Abstract
Identification and characterization of the molecular mechanisms contributing to the high incidence of insulin resistance in HIV infected patients treated with combined antiretroviral therapy remains a critically important goal in the quest to improve the safety of antiretroviral treatment regimens. The use of in vitro model systems together with the investigation of drug-mediated effects on glucose homeostasis in animals and healthy human volunteers has provided important insight into the contribution of individual drugs to insulin resistance and affected cellular pathways. HIV protease inhibitor mediated blockade of glucose transport and nucleoside reverse transcriptase inhibitor mediated mitochondrial toxicity have been well characterized. Together with growing understanding of mediators of insulin resistance in non-HIV metabolic syndrome, additional cellular effects including the induction of endoplasmic reticulum and oxidative stress, altered adipocytokine secretion, and lipotoxicity have been integrated into this developing picture. Further elucidation of these mechanisms provides potential for the continued development of safer antiviral drugs and targeted treatment of insulin resistance in affected patients.
Keywords: Insulin sensitivity, glucose transport, in vitro models, HIV protease inhibitors, nucleoside reverse transcriptase inhibitors, adipocytokines, ER stress, lipotoxicity
Introduction
With the emergence and growth of serious adverse metabolic changes in HIV infected individuals treated with anti-retroviral therapy (ART), considerable attention has been devoted to the characterization of insulin resistance and the elucidation of specific drug-induced alterations of cellular pathways that affect glucose homeostasis in this patient population. What has emerged from these efforts, in many ways, closely follows the search for mechanisms of insulin resistance in non-HIV infected individuals. This includes the identification of multiple inter-related perturbations in a highly complex and tightly regulated network of cellular signals that lead to significant changes in adipocyte function, peripheral glucose disposal, hepatic glucose production and insulin secretion. In addition to a growing recognition of the existence and role of specific molecular mediators in these effects, understanding of the unique way in which individual antiretroviral agents contribute to these changes continues to expand. As the HIV therapeutic landscape continues to change with the development of newer drug classes and agents within existing drug classes, the need for a deeper understanding of the molecular basis for insulin resistance in treated HIV infection continues to increase.
A particular challenge in studying insulin resistance in treated HIV infected patients is the wide heterogeneity found in individuals who have been exposed to multiple different drugs in varying combinations and durations. Furthermore, in addition to specific effects of anti-retroviral therapy, the development of insulin resistance in treated patients is also contributed by underlying genetic susceptibility, age, environmental influences, and multiple disease-related factors. In some settings, these factors may overshadow antiviral-mediated effects [1]. Nevertheless, treatment with ART is directly associated with the development of diabetes [2] and efforts to understand the molecular mechanisms leading to these changes provide opportunities for developing safer treatment regimens.
This review aims to synthesize current understanding of the molecular basis for insulin resistance in treated HIV infection and to set forth an agenda for continuing studies over the next decade. While an emphasis is placed on the most proximate steps in the cascade leading ultimately to the development of diabetes mellitus, indirect mechanisms contributing to insulin resistance are also discussed. Where possible, the extension of in vitro findings to established in vivo experimental models and then to the clinical arena is considered. Underlying etiologic factors unrelated to ART are discussed primarily in relation to their potential for additively or synergistically contributing to drug-mediated effects.
Multiple tissues that are normally involved in glucoregulation (muscle, fat, liver, and pancreas) have been found to be affected by ART. While each of these tissues can be considered individually, an understanding of the relationships that exist between each of these organs in modulating insulin sensitivity must be considered. The progress that has been made in elucidating these interactions in HIV negative patients with features of the metabolic syndrome can serve as a foundation for understanding metabolic changes in patients with HIV infection and aid efforts to distinguish changes induced by drug therapy and those that are independent of viral infection.
Adipose tissue
Fat plays a critical role in the maintenance of normal glucose homeostasis both through its role as an energy storage repository and by acting as the largest endocrine organ in the body [3]. Changes in fat mass or adipocyte function are strongly associated with non-HIV metabolic syndrome [4]. Recognition that HIV infected individuals with similar phenotypic changes also have significant metabolic dysregulation has prompted considerable attention to the study of abnormal adipose function in this patient population. The development of lipodystrophy (increased visceral adiposity and peripheral lipoatrophy) has been directly linked to the use of ART and contributing mechanisms have been explored using both in vitro and in vivo models [5]. Several studies in cultured adipocytes have clearly demonstrated that some but not all HIV protease inhibitors (PIs) inhibit adipocyte differentiation [6-9]. It is also known that several PIs alter the expression and localization of sterol response element binding protein 1 (SREBP-1), which influences the expression of adipogenic factors [10, 11]. The integrase inhibitor raltegravir does not appear to influence 3T3L1 adipocyte differentiation [12]. More recently, the zinc metalloproteinase ZMPSTE24, which is necessary for lamin A biogenesis, has been shown to be a direct molecular target of some, but again not all, PIs [13, 14]. This has provided a mechanistic explanation for the observation that treatment of preadipocytes with indinavir or nelfinavir leads to accumulation of prelamin A [15]. Mutations in ZMPSTE24 are associated with a form of congenital lipodystrophy [16] providing support for a clinical link to these in vitro findings.
Elucidation of the genetic basis and molecular phenotype in several rare non-HIV lipodystrophy syndromes has assisted efforts to understand the metabolic changes that occur in treated HIV infection [17, 18]. In these patients it is hypothesized that limitations in the ability to store triglycerides in fat may lead abnormal lipid accumulation in insulin-responsive tissues such liver and skeletal muscle with subsequent interference of normal insulin signaling [19]. Alternatively, insulin resistance can be mediated through deficiency or abnormal regulation of adipocyte secreted hormones such as leptin and adiponectin. This is supported by the dramatic improvement in insulin sensitivity produced by leptin administration to patients with severe forms of congenital lipodystrophy [20]. Recent studies have similarly shown that leptin administration can also improve lipid profiles and insulin sensitivity in lipodystrophic HIV-infected patients with low baseline leptin levels [21].
The significant metabolic and functional differences that are known to exist between subcutaneous and visceral fat depots [22] are important in consideration of the specific changes in insulin sensitivity in treated HIV infection. While visceral adipose tissue (VAT) appears to be most closely associated with insulin resistance, this tissue is heterogeneous with respect to effects on glucose homeostasis. In HIV infected patients, omental-mesenteric fat is more closely correlated with insulin resistance than other VAT depots [23]. While the molecular basis for the metabolic differences between fat depots remains incompletely understood, regional blood flow, cellular composition of different tissues (adipocytes, fibroblasts, endothelial cells, macrophages), and relative metabolic activity are likely contributing factors.
Glucose transport
Cultured 3T3-L1 adipocytes have been used extensively in the investigation of insulin signaling and glucose transport. These cells have proven useful in the investigation of insulin resistance due to antiretroviral treatment and many of the observed drug-mediated effects have been validated in other in vitro and in vivo models. Several of the first generation HIV protease inhibitors (indinavir, ritonavir, nelfinavir, and amprenavir) have been shown to directly block insulin-stimulated glucose uptake in 3T3-L1 adipocytes through direct inhibition of the insulin-responsive facilitative glucose transporter GLUT4 [24]. Importantly, with acute drug exposure, insulin signaling and GLUT4 translocation are not affected. These PIs also block glucose transport in cultured primary adipocytes at therapeutically relevant drug concentrations [25]. Inhibition is non-competitive with respect to zero-trans glucose influx and readily reversible. Inhibitory binding constants for first-generation PIs are in the low micromolar range concordant with peak therapeutic drug levels in treated patients. It is now clear that the ability to inhibit GLUT4 is not shared equally among all of the available PIs. Specifically, ritonavir and indinavir appear to inhibit the transporter most strongly whereas other PIs such as atazanavir have little to no effect on GLUT4 activity at therapeutically relevant drug levels. Structural analysis has implicated the peptidomimetic character found within each of the first generation PIs as mediating direct interaction with GLUT4 [26]. This provides a basis for the observation that non-peptidomimetic PIs such as tipranavir are without effect [27]. The reason for the relative lack of GLUT4 inhibitory activity of atazanavir has not been definitively established. It has been hypothesized that this may be due either to differences in hydrophobicity or steric interference caused by the additional pyridine ring attached to one of the phenylalanine-like moieties in this drug, thereby hindering access to the transporter. While the PI-binding domain in GLUTs has not been definitively identified, indirect evidence suggests that these drugs interact at that cytoplasmic surface of the transporter [26]. Thus, differences in membrane permeability may in part be responsible for the limited GLUT4 inhibitory effect of atazanavir [28].
Although inhibition of glucose transport is a primary mechanism for acute induction of insulin resistance, longer exposure to PIs can perturb insulin signaling and thereby lead indirectly to altered glucose uptake. Similar to the differential effects of PIs on acute GLUT4 inhibition, the ability to impair insulin signaling following prolonged drug exposure also differs between PIs [29]. Saquinavir has been shown to alter early insulin signaling events including insulin receptor substrate-1 (IRS-1) phosphorylation in cultured adipocytes, but only at relatively toxic drug levels following 48 hour drug exposure [30]. Exposure of 3T3-L1 adipocytes to nelfinavir for 18 hours leads to impaired activation of AKT without affecting activation of PI3 kinase [31]. These effects may be due in part to the induction of oxidative stress [32]. Even longer exposure (3 weeks) to indinavir has been associated with activation of the suppressor of cytokine signaling-1 (SOCS-1) signaling cascade in diabetes prone rats [33]. This is accompanied by elevated levels of the sterol response element binding protein-1 (SREBP-1) and the inflammatory cytokine TNFα .
Inflammatory changes
The link between adipose tissue and inflammation is well described in non-HIV infected populations [34]. Macrophages present within adipose tissue secrete inflammatory cytokines such as TNF-alpha and IL-6. These cytokines are frequently elevated and are likely mediators of insulin resistance in HIV infection [35]. TNF-alpha is known to contribute to insulin resistance both through activation of mitogen activated protein kinase (MAPK) and inhibitor of kappa B kinase (IKK) resulting in serine phosphorylation of IRS-1 and through activation of protein tyrosine phosphorylase b which leads to dephosphorylation and inactivation of the insulin receptor and IRS-1 [36]. Indirectly, TNF-α can also increase hepatic triglyceride release, thereby contributing to abnormal fatty acid accumulation in skeletal muscle [37].
Endoplasmic Reticulum Stress
Increased understanding of the role of ER stress with resulting induction of the unfolded protein response has provided a link between inflammation and insulin resistance in non-HIV associated metabolic syndrome [38]. The proteasome has been shown to be directly inhibited by some PIs in vitro and this can contribute to ER stress and the resulting unfolded protein response in adipocytes [39]. This could further exacerbate obesity associated ER stress in treated HIV patients with environmental contribution to visceral adiposity through excess caloric intake. Among the many effects of ER stress, activation of c-Jun N-terminal kinase (JNK) or IKK either directly or through increased generation of reactive oxygen species (ROS) can serine phosphorylate IRS-1. NRTI-induced mitochondrial dysfunction can also contribute to ROS generation, providing an additional influence on insulin signaling. Similar to differences in PI-mediated toxicities, effects on mitochondrial function vary among the different drugs within this class [40]. Thymidine containing NRTIs (zidovudine and stavudine) appear to have the greatest mitochondrial toxicity and have also been shown to be most closely associated with lipoatrophy [41]. Despite these advances, the precise mechanisms for induction of mitochondrial toxicity remains incompletely understood. While it is widely believed that inhibition of DNA pol-γ by NRTIs contributes to mitochondrial DNA depletion, other factors are also likely to contribute [42].
Adipocytokines
Understanding of how adipose tissue influences glucose homeostasis beyond its role in energy storage is increasing rapidly with the discovery and characterization of adipocytokines such as adiponectin, leptin, and resistin. Specific mechanisms by which these hormones influence glucose homeostasis can be found in several recent reviews [43]. The recognition of altered adipocytokine levels in treated HIV infection has led to efforts to understand the molecular mechanisms that contribute to these changes [44]. The etiology appears to be complex but is likely reflective of both changes in adipocyte mass and function. It remains unclear whether antiretroviral-mediated effects on insulin signaling and glucose transport in adipocytes directly contribute to changes in adipokine secretion. Reduced adiponectin and higher leptin levels are associated with increased visceral adiposity in HIV infected patients [45] similar to changes that are observed in non-HIV metabolic syndrome. In cultured 3T3-F442A adipocytes, nelfinavir, ritonavir and saquinavir were all shown to reduce adiponectin expression [46]. Studies in cultured human adipocytes with several PIs have demonstrated that altered adipokine secretion is correlated with induction of reactive oxygen species (ROS) [47]. Exposure of HIV infected patients with NRTIs (stavudine and zidovudine) is also associated with lower levels of adiponectin and SREBP1c [48]. Further investigation of direct effects of ART on some of the more recently identified adipokines such as retinol binding protein 4, omentin, and visfatin will be helpful in further elucidating the role of these hormones in the development of insulin resistance in treated HIV infection.
Skeletal Muscle
Skeletal muscle accounts for the majority of peripheral glucose disposal. Both insulin and contraction stimulate the translocation of GLUT4 from intracellular storage vesicles to the plasma membrane. Indinavir has been shown to impair glucose uptake with both stimuli in isolated perfused muscle [49]. With the shared insulin-signaling pathways found in myocytes and adipocytes, it is not surprising that PIs that inhibit glucose transport in fat also directly impair glucose disposal in muscle. Using the gold standard test for direct assessment of peripheral glucose disposal, the hyperinsulinemic euglycemic clamp [50], impaired skeletal muscle glucose uptake has been linked to combined antiretroviral therapy in HIV infected patients [51]. Studies in both rodents [27, 52] and healthy human volunteers [53] have allowed dissociation of drug-induced effects from those induced by HIV infection. These studies have shown that some but not all PIs acutely induce peripheral insulin resistance. In rodents, assessment of tissue glucose uptake has confirmed a direct effect on skeletal muscle [52]. In this acute setting, impairment of muscle glucose uptake directly correlates with the ability of PIs to block GLUT4 activity in vitro [28] providing a direct mechanistic explanation for insulin resistance.
In addition to PI-mediated changes in peripheral glucose disposal, there are several studies in treated HIV patients examining specific effects of NRTIs on glucose disposal. While in most of these studies, the strongest correlations to insulin sensitivity have been drawn between lipodystrophic changes and thymidine NRTI containing regimens [54] , treatment with zidovudine/lamivudine has been shown to be significantly associated with insulin resistance independent of body composition changes when compared to patients receiving an NRTI-sparing regimen [55]. Validating a link between NRTI use and insulin resistance, treatment of HIV negative human volunteers with stavudine for 4 weeks reduced mitochondrial function and induced detectable insulin resistance [56]. While the precise molecular mechanism for this effect remains to be determined, there is growing evidence for mitochondrial dysfunction as a mediator of insulin resistance in non-HIV patients with type 2 diabetes [57].
In chronically treated humans several additional mechanisms may contribute additively to impaired skeletal muscle insulin sensitivity. Antiretroviral mediated changes in adipose tissue lipolysis together with impaired adipocyte storage capacity in patients with significant lipoatrophy are associated with ectopic skeletal muscle fat accumulation [58, 59]. Several PIs have also been shown to impair fatty acid oxidation in cultured differentiated C2C12 myotubes [60]. The effects of abnormal fat partitioning into skeletal muscle in patients without HIV infection provides insight into potential effects in HIV-infected patients [61]. Lipid derivatives are known to impair insulin signaling by activating protein kinase θ, JNK and IKK which phosphorylate IRS-1 at serine 307, leading to IRS-1inactivation [62]. More recently, evidence has accumulated specifically implicating excess diacylglycerol in IRS-1 inactivation [19]. Direct measurement of insulin signaling in muscle harvested from lipodystrophic HIV-infected patients under hyperinsulinemic clamp conditions has demonstrated defects downstream of IRS at the level of AKT phosphorylation [63]. While this in vivo study was not able to directly assess the contribution of PIs to this effect, the ability of nelfinavir to impair AKT activation in cultured adipocytes supports the potential for a similar effect of this PI on insulin signaling in skeletal muscle [31] .
Relating the previously discussed association between reduced adiponectin and leptin levels in patients receiving ART, these adipokines have direct effects on muscle insulin sensitivity [64]. Studies performed in HIV-negative human volunteers have underscored the potential importance of PI-induced changes in adipokine levels. When lopinavir was administered to healthy human volunteers as a single dose, insulin sensitivity as measured by hyperinsulinemic euglycemic clamps was reduced, but no changes were observed in adiponectin levels [65]. However, after 4 weeks of lopinavir exposure, no insulin resistance was detected but adiponectin levels were increased [66] suggesting that compensatory changes may have been elicited in this otherwise healthy patient model.
β-cell Insulin secretion
In the setting of isolated peripheral insulin resistance, compensatory increases in β-cell insulin secretion normally allow maintenance of normal blood glucose levels. The development of relative or absolute insulin deficiency is generally required for the manifestation of overt diabetes mellitus. While prolonged insulin resistance and insulin hypersecretion alone may lead to β-cell dysfunction, several studies have provided evidence that PIs directly impair insulin secretion. This includes homeostasis model assessment and hyperglycemic clamp experiments in treated HIV patients [67, 68] and measurement of glucose stimulated insulin secretion from cultured islets [69]. In these studies, insulin secretion was not increased with the induction of peripheral insulin resistance reflecting relative β-cell insufficiency. While concomitant inhibition of glucose uptake following acute indinavir exposure had led to the hypothesis that GLUT2 blockade may contribute to this effect, glucokinase activity and not glucose transport is understood to be the rate limiting step in ATP production [70]. Nelfinavir and ritonavir have been shown to cause perturbation of voltage dependent K+ channels and anion channels providing an alternative mechanistic explanation for these acute effects [71]. More chronic treatment of INS-1 cells with PIs has revealed impairment of insulin signaling [72]. Pharmacologic reduction in ROS ameliorates the adverse effect of nelfinavir on insulin release [73]. Chronic in vitro exposure to PIs has also been shown to induce β cell apoptosis [74]. Similar to the effects on adipocyte function and peripheral glucose disposal, differences have been observed in the cellular perturbations of the various PIs on insulin secretion suggesting that multiple distinct but inter-related pathways may be involved and highlighting the existence of drug-specific rather than class-wide effects.
Liver
Hepatic function also contributes, both directly and indirectly, to normal glucose homeostasis. Direct mechanisms are mediated through regulation of hepatic glucose production. In the fed state insulin normally suppresses hepatic glucose production (HGP). In addition to reflecting relative β-cell insufficiency, elevations in fasting glucose levels in treated HIV patients indicate the presence of hepatic insulin resistance [2]. This has been confirmed in lipodystrophic patients subjected to hyperinsulinemic euglycemic clamps [75]. A role of some PIs in mediating this change has been established from studies in HIV negative men given indinavir. Elevated fasting blood sugars and blunted suppression of HGP during hyperinsulinemic clamps was detected after 4 weeks of drug exposure [76]. The mechanistic basis for this effect remains to be fully elucidated. Impaired insulin signaling has been observed in cultured HepG2 cells treated with indinavir [77]. Direct in vitro effects of indinavir on GLUT2 [25] has led to speculation that blockade of this hepatic facilitative glucose transporter may contribute to these observed changes. Studies in GLUT2 knockout mice have shown that this transporter is important in the normal function of the hepatoportal glucose sensor [78]. However, in vitro blockade of GLUT2 by indinavir was only observed at drug levels exceeding those clinically achieved. To date there has been no experimental confirmation of PI-induced GLUT2 blockade in vivo. An alternative potential mechanism is alteration in hypothalamic insulin sensitivity which is known to influence HGP [79]. This may be mediated through effects on GLUT4 activity in the central nervous system [80].
Hepatic steatosis is frequently encountered in both non-HIV infected patients with features of the metabolic syndrome and those receiving ART [81]. The relationship between non alcoholic fatty liver disease and insulin sensitivity (i.e. whether steatosis is a cause or consequence of insulin resistance) remains unclear in non-HIV infected individuals [82]. However, similar to effects of ectopic fat in skeletal muscle, excess triglycerides and diacylglycerol in the liver activate PKC-ε and JNK leading to inhibition of IRS-2 [83]. Therefore, effects of PIs on peripheral lipolysis and NRTIs on mitochondrial function may also contribute to the induction of hepatic insulin resistance in HIV patients receiving ART.
Summary
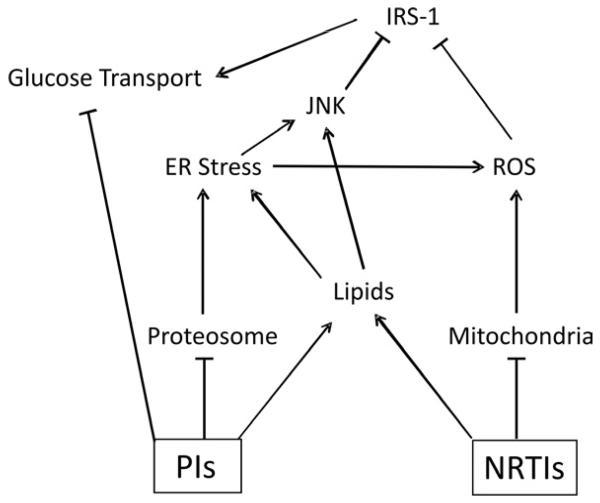
While the molecular mechanisms leading to insulin resistance in treated HIV infection are complex and are contributed by multiple disease-related and independent factors, current insight into changes in specific cellular pathways allows general conclusions to be reached regarding etiology which can be helpful in the ongoing development of strategies to both treat and prevent insulin resistance in these patients. The identification of molecular targets of antiretroviral drugs remains limited. Proteins directly inhibited by HIV protease inhibitors include GLUT4, the proteasome, and ZMPSTE24. For NRTIs, inhibition of DNA polymerase γ contributes to mitochondrial toxicity. Integrating these proximal defects to downstream changes in pathways that influence insulin sensitivity, specific drugs within both antiretroviral classes contribute to the development of ER stress, generation of reactive oxygen species, altered lipid metabolism, changes in adipocytokine secretion, and inhibition of insulin signaling (Figure 1). This ultimately leads to dysregulation of hepatic glucose production and reduced peripheral glucose disposal. When pancreatic β-cells are unable to compensate with increased insulin secretion, serum glucose levels rise.
Figure 1.
Schematic representation of the contribution of antiretroviral therapy to altered glucose transport in treated HIV infected patients. Arrows represent activation and bars depict inactivation of specific cellular pathways.
Efforts to effectively manage impaired glucose tolerance in treated HIV infected patients currently relies upon diabetes treatment strategies established for the treatment of non-HIV infected patients [84]. The effectiveness of this approach in patients receiving ART is dependent upon the extent to which these pathways are shared between these two clinical groups. The growing body of research on HIV and glucose homeostasis has demonstrated many areas of overlap. Future studies will aid efforts to distinguish drug related toxicities from background risk in the aging HIV population. Use of this information in designing improved treatment regimens holds great potential in further improving the quality of life of affected patients.
Practice Points
Altered glucose homeostasis in treated HIV infection is mediated by complex and inter-related changes in adipocyte function, peripheral glucose disposal, hepatic insulin resistance and impaired β-cell insulin secretion
In treating affected patients, it is important to distinguish general non-HIV associated risk factors for the development of insulin resistance (age, diet, exercise, genetic risk) from those specifically induced by ART
Significant differences in the ability to alter specific metabolic pathways exist between antiretroviral classes and individual drugs.
Many of the newer anti-retroviral agents appear to have more favorable metabolic profiles with respect to insulin sensitivity and lipotoxicity.
Research Agenda
Despite recent advances in characterizing ART-induced cellular changes in vitro, in many areas there is a general need for confirmation of these findings in vivo.
As understanding of the role of lipotoxicity, ER stress, and altered adipocytokine function increases in relation to non-HIV associated insulin resistance, there is a need for translation to HIV infected patients.
Further identification and characterization of direct molecular targets of individual antiretroviral agents is needed to facilitate the development of newer drugs with reduced potential for inducing insulin resistance.
Acknowledgements
This work is supported in part by NIH grants DK064572 and HL092798.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The author has received grant support from Bristol Myers Squibb and Gilead Sciences
References
- 1*.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 3.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Bruce KD, Byrne CD. The metabolic syndrome: common origins of a multifactorial disorder. Postgrad Med J. 2009;85:614–21. doi: 10.1136/pgmj.2008.078014. [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Misra A, Garg A. Clinical review 153: Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87:4845–56. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 6.Dowell P, Flexner C, Kwiterovich PO, et al. Suppression of Preadipocyte Differentiation and Promotion of Adipocyte Death by HIV Protease Inhibitors. J Biol Chem. 2000 Dec 29;275(52):41325–32. doi: 10.1074/jbc.M006474200. [DOI] [PubMed] [Google Scholar]
- 7.Kim RJ, Wilson CG, Wabitsch M, et al. HIV Protease Inhibitor-Specific Alterations in Human Adipocyte Differentiation and Metabolism[ast] Obesity. 2006;14:994–1002. doi: 10.1038/oby.2006.114. [DOI] [PubMed] [Google Scholar]
- 8.Lenhard JM, Furfine ES, Jain RG, et al. HIV protease inhibitors block adipogenesis and increase lipolysis in vitro. Antiviral Research. 2000;47:121–9. doi: 10.1016/s0166-3542(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Macnaul K, Szalkowski D, et al. Inhibition of Adipocyte Differentiation by HIV Protease Inhibitors. Journal of Clinical Endocrinology and Metabolism. 1999;84:4274–7. doi: 10.1210/jcem.84.11.6234. [DOI] [PubMed] [Google Scholar]
- 10.Bastard JP, Caron M, Vidal H, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–31. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 11.Caron M, Auclair M, Vigouroux C, et al. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001;50:1378–88. doi: 10.2337/diabetes.50.6.1378. [DOI] [PubMed] [Google Scholar]
- 12.Minami R, Yamamoto M, Takahama S, et al. Comparison of the influence of four classes of HIV antiretrovirals on adipogenic differentiation: the minimal effect of raltegravir and atazanavir. J Infect Chemother. 2010 doi: 10.1007/s10156-010-0101-5. [DOI] [PubMed] [Google Scholar]
- 13*.Coffinier C, Hudon SE, Farber EA, et al. HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells. Proc Natl Acad Sci U S A. 2007;104:13432–7. doi: 10.1073/pnas.0704212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulbourne CN, Vaux DJ. HIV protease inhibitors inhibit FACE1/ZMPSTE24: a mechanism for acquired lipodystrophy in patients on highly active antiretroviral therapy? Biochem Soc Trans. 2010;38:292–6. doi: 10.1042/BST0380292. [DOI] [PubMed] [Google Scholar]
- 15.Caron M, Auclair M, Sterlingot H, et al. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS. 2003;17:2437–44. doi: 10.1097/00002030-200311210-00005. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal AK, Fryns JP, Auchus RJ, et al. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- 17.Simha V, Garg A. Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol. 2006;17:162–9. doi: 10.1097/01.mol.0000217898.52197.18. [DOI] [PubMed] [Google Scholar]
- 18.Garg A, Misra A. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am. 2004;33:305–31. doi: 10.1016/j.ecl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract. 2010;16:324–33. doi: 10.4158/EP09155.RA. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan K, Khatami H, Schwarz JM, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94:1137–44. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 23.He Q, Engelson ES, Albu JB, et al. Preferential loss of omental-mesenteric fat during growth hormone therapy of HIV-associated lipodystrophy. J Appl Physiol. 2003;94:2051–7. doi: 10.1152/japplphysiol.00845.2002. [DOI] [PubMed] [Google Scholar]
- 24*.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–4. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 25.Murata H, Hruz PW, Mueckler M. Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS. 2002;16:859–63. doi: 10.1097/00002030-200204120-00005. [DOI] [PubMed] [Google Scholar]
- 26.Hertel J, Struthers H, Horj CB, et al. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J Biol Chem. 2004;279:55147–52. doi: 10.1074/jbc.M410826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hruz PW, Yan Q. Tipranavir without ritonavir does not acutely induce peripheral insulin resistance in a rodent model. J Acquir Immune Defic Syndr. 2006;43:624–5. doi: 10.1097/01.qai.0000245883.66509.b4. [DOI] [PubMed] [Google Scholar]
- 28.Yan Q, Hruz PW. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J Acquir Immune Defic Syndr. 2005;40:398–403. doi: 10.1097/01.qai.0000176654.97392.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Romano R, Rudich A, Torok D, et al. Agent and cell-type specificity in the induction of insulin resistance by HIV protease inhibitors. AIDS. 2003;17:23–32. doi: 10.1097/00002030-200301030-00005. [DOI] [PubMed] [Google Scholar]
- 30.Algenstaedt P, Daneshi S, Schwarzloh B, et al. Therapeutic dose of HIV-1 protease inhibitor saquinavir does not permanently influence early insulin signaling. Exp Clin Endocrinol Diabetes. 2003;111:491–8. doi: 10.1055/s-2003-44709. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Romano R, Rudich A, Tirosh A, et al. Nelfinavir-induced insulin resistance is associated with impaired plasma membrane recruitment of the PI 3-kinase effectors Akt/PKB and PKC-zeta. Diabetologia. 2004;47:1107–17. doi: 10.1007/s00125-004-1408-5. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Romano R, Rudich A, Etzion S, et al. Nelfinavir induces adipocyte insulin resistance through the induction of oxidative stress: differential protective effect of antioxidant agents. Antivir Ther. 2006;11:1051–60. [PubMed] [Google Scholar]
- 33.Carper MJ, Cade WT, Cam M, et al. HIV-protease inhibitors induce expression of suppressor of cytokine signaling-1 in insulin-sensitive tissues and promote insulin resistance and type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2008;294:E558–67. doi: 10.1152/ajpendo.00167.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–31. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown TT, Tassiopoulos K, Bosch RJ, et al. Association between Systemic Inflammation and Incident Diabetes Mellitus in HIV-infected Patients after Initiation of Antiretroviral Therapy. Diabetes Care. 2010 doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin B, Anderson RA, Adeli K. Tumor necrosis factor-alpha directly stimulates the overproduction of hepatic apolipoprotein B100-containing VLDL via impairment of hepatic insulin signaling. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1120–9. doi: 10.1152/ajpgi.00407.2007. [DOI] [PubMed] [Google Scholar]
- 38*.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Parker RA, Flint OP, Mulvey R, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol Pharmacol. 2005;67:1909–19. doi: 10.1124/mol.104.010165. [DOI] [PubMed] [Google Scholar]
- 40.Maagaard A, Kvale D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: a continuum or distinct underlying mechanisms? J Antimicrob Chemother. 2009;64:901–9. doi: 10.1093/jac/dkp316. [DOI] [PubMed] [Google Scholar]
- 41.McComsey GA, Walker UA. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion. 2004;4:111–8. doi: 10.1016/j.mito.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Feeney Eoin R, Mallon Patrick WG. Impact of Mitochondrial Toxicity of HIV-1 Antiretroviral Drugs on Lipodystrophy and Metabolic Dysregulation. Curr Pharm Des. 2010 doi: 10.2174/138161210793563482. [DOI] [PubMed] [Google Scholar]
- 43.Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- 44.Tsiodras S, Perelas A, Wanke C, et al. The HIV-1/HAART associated metabolic syndrome - novel adipokines, molecular associations and therapeutic implications. J Infect. 2010;61:101–13. doi: 10.1016/j.jinf.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Kosmiski LA, Bacchetti P, Kotler DP, et al. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab. 2008;93:216–24. doi: 10.1210/jc.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones SP, Janneh O, Back DJ, et al. Altered adipokine response in murine 3T3-F442A adipocytes treated with protease inhibitors and nucleoside reverse transcriptase inhibitors. Antivir Ther. 2005;10:207–13. [PubMed] [Google Scholar]
- 47.Lagathu C, Eustace B, Prot M, et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- 48.Jones SP, Qazi N, Morelese J, et al. Assessment of adipokine expression and mitochondrial toxicity in HIV patients with lipoatrophy on stavudine- and zidovudine-containing regimens. J Acquir Immune Defic Syndr. 2005;40:565–72. doi: 10.1097/01.qai.0000187443.30838.3e. [DOI] [PubMed] [Google Scholar]
- 49.Nolte LA, Yarasheski KE, Kawanaka K, et al. The HIV protease inhibitor indinavir decreases insulin- and contraction-stimulated glucose transport in skeletal muscle. Diabetes. 2001;50:1397–401. doi: 10.2337/diabetes.50.6.1397. [DOI] [PubMed] [Google Scholar]
- 50.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 51.Behrens GM, Boerner AR, Weber K, et al. Impaired glucose phosphorylation and transport in skeletal muscle cause insulin resistance in HIV-1-infected patients with lipodystrophy. J Clin Invest. 2002;110:1319–27. doi: 10.1172/JCI15626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hruz PW, Murata H, Qiu H, et al. Indinavir induces acute and reversible peripheral insulin resistance in rats. Diabetes. 2002;51:937–42. doi: 10.2337/diabetes.51.4.937. [DOI] [PubMed] [Google Scholar]
- 53.Noor MA, Flint OP, Maa JF, et al. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20:1813–21. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 54.Shlay JC, Visnegarwala F, Bartsch G, et al. Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs. abacavir and lamivudine. J Acquir Immune Defic Syndr. 2005;38:147–55. doi: 10.1097/01.qai.0000143599.64234.15. [DOI] [PubMed] [Google Scholar]
- 55.Blumer RM, van Vonderen MG, Sutinen J, et al. Zidovudine/lamivudine contributes to insulin resistance within 3 months of starting combination antiretroviral therapy. AIDS. 2008;22:227–36. doi: 10.1097/QAD.0b013e3282f33557. [DOI] [PubMed] [Google Scholar]
- 56.Fleischman A, Johnsen S, Systrom DM, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–73. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelley DE, He J, Menshikova EV, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 58.Gan SK, Samaras K, Thompson CH, et al. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51:3163–9. doi: 10.2337/diabetes.51.11.3163. [DOI] [PubMed] [Google Scholar]
- 59.Luzi L, Perseghin G, Tambussi G, et al. Intramyocellular lipid accumulation and reduced whole body lipid oxidation in HIV lipodystrophy. Am J Physiol Endocrinol Metab. 2003;284:E274–80. doi: 10.1152/ajpendo.00391.2001. [DOI] [PubMed] [Google Scholar]
- 60.Richmond SR, Carper MJ, Lei X, et al. HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters. Biochim Biophys Acta. 2010;1801:559–66. doi: 10.1016/j.bbalip.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Unger RH, Clark GO, Scherer PE, et al. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–14. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Gao Z, Zhang X, Zuberi A, et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2024–34. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 63.Haugaard SB, Andersen O, Madsbad S, et al. Skeletal muscle insulin signaling defects downstream of phosphatidylinositol 3-kinase at the level of Akt are associated with impaired nonoxidative glucose disposal in HIV lipodystrophy. Diabetes. 2005;54:3474–83. doi: 10.2337/diabetes.54.12.3474. [DOI] [PubMed] [Google Scholar]
- 64.Rabe K, Lehrke M, Parhofer KG, et al. Adipokines and insulin resistance. Mol Med. 2008;14:741–51. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee GA, Lo JC, Aweeka F, et al. Single-dose lopinavir-ritonavir acutely inhibits insulin-mediated glucose disposal in healthy volunteers. Clin Infect Dis. 2006;43:658–60. doi: 10.1086/505974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee GA, Mafong DD, Noor MA, et al. HIV Protease Inhibitors Increase Adiponectin Levels in HIV-Negative Men. J Acquir Immune Defic Syndr. 2004;36:645–7. doi: 10.1097/00126334-200405010-00017. [DOI] [PubMed] [Google Scholar]
- 67*.Woerle HJ, Mariuz PR, Meyer C, et al. Mechanisms for the deterioration in glucose tolerance associated with HIV protease inhibitor regimens. Diabetes. 2003;52:918–25. doi: 10.2337/diabetes.52.4.918. [DOI] [PubMed] [Google Scholar]
- 68.Dube MP, Edmondson-Melancon H, Qian D, et al. Prospective evaluation of the effect of initiating indinavir-based therapy on insulin sensitivity and B-cell function in HIV-infected patients. J Acquir Immune Defic Syndr. 2001;27:130–4. doi: 10.1097/00126334-200106010-00006. [DOI] [PubMed] [Google Scholar]
- 69.Koster JC, Remedi MS, Qiu H, et al. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes. 2003;52:1695–700. doi: 10.2337/diabetes.52.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–41. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 71.Neye Y, Dufer M, Drews G, et al. HIV protease inhibitors: suppression of insulin secretion by inhibition of voltage-dependent K+ currents and anion currents. J Pharmacol Exp Ther. 2006;316:106–12. doi: 10.1124/jpet.105.090589. [DOI] [PubMed] [Google Scholar]
- 72.Schutt M, Zhou J, Meier M, et al. Long-term effects of HIV-1 protease inhibitors on insulin secretion and insulin signaling in INS-1 beta cells. J Endocrinol. 2004;183:445–54. doi: 10.1677/joe.1.05620. [DOI] [PubMed] [Google Scholar]
- 73.Chandra S, Mondal D, Agrawal KC. HIV-1 protease inhibitor induced oxidative stress suppresses glucose stimulated insulin release: protection with thymoquinone. Exp Biol Med (Maywood) 2009;234:442–53. doi: 10.3181/0811-RM-317. [DOI] [PubMed] [Google Scholar]
- 74.Zhang S, Carper MJ, Lei X, et al. Protease inhibitors used in the treatment of HIV+ induce beta-cell apoptosis via the mitochondrial pathway and compromise insulin secretion. Am J Physiol Endocrinol Metab. 2009;296:E925–35. doi: 10.1152/ajpendo.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Valk M, Bischop PH, Romijn JA, et al. Lipodystrophy in HIV-1-positive patients is associated with insulin resistance in multiple metabolic pathways. Aids. 2001;15:2093–100. doi: 10.1097/00002030-200111090-00004. [DOI] [PubMed] [Google Scholar]
- 76.Schwarz JM, Lee GA, Park S, et al. Indinavir increases glucose production in healthy HIV-negative men. AIDS. 2004;18:1852–4. doi: 10.1097/00002030-200409030-00017. [DOI] [PubMed] [Google Scholar]
- 77.Schutt M, Meier M, Meyer M, et al. The HIV-1 protease inhibitor indinavir impairs insulin signalling in HepG2 hepatoma cells. Diabetologia. 2000;43:1145–8. doi: 10.1007/s001250051505. [DOI] [PubMed] [Google Scholar]
- 78.Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643–8. doi: 10.2337/diabetes.49.10.1643. [DOI] [PubMed] [Google Scholar]
- 79.Buettner C, Camacho RC. Hypothalamic control of hepatic glucose production and its potential role in insulin resistance. Endocrinol Metab Clin North Am. 2008;37:825–40. doi: 10.1016/j.ecl.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 80.El Messari S, Leloup C, Quignon M, et al. Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J Comp Neurol. 1998;399:492–512. doi: 10.1002/(sici)1096-9861(19981005)399:4<492::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 81.Bongiovanni M, Tordato F. Steatohepatitis in HIV-infected subjects: pathogenesis, clinical impact and implications in clinical management. Curr HIV Res. 2007;5:490–8. doi: 10.2174/157016207781662407. [DOI] [PubMed] [Google Scholar]
- 82.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 84.Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]