |||
Future directions of antidepressant treatment for children and adolescents
Improvements to treatment practices are still needed to optimize care in the long-term. These improvements could benefit from guidance by the “equal, but opposite” hypothesis if we were able to identify which mechanisms are changed with earlier intervention. Currently, the “equal, but opposite” heuristic predicts – and the preclinical data suggests – that the developmental application of SSRIs based on effective adult-based studies predicts elevated depressive symptoms later in life. “The glass is half full” approach, however, suggests that this heuristic could guide novel antidepressant treatment that could be maximized with specific developmental windows.
Atypical antipsychotics
Animal studies aimed at understanding the enduring effects of antipsychotics following juvenile exposure are relatively rare. Early research in this area focused on prenatal exposure and found elevated anxiety, stereotypies, and spatial memory issues in adulthood (Rosengarten and Quartermain, 2002, Singh et al., 1998, Singh and Singh, 2002, Zuo et al., 2008). Juvenile exposure to haloperidol or olanzipine reduces dendritic branching patterns (Frost et al., 2010). Juvenile exposure to risperidol produces dose-dependent increases in 5-HT1a and dopamine receptors in the frontal cortex of rats when assessed after three weeks of treatment (Choi et al., 2010). Behavioral effects, however, have not been studied. The observation that striatal D4, but not D2, receptors were elevated in juveniles, but not adults, is likely to have developmental consequences that were not investigated in this study (Moran-Gates et al., 2006). Transient D2 over-expression, produced by inducible over-expression of the D2DR gene in the striatum, prenatally produces working memory impairment in adulthood (Kellendonk et al., 2006). Given that D4 receptors are elevated in adult patients with schizophrenia and clozapine reduces D4 receptor binding (Seeman et al., 1993), the possibility exists that increased D4 expression following juvenile exposure to atypical antipsychotics could produce later impairment. Again, these exposure studies were conducted in normal animals.
While studies with the antipsychotics indicate modest efficacy, untoward side effects are prohibitive. Childhood exposure in early-onset cases of schizophrenia suggests that olanzapine increases right prefrontal cortical thickness, whereas no effect on gray matter density is observed in clozapine-treated individuals (Mattai et al., 2010). Overall, no medication effect was found, however, in the Mattai and colleagues (2010) study on the trajectory of gray matter development. As Figure 1 reminds us, changes can occur at multiple levels of analysis, which are likely to occur based on the preclinical data.
Early identification of individuals at risk is paramount for effective early intervention or prevention
Early identification is our best first step towards effective treatment, but requires a certain degree of certainty that the individual is truly at risk in the first place. Treatment of prodromal cases of schizophrenia has been promising thus far and the evidence is accumulating for continued success (Richtand and McNamara, 2008). Identification and prevention of problems in children at risk for different subtypes of ADHD symptoms could also be realized with reduced SUD rates, conduct disorder, and other dysfunction in adulthood (i.e., Mannuzza et al., 2008). Finally, preventive treatment for depression remains elusive. Targeting at-risk populations, when the greatest surge in the number of newly emergent cases occurs between 15–18 years (Hankin et al., 1998), would greatly reduce adult prevalence rates. Two high-risk populations can be identified and account for earlier-onset cases of depression relative to the average population (Beesdo et al., 2007, Teicher et al., 2009). First, childhood social anxiety disorder, or anxiety itself, increases the risk for depression in adolescence by 3- and 2-fold, respectively, compared with non-anxious, age-matched children (Beesdo et al., 2007). Vulnerable populations may be identifiable with fMRI whereby individual, atypical patterns of fronto-limbic circuits may be an early indicator of cognitive and behavioral inflexibility (e.g., McClure-Tone, 2009, Beesdo et al., 2009). Second, exposure to childhood sexual abuse is associated with the first episodes of depression occurring between 12–15 years of age (Teicher et al., 2009, Widom et al., 2007). In a cohort of exclusively female subjects exposed to childhood sexual abuse, but no other forms of abuse or trauma, depression onset in 62% of the subjects was delayed 9.2±3.6 years from the first episode of abuse (Teicher et al., 2009). These findings indicate that early stress not only increases risk of developing depression, but also accelerates onset into early adolescence. Children with an abuse history may be relatively asymptomatic until young adolescence when depressive symptoms emerge. In toto, preventive interventions for both of these trajectories leading to depression may be possible before symptoms emerge.
Missing pieces to the story of developmental drug exposure exist. For example, what happens in the interim periods of exposure? Does drug exposure produce beneficial effects initially before causing adverse effects in adulthood? Can effects that suggest increased plasticity (e.g., dendritic branching) be observed initially and then re-program the developmental circuitry resulting in decreased plasticity in adulthood? What can we learn about the regional nature of drug effects (e.g., Sarkissian et al., 1990, Trouvin et al., 1993)?
Conclusions
Human studies on the effects of medication exposure are thus limited to inform clinical practice for the long-term primarily due to reasons of efficiency and practicality. The time course to fully document enduring drug exposure effects just cannot keep pace with the increasing use of new (and old) medications in pediatric populations. More importantly, the limitations of human studies to control for the myriad of variables that may influence outcome will continue to obscure any findings of long-term effects. For these reasons, conducting research on the enduring effects in animal models is prudent in which observation of any sustained effects – beneficial or otherwise – may be more detectable.
In agreement with Cicchetti and Toth (Cicchetti and Toth, 2009), shifting to a framework based on individualized treatment that incorporates both underlying risk and appropriate intervention at multiple levels may provide effective intervention and prevention for subsequent problems later in life. Manipulating developmental processes to correct a trajectory that has gone awry (or is predictably about to do so) may allow clinicians to treat mental health problems in a novel way that leads to a decline in symptoms with maturation. Alternatively, drug exposure may lead to additional psychopathology that has yet to be identified. As new drugs become available, pharmaceutical companies need to perform longitudinal studies to determine any delayed untoward effects. Rather than studying drug effects on our children, animal studies have and will continue to provide a greater depth of understanding of both causal pathways that underlie the consequences of developmental drug exposure. We can use this information to explain why certain psychopathologies emerge at different points in development, but more importantly, this information can be used to harness these mechanisms and reduce suffering long-term.
Acknowledgments
The authors gratefully acknowledge the support of NIH (DA-15403), the Simches and Rosenberg families. We also wish to thank our anonymous reviewers who provided thoughtful and constructive comments that finely pruned this manuscript to allow it to fully mature.
Footnotes
Conflicts of Interest and Code of Conduct: The authors declare no conflict of interest and abide by the ethical principles of psychologists and code of conduct as stated by the American Psychological Association, 1992.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andersen S, Dumont N, Teicher M. Differences in behavior and monoamine laterality following neonatal clomipramine treatment. Developmental Psychobiology. 2002a;41:50–57. doi: 10.1002/dev.10055. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, Leblanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002b;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by 7-OHDPAT. Naunyn Schmiedeberg’s Archives of Pharmacology. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Green-Collozi E, Sonntag KC. A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry. 2010;68:741–747. doi: 10.1016/j.biopsych.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Napierata L, Brenhouse HC, Sonntag KC. Juvenile methylphenidate modulates reward-related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur J Neurosci. 2008a;27:2962–2972. doi: 10.1111/j.1460-9568.2008.06254.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–534. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Current Op Pharmacol. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- Auerbach J, Geller V, Lezer S, Shinwell E, Belmaker RH, Levine J, Ebstein R. Dopamine D4 receptor (D4DR) and serotonin transporter promoter (5-HTTLPR) polymorphisms in the determination of temperament in 2-month-old infants. Mol Psychiatry. 1999;4:369–373. doi: 10.1038/sj.mp.4000531. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Whitaker-Azmitia PM. Awakening the sleeping giant: anatomy and plasticity of the brain serotonergic system. J Clin Psychiatry. 1991;52(Suppl):4–16. [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Banerjee PS, Aston J, Khundakar AA, Zetterstrom TS. Differential regulation of psychostimulant-induced gene expression of brain derived neurotrophic factor and the immediate-early gene Arc in the juvenile and adult brain. Eur J Neurosci. 2009;29:465–476. doi: 10.1111/j.1460-9568.2008.06601.x. [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27:1–10. doi: 10.1097/00004703-200602000-00001. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R, Wittchen HU. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, Mcclure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Benes FM. Neural circuitry models of schizophrenia: is it dopamine, GABA, glutamate, or something else? Biol Psychiatry. 2009;65:1003–1005. doi: 10.1016/j.biopsych.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Benes FM, Majocha R, Bird ED, Marotta CA. Increased vertical axon numbers in cingulate cortex of schizophrenics. Archives of General Psychiatry. 1987;44:1017–1021. doi: 10.1001/archpsyc.1987.01800230097015. [DOI] [PubMed] [Google Scholar]
- Benjamin CL, O’neil KA, Crawley SA, Beidas RS, Coles M, Kendall PC. Patterns and predictors of subjective units of distress in anxious youth. Behav Cogn Psychother. 2010;38:497–504. doi: 10.1017/S1352465810000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Benes FM. A rat model for neural circuitry abnormalities in schizophrenia. Nat Protoc. 2006;1:833–839. doi: 10.1038/nprot.2006.110. [DOI] [PubMed] [Google Scholar]
- Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14:876–894. doi: 10.1002/hipo.20002. [DOI] [PubMed] [Google Scholar]
- Berretta S, Munno DW, Benes FM. Amygdalar activation alters the hippocampal GABA system: “partial” modelling for postmortem changes in schizophrenia. J Comp Neurol. 2001;431:129–138. doi: 10.1002/1096-9861(20010305)431:2<129::aid-cne1060>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bethancourt JA, Camarena ZZ, Britton GB. Exposure to oral methylphenidate from adolescence through young adulthood produces transient effects on hippocampal-sensitive memory in rats. Behav Brain Res. 2009;202:50–57. doi: 10.1016/j.bbr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Spencer T, Wilens TE, Faraone SV. Do stimulants protect against psychiatric disorders in youth with ADHD? A 10-year follow-up study. Pediatrics. 2009;124:71–78. doi: 10.1542/peds.2008-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Willey MD, Maffeo ML, Powers KD, Kinka DW, Grausam KB, Henderson RP. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methylphenidate. Biol Psychiatry. 2008;63:309–316. doi: 10.1016/j.biopsych.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Borycz J, Zapata A, Quiroz C, Volkow ND, Ferre S. 5-HT 1B receptor-mediated serotoninergic modulation of methylphenidate-induced locomotor activation in rats. Neuropsychopharmacology. 2008;33:619–626. doi: 10.1038/sj.npp.1301445. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Arnold AP. Developmental plasticity in neural circuits for a learned behavior. Annu Rev Neurosci. 1997;20:459–481. doi: 10.1146/annurev.neuro.20.1.459. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H, Sonntag KC, Andersen SL. Transient D1 dopamine receptor over-expression on prefrontal cortex projection neurons: A mechanism for enhanced motivational salience of drug cues in adolescence. Journal of Neuroscience. 2008a;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2011.04.013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Dumais K, Andersen SL. Enhancing the salience of dullness: Behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience. 2010;168:628–636. doi: 10.1016/j.neuroscience.2010.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Napierata L, Kussmaul L, Leussis M, Andersen SL. Juvenile methylphenidate exposure and factors that influence incentive processing. Dev Neurosci. 2009;31:95–106. doi: 10.1159/000207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008b;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychol Rev. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Brunello N, Mendlewicz J, Kasper S, Leonard B, Montgomery S, Nelson J, Paykel E, Versiani M, Racagni G. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur Neuropsychopharmacol. 2002;12:461–475. doi: 10.1016/s0924-977x(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Brunner D, Hen R. Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Ann N Y Acad Sci. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Bussing R, Zima BT, Gary FA, Garvan CW. Barriers to detection, help-seeking, and service use for children with ADHD symptoms. J Behav Health Serv Res. 2003;30:176–189. doi: 10.1007/BF02289806. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Reed AL. Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? Neurochem Int. 2007;51:246–253. doi: 10.1016/j.neuint.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat. A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Mcclay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Choi YK, Moran-Gates T, Gardner MP, Tarazi FI. Effects of repeated risperidone exposure on serotonin receptor subtypes in developing rats. Eur Neuropsychopharmacol. 2010;20:187–194. doi: 10.1016/j.euroneuro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Raggi VL, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. The emergence of developmental psychopathology. Child Dev. 1984;55:1–7. [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: the coming of age of a discipline. J Child Psychol Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci. 2004;24:412–419. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Antischizophrenic drugs: Chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196:326–328. doi: 10.1126/science.847477. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic evaluation of the antidepressant citalopram in the mouse tail suspension test. Neuropsychopharmacology. 2006;31:2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Connor CM, Zhang K, Benes FM. Diminished serotonergic innervation of adult medial prefrontal cortex after 6-OHDA lesions in the newborn rat. Brain Res Dev Brain Res. 2005;157:124–131. doi: 10.1016/j.devbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Dasbanerjee T, Middleton FA, Berger DF, Lombardo JP, Sagvolden T, Faraone SV. A comparison of molecular alterations in environmental and genetic rat models of ADHD: a pilot study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1554–1563. doi: 10.1002/ajmg.b.30877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Day M, Pan JB, Buckley MJ, Cronin E, Hollingsworth PR, Hirst WD, Navarra R, Sullivan JP, Decker MW, Fox GB. Differential effects of ciproxifan and nicotine on impulsivity and attention measures in the 5-choice serial reaction time test. Biochem Pharmacol. 2007;73:1123–1134. doi: 10.1016/j.bcp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- de Montigny C, Chaput Y, Blier P. Modification of serotonergic neuron properties by long-term treatment with serotonin reuptake blockers. J Clin Psychiatry. 1990;51(Supplement B):4–8. [PubMed] [Google Scholar]
- Deupree JD, Reed AL, Bylund DB. Differential effects of the tricyclic antidepressant desipramine on the density of adrenergic receptors in juvenile and adult rats. J Pharmacol Exp Ther. 2007;321:770–776. doi: 10.1124/jpet.106.118935. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur J Neurosci. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15:561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Kennard BD, Mayes TL, Nightingale-Teresi J, Carmody T, Hughes CW, Rush AJ, Tao R, Rintelmann JW. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165:459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Liebenauer LL, King AC, Fitzgerald GA, Cohen RM, Zametkin AJ. Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry. 1994;33:858–868. doi: 10.1097/00004583-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Westerberg VS. Norepinephrine and brain damage: alpha noradrenergic pharmacology alters functional recovery after cortical trauma. Can J Psychol. 1990;44:233–252. doi: 10.1037/h0084243. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Van Galen H, Boer GJ. Early postnatal clonidine treatment results in altered regional catecholamine utilisation in adult rat brain. Psychopharmacology (Berl) 1992;106:19–25. doi: 10.1007/BF02253583. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Feng P, Ma Y, Vogel GW. The critical window of brain development from susceptive to insusceptive. Effects of clomipramine neonatal treatment on sexual behavior. Brain Res Dev Brain Res. 2001;129:107–110. doi: 10.1016/s0165-3806(01)00158-4. [DOI] [PubMed] [Google Scholar]
- Feuerstein TJ, Huber B, Vetter J, Aranda H, Van Velthoven V, Limberger N. Characterization of the alpha(2)-adrenoceptor subtype, which functions as alpha(2)-autoreceptor in human neocortex. J Pharmacol Exp Ther. 2000;294:356–362. [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Fox GB, Pan JB, Esbenshade TA, Bennani YL, Black LA, Faghih R, Hancock AA, Decker MW. Effects of histamine H(3) receptor ligands GT-2331 and ciproxifan in a repeated acquisition avoidance response in the spontaneously hypertensive rat pup. Behav Brain Res. 2002;131:151–161. doi: 10.1016/s0166-4328(01)00379-5. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AF. The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AF. Actions of alpha-2 noradrenergic agonists on spatial working memory and blood pressure in rhesus monkeys appear to be mediated by the same receptor subtype. Psychopharmacology (Berl) 2002;162:304–312. doi: 10.1007/s00213-002-1110-6. [DOI] [PubMed] [Google Scholar]
- Frost DO, Page SC, Carroll C, Kolb B. Early exposure to haloperidol or olanzapine induces long-term alterations of dendritic form. Synapse. 2010;64:191–199. doi: 10.1002/syn.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alcocer G, Segura LC, Garcia Pena M, Martinez-Torres A, Miledi R. Ontogenetic distribution of 5-HT2C, 5-HT5A, and 5-HT7 receptors in the rat hippocampus. Gene Expr. 2006;13:53–57. doi: 10.3727/000000006783991935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Baldessarini RJ, Gallitano A, Marsh ER, Zorc J, Faedda G. Dopamine D1 receptor development depends on endogenous dopamine. Brain Res Dev Brain Res. 1990;56:137–140. doi: 10.1016/0165-3806(90)90173-v. [DOI] [PubMed] [Google Scholar]
- Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010;27:675–686. doi: 10.1002/da.20706. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gisabella B, Cunningham MG, Bolshakov VY, Benes FM. Amygdala-dependent regulation of electrical properties of hippocampal interneurons in a model of schizophrenia. Biol Psychiatry. 2009;65:464–472. doi: 10.1016/j.biopsych.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006 doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Grace A. Psychostimulant actions on dopamine and limbic system function: Relvance to pathophysiology and treatment of ADHD. In: SOLANTO M, ARNSTEN A, CASTELLANOS F, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University; 2000. [Google Scholar]
- Gray JD, Punsoni M, Tabori NE, Melton JT, Fanslow V, Ward MJ, Zupan B, Menzer D, Rice J, Drake CT, Romeo RD, Brake WG, Torres-Reveron A, Milner TA. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J Neurosci. 2007;27:7196–7207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Haber SN, Rauch SL. Neurocircuitry: a window into the networks underlying neuropsychiatric disease. Neuropsychopharmacology. 2010;35:1–3. doi: 10.1038/npp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, Mcgee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Happe HK, Coulter CL, Gerety ME, Sanders JD, O’rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Harro J, Merenakk L, Nordquist N, Konstabel K, Comasco E, Oreland L. Personality and the serotonin transporter gene: Associations in a longitudinal population-based study. Biol Psychol. 2009;81:9–13. doi: 10.1016/j.biopsycho.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Emily Durbin C, Olino TM, Nurnberger JI, Jr, Lahiri DK, Klein DN. Temperamental fearfulness in childhood and the serotonin transporter promoter region polymorphism: a multimethod association study. Psychiatr Genet. 2007;17:135–142. doi: 10.1097/YPG.0b013e3280147847. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(Suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Horner WE, Johnson DE, Schmidt AW, Rollema H. Methylphenidate and atomoxetine increase histamine release in rat prefrontal cortex. Eur J Pharmacol. 2007;558:96–97. doi: 10.1016/j.ejphar.2006.11.048. [DOI] [PubMed] [Google Scholar]
- Hugtenburg JG, Heerdink ER, Tso YH. Psychoactive drug prescribing by Dutch child and adolescent psychiatrists. Acta Paediatr. 2005;94:1484–1487. doi: 10.1111/j.1651-2227.2005.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Hunkeler EM, Fireman B, Lee J, Diamond R, Hamilton J, He CX, Dea R, Nowell WB, Hargreaves WA. Trends in use of antidepressants, lithium, and anticonvulsants in Kaiser Permanente-insured youths, 1994–2003. J Child Adolesc Psychopharmacol. 2005;15:26–37. doi: 10.1089/cap.2005.15.26. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Stimulus-specific plasticity of prefrontal cortex dopamine neurotransmission. J Neurochem. 2004;88:1327–1334. doi: 10.1046/j.1471-4159.2003.02205.x. [DOI] [PubMed] [Google Scholar]
- Jezierski G, Zehle S, Bock J, Braun K, Gruss M. Early stress and chronic methylphenidate cross-sensitize dopaminergic responses in the adolescent medial prefrontal cortex and nucleus accumbens. J Neurochem. 2007;103:2234–2244. doi: 10.1111/j.1471-4159.2007.04927.x. [DOI] [PubMed] [Google Scholar]
- Ji XH, Ji JZ, Zhang H, Li BM. Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–2271. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Eshleman AJ, Meyers T, Neve KA, Janowsky A. [3H]substrate- and cell-specific effects of uptake inhibitors on human dopamine and serotonin transporter-mediated efflux. Synapse. 1998;30:97–106. doi: 10.1002/(SICI)1098-2396(199809)30:1<97::AID-SYN12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Karpova N, Lindholm J, Pruunslid P, Timmusk T, Castren E. Long-lasting behavioral and olecular alterationsinduced by early postnatal fluoxetne are restored by chronic fluoxetine treatment in mice. Eur Neuropsychopharmacol. 2009;19:97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim BN, Lee JS, Cho SC, Lee DS. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Med J. 2001;42:19–29. doi: 10.3349/ymj.2001.42.1.19. [DOI] [PubMed] [Google Scholar]
- Kim BN, Lee JS, Shin MS, Cho SC, Lee DS. Regional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysis. Eur Arch Psychiatry Clin Neurosci. 2002;252:219–225. doi: 10.1007/s00406-002-0384-3. [DOI] [PubMed] [Google Scholar]
- Kline AE, Chen MJ, Tso-Olivas DY, Feeney DM. Methylphenidate treatment following ablation-induced hemiplegia in rat: experience during drug action alters effects on recovery of function. Pharmacol Biochem Behav. 1994;48:773–779. doi: 10.1016/0091-3057(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, Slutske WS, Grant JD, Mclaughlin TL, Todorov A, Todd RD, Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005;35:625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- Koehl M, Lemaire V, Mayo W, Abrous DN, Maccari S, Piazza PV, Le Moal M, Vallee M. Individual vulnerability to substance abuse and affective disorders: role of early environmental influences. Neurotox Res. 2002;4:281–296. doi: 10.1080/1029842021000010866. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Seidler FJ, Cousins MM, Tate CA, Slotkin TA. Transiently overexpressed alpha2-adrenoceptors and their control of DNA synthesis in the developing brain. Brain Res Dev Brain Res. 2004;152:233–239. doi: 10.1016/j.devbrainres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppermann BD, Kasamatsu T. Enhanced binocular interaction in the visual cortex of normal kittens subjected to intracortical nnorepinephrine perfusion. Brain Research. 1984;302:91–99. doi: 10.1016/0006-8993(84)91288-5. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Yee JK, Bolanos CA, Eisch AJ. Juvenile administration of methylphenidate attenuates adult hippocampal neurogenesis. Biol Psychiatry. 2006;60:1121–1130. doi: 10.1016/j.biopsych.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lankford KL, Demello FG, Klein WL. D1-type dopamine receptors inhibit growth cone motility in cultured retina neurons: evidence that neurotransmitters act as morphogenic growth regulators in the developing central nervous system. Proc Natl Acad Sci U S A. 1988;85:4567–4571. doi: 10.1073/pnas.85.12.4567-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Leblanc-Duchin D, Taukulis HK. Chronic oral methylphenidate administration to periadolescent rats yields prolonged impairment of memory for objects. Neurobiol Learn Mem. 2007;88:312–320. doi: 10.1016/j.nlm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, Kim CJ, Chung JH. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6:610, 725–618. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hohn A, Shatz CJ. Dynamic regulation of BDNF and NT-3 expression during visual system development. J Comp Neurol. 2000;420:1–18. doi: 10.1002/(sici)1096-9861(20000424)420:1<1::aid-cne1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Bozian D, Song ZM. Cocaine affects cerebral neocortical cytoarchitecture in primates only if administered during neocortical neuronogenesis. Brain Res Dev Brain Res. 2001;128:45–52. doi: 10.1016/s0165-3806(01)00139-0. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Noam GG, Lee CK, Lee HK, Kennedy BP, Renshaw PF. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biol Psychiatry. 1996;40:1060–1063. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, Sharp WS, Giedd JN, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, Mccammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mague SD, Andersen SL, Carlezon WA., Jr Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57:120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Moulton JL., 3rd Does stimulant treatment place children at risk for adult substance abuse? A controlled, prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13:273–282. doi: 10.1089/104454603322572606. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, 3rd, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JS, Devane CL, Pestreich LK, Patrick KS, Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J Child Adolesc Psychopharmacol. 2006;16:687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- Mattai A, Chavez A, Greenstein D, Clasen L, Bakalar J, Stidd R, Rapoport J, Gogtay N. Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr Res. 116:44–48. doi: 10.1016/j.schres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70:15–40. doi: 10.2165/11530540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- Mcclure-Tone E. Socioemotional functioning in bipolar disorder versus typical development: Behavioral and neural differences. Clinical Psychology: Science and Practice. 2009;16:98–113. [Google Scholar]
- Melnick SM, Dow-Edwards DL. Differential behavioral responses to chronic amphetamine in adult male and female rats exposed to postnatal cocaine treatment. Pharmacol Biochem Behav. 2001;69:219–224. doi: 10.1016/s0091-3057(01)00545-7. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Van De Poll NE, Corner MA, Van Oyen HG, Bour HL. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–146. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, Hechtman L, Jensen PS, Vitiello B, Hoza B, Pelham WE, Elliott GR, Wells KC, Abikoff HB, Gibbons RD, Marcus S, Conners CK, Epstein JN, Greenhill LL, March JS, Newcorn JH, Severe JB, Wigal T. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007;46:1028–1040. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Gates T, Gan L, Park YS, Zhang K, Baldessarini RJ, Tarazi FI. Repeated antipsychotic drug exposurein developing rats: Dopamine receptor effects. Synapse. 2006;59:92–100. doi: 10.1002/syn.20220. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LK, Kollins SH. Effects of methylphenidate on sensitivity to reinforcement in children diagnosed with attention deficit hyperactivity disorder: an application of the matching law. J Appl Behav Anal. 2000;33:573–591. doi: 10.1901/jaba.2000.33-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, Vaidya VA. Stressor-Specific Regulation of Distinct Brain-Derived Neurotrophic Factor Transcripts and Cyclic AMP Response Element-Binding Protein Expression in the Postnatal and Adult Rat Hippocampus. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res. 2000;883:205–215. doi: 10.1016/s0006-8993(00)02909-7. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Hayes NL. CNS development: an overview. Dev Psychopathol. 1999;11:395–417. doi: 10.1017/s0954579499002126. [DOI] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG, Kulin N, Koren G. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry. 2002;159:1889–1895. doi: 10.1176/appi.ajp.159.11.1889. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau R, Mayes L, Riggs W, Rurak D, Papsdorf M, Misri S, Weinberg J. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164:444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- Oh JE, Zupan B, Gross S, Toth M. Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology. 2009;34:2197–2207. doi: 10.1038/npp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE., Jr The NIMH multimodal treatment study for attention-deficit hyperactivity disorder: just say yes to drugs alone? Can J Psychiatry. 1999;44:981–990. doi: 10.1177/070674379904401004. [DOI] [PubMed] [Google Scholar]
- Penner MR, Mcfadyen MP, Pinaud R, Carrey N, Robertson HA, Brown RE. Age-related distribution of c-fos expression in the striatum of CD-1 mice after acute methylphenidate administration. Brain Res Dev Brain Res. 2002;135:71–77. doi: 10.1016/s0165-3806(02)00308-5. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, Mcdermott JM, Gorodetsky E, Hodgkinson CA, Goldman D, Ernst M, Pine DS, Fox NA. Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biol Psychol. 2010;83:269–271. doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Moorjani P, Fagerness J, Purcell S, Trivedi MH, Fava M, Rush AJ, Smoller JW. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pires VA, Pamplona FA, Pandolfo P, Fernandes D, Prediger RD, Takahashi RN. Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: a rodent model of attention-deficit hyperactivity disorder. Behav Pharmacol. 2009;20:134–145. doi: 10.1097/FBP.0b013e32832a80bf. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered Responsiveness to Cocaine and Increased Immobility in the Forced Swim Test Associated with Elevated cAMP Response Element-Binding Protein Expression in Nucleus Accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, Rohde LA. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry. 2007;20:386–392. doi: 10.1097/YCO.0b013e3281568d7a. [DOI] [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Post RM, Luckenbaugh DA, Leverich GS, Altshuler LL, Frye MA, Suppes T, Keck PE, Mcelroy SL, Nolen WA, Kupka R, Grunze H, Walden J. Incidence of childhood-onset bipolar illness in the USA and Europe. Br J Psychiatry. 2008;192:150–151. doi: 10.1192/bjp.bp.107.037820. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Fernandes D, Takahashi RN. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res. 2005;159:197–205. doi: 10.1016/j.bbr.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: inter-species and intra-species differences. Brain Res Rev. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Quay HC. Inhibition and attention deficit hyperactivity disorder. J Abnorm Child Psychol. 1997;25:7–13. doi: 10.1023/a:1025799122529. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, Nicolson R, Bedwell J, Lenane M, Zijdenbos A, Paus T, Evans A. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology. 2008;33:181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- Reed AL, Anderson JC, Bylund DB, Petty F, El Refaey H, Happe HK. Treatment with escitalopram but not desipramine decreases escape latency times in a learned helplessness model using juvenile rats. Psychopharmacology (Berl) 2009;205:249–259. doi: 10.1007/s00213-009-1535-2. [DOI] [PubMed] [Google Scholar]
- Reinecke MA, Curry JF, March JS. Findings from the Treatment for Adolescents with Depression Study (TADS): what have we learned? What do we need to know? J Clin Child Adolesc Psychol. 2009;38:761–767. doi: 10.1080/15374410903258991. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Mcnamara RK. Serotonin and dopamine interactions in psychosis prevention. Prog Brain Res. 2008;172:141–153. doi: 10.1016/S0079-6123(08)00907-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D. Effect of prenatal administration of haloperidol, risperidone, quetiapine and olanzapine on spatial learning and retention in adult rats. Pharmacol Biochem Behav. 2002;72:575–579. doi: 10.1016/s0091-3057(02)00727-x. [DOI] [PubMed] [Google Scholar]
- Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Psychosocial influences: critiques, findings, and research needs. Dev Psychopathol. 2000;12:375–405. doi: 10.1017/s0954579400003072. [DOI] [PubMed] [Google Scholar]
- Ryan ND. The pharmacologic treatment of child and adolescent depression. Psychiatr Clin North Am. 1992;15:29–40. [PubMed] [Google Scholar]
- Ryan ND. Continuation treatment with antidepressants in child and adolescent major depression. Am J Psychiatry. 2008;165:411–412. doi: 10.1176/appi.ajp.2008.08010115. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol Disord Drug Targets. 2007;6:127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Murrin LC. A transient expression of functional alpha2-adrenergic receptors in white matter of the developing brain. Synapse. 2005;57:213–222. doi: 10.1002/syn.20174. [DOI] [PubMed] [Google Scholar]
- Sarkissian CF, Wurtman RJ, Morse AN, Gleason R. Effects of fluoxetine or D-fenfluramine on serotonin release from, and levels in, rat frontal cortex. Brain Res. 1990;529:294–301. doi: 10.1016/0006-8993(90)90840-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [see comments] [DOI] [PubMed] [Google Scholar]
- Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3:386–396. doi: 10.1038/sj.mp.4000421. [see comments] [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, Mouly AM, Sullivan RM. Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. 2007;62:1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KP, Jaiswal AK, Singh M, Bhattacharya SK. Behavioural alterations in rats induced by single prenatal exposure of haloperidol. Indian J Exp Biol. 1998;36:1102–1107. [PubMed] [Google Scholar]
- Singh KP, Singh M. Effect of prenatal haloperidol exposure on behavioral alterations in rats. Neurotoxicol Teratol. 2002;24:497–502. doi: 10.1016/s0892-0362(02)00189-7. [DOI] [PubMed] [Google Scholar]
- Skaer TL, Sclar DA, Robison LM. Trends in prescriptions for antidepressant pharmacotherapy among US children and adolescents diagnosed with depression, 1990 through 2001: an assessment of accordance with treatment recommendations from the American Academy of Child and Adolescent Psychiatry. Clin Ther. 2009;31(Pt 1):1478–1487. doi: 10.1016/j.clinthera.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Doucet G, Descarries L. Serotonin innervation in adult rat neostriatum. I. Quantified regional distribution. Brain Res. 1987;425:85–100. doi: 10.1016/0006-8993(87)90486-0. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Editorial: ‘It’s the environment stupid!’ On epigenetics, programming and plasticity in child mental health. J Child Psychol Psychiatry. 2010;51:113–115. doi: 10.1111/j.1469-7610.2009.02213.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Soutullo CA, Chang KD, Diez-Suarez A, Figueroa-Quintana A, Escamilla-Canales I, Rapado-Castro M, Ortuno F. Bipolar disorder in children and adolescents: international perspective on epidemiology and phenomenology. Bipolar Disord. 2005;7:497–506. doi: 10.1111/j.1399-5618.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Steingard RJ, Renshaw PF, Hennen J, Lenox M, Cintron CB, Young AD, Connor DF, Au TH, Yurgelun-Todd DA. Smaller frontal lobe white matter volumes in depressed adolescents. Biol Psychiatry. 2002;52:413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Developing a sense of safety: the neurobiology of neonatal attachment. Ann N Y Acad Sci. 2003;1008:122–131. doi: 10.1196/annals.1301.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Greengard P. p11 (S100A10)--an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Offord DR, Boyle MH. Ontario child health study: prevalence of attention deficit disorder with hyperactivity. J Child Psychol Psychiatry. 1989;30:219–230. doi: 10.1111/j.1469-7610.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Navalta CP, Polcari A, Kim D. Neuropsychiatric disorders of childhood and adolescence. In: YUDOFSY S, HALES RE, editors. American Psychiatric Association Textbook of Neuropsychiatry. Washington DC: APA Press; 2002. pp. 1069–1119. [Google Scholar]
- Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD. Neural development is regulated by classical neurotransmitters: dopamine D2 receptor stimulation enhances neurite outgrowth. Biol Psychiatry. 1992;31:794–807. doi: 10.1016/0006-3223(92)90311-m. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Dow-Edwards DL. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacology (Berl) 2005;181:38–47. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- Trouvin JH, Gardier AM, Chanut E, Pages N, Jacquot C. Time course of brain serotonin metabolism after cessation of long-term fluoxetine treatment in the rat. Life Sci. 1993;52:PL187–192. doi: 10.1016/0024-3205(93)90116-k. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Oord S, Prins PJM, Oosterlaan J, Emmelkamp PMG. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: A meta-analysis. Clinical Psychology Review. 2008;28:783–800. doi: 10.1016/j.cpr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Vasconcelos MM, Werner J, Jr, Malheiros AF, Lima DF, Santos IS, Barbosa JB. Attention deficit/hyperactivity disorder prevalence in an inner city elementary school. Arq Neuropsiquiatr. 2003;61:67–73. doi: 10.1590/s0004-282x2003000100012. [DOI] [PubMed] [Google Scholar]
- Vaughan BS, Wetzel MW, Kratochvil CJ. Beyond the ‘typical’ patient: treating attention-deficit/hyperactivity disorder in preschoolers and adults. Int Rev Psychiatry. 2008;20:143–149. doi: 10.1080/09540260801887751. [DOI] [PubMed] [Google Scholar]
- Velves T, Kaasenbrood H, Visser G, Schobben F, de Jong-van den Berg L, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62:863–870. doi: 10.1007/s00228-006-0177-0. [DOI] [PubMed] [Google Scholar]
- Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267:87–93. doi: 10.1002/ar.10089. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Vinkers C, Oosting R, Van Bogaert M, Oliver B, Groenin L. Early-life blockdade of 5-HT1a receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biological Psychiatry. 2010;67:309–316. doi: 10.1016/j.biopsych.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Ethical considerations in psychopharmacological research involving children and adolescents. Psychopharmacology (Berl) 2003;171:86–91. doi: 10.1007/s00213-003-1400-7. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Traiffort E, Vargas C, Machado A, Cano J. Expression of 5-HT7 receptor mRNA in rat brain during postnatal development. Neurosci Lett. 1997;227:53–56. doi: 10.1016/s0304-3940(97)00302-9. [DOI] [PubMed] [Google Scholar]
- Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol Psychiatry. 2003;54:1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Wang JW, Dranovsky A, Hen R. The when and where of BDNF and the antidepressant response. Biol Psychiatry. 2008;63:640–641. doi: 10.1016/j.biopsych.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, Kraemer G, Breese GR. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983;226:382–386. [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7:985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Wegerer V, Moll GH, Bagli M, Rothenberger A, Ruther E, Huether G. Persistently increased density of serotonin transporters in the frontal cortex of rats treated with fluoxetine during early juvenile life. J Child Adolesc Psychopharmacol. 1999;9:13–24. doi: 10.1089/cap.1999.9.13. discussion 25–16. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Azmitia EC. Autoregulation of fetal serotonergic neuronal development: role of high affinity serotonin receptors. Neurosci Lett. 1986;67:307–312. doi: 10.1016/0304-3940(86)90327-7. [DOI] [PubMed] [Google Scholar]
- Widom CS, Dumont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Versican in the developing brain: lamina-specific expression in interneuronal subsets and role in presynaptic maturation. J Neurosci. 2005;25:8457–8467. doi: 10.1523/JNEUROSCI.1976-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113:368–375. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- Zhang K, Davids E, Tarazi FI, Baldessarini RJ. Serotonin transporter binding increases in caudate-putamen and nucleus accumbens after neonatal 6-hydroxydopamine lesions in rats: implications for motor hyperactivity. Brain Res Dev Brain Res. 2002;137:135–138. doi: 10.1016/s0165-3806(02)00436-4. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhao N, Wang HY, Dow-Edwards D. Cocaine exposure during the early postnatal period diminishes medial frontal cortex Gs coupling to dopamine D1-like receptors in adult rat. Neurosci Lett. 2008;438:159–162. doi: 10.1016/j.neulet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, De Jong-Van Den Berg LT, Janhsen K, Fegert JM, Gardner JF, Glaeske G, Valluri SC. A three-country comparison of psychotropic medication prevalence in youth. Child Adolesc Psychiatry Ment Health. 2008;2:26. doi: 10.1186/1753-2000-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Gardner JF, Soeken K, Ryu J. Anticonvulsant treatment for psychiatric and seizure indications among youths. Psychiatr Serv. 2006;57:681–685. doi: 10.1176/ps.2006.57.5.681. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Valluri S, Gardner JF, Korelitz JJ, Mattison DR. Psychotherapeutic medication prevalence in Medicaid-insured preschoolers. J Child Adolesc Psychopharmacol. 2007;17:195–203. doi: 10.1089/cap.2007.0006. [DOI] [PubMed] [Google Scholar]
- Zuo J, Liu Z, Ouyang X, Liu H, Hao Y, Xu L, Lu XH. Distinct neurobehavioral consequences of prenatal exposure to sulpiride (SUL) and risperidone (RIS) in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:387–397. doi: 10.1016/j.pnpbp.2007.09.005. [DOI] [PubMed] [Google Scholar]