Abstract
Serotonin (5-HT) controls affective and motivational aspects of palatable food and drug reward and the 5-HT2C receptor (5-HT2CR) has emerged as a key regulator in this regard. We have evaluated the efficacy of a selective 5-HT2CR agonist, WAY 163909, in cocaine and sucrose self-administration and reinstatement assays employing parallel experimental designs in free-fed rats. WAY 163909 dose-dependently reduced the reinforcing efficacy of cocaine (ID50=1.19 mg/kg) and sucrose (ID50=0.7 mg/kg) as well as reinstatement (ID50=0.5 mg/kg) elicited by exposure to cocaine-associated contextual cues, but not sucrose-associated contextual cues. The ID50 of WAY 163909 predicted to decrease the reinforcing efficacy of cocaine or sucrose as well as reinstatement upon exposure to cocaine-associated cues was ~5–12-fold lower than that predicted to suppress horizontal ambulation (ID50 = 5.89 mg/kg) and ~2-5-fold lower than that predicted to suppress vertical activity (ID50= 2.3 mg/kg). Thus, selective stimulation of the 5-HT2CR decreases the reinforcing efficacy of cocaine and sucrose in freely-fed rats, but differentially alters the incentive-salience value of cocaine- vs. sucrose-associated cues at doses that do not impair locomotor activity. Future research is needed to tease apart the precise contribution of 5-HT2CR neurocircuitry in reward and motivation and the learning and memory processes that carry the encoding for associations between environmental cues and consumption of rewarding stimuli. A more complete preclinical evaluation of these questions will ultimately allow educated proof-of-concept trials to test the efficacy of selective 5-HT2CR agonists as adjunctive therapy in chronic health maladies including obesity, eating disorders and drug addiction.
Keywords: 5-HT2C receptor, Addiction, Cocaine, Reward, Self-administration, Serotonin, Sucrose, WAY 163909
1. INTRODUCTION
Survival is ensured by neural circuits engaged by basic needs (e.g., food, water) and states (e.g., hunger, thirst) that motivate the organism to sustain important biological functions. Brain motivational systems are sensitive to endogenous and exogenous stimuli that become linked to natural and drug rewards, and neuroplasticity in these circuits engender affective states that powerfully drive such behaviors as bingeing on palatable food (e.g., fat, sucrose) or abused drugs (e.g., cocaine). A complex appetitive process links the motivation to consume (e.g., hunger) with the palatability and incentive salience of the rewarding stimulus (drug or food) and its associated conditioned cue properties as well as with the satiety signals that terminate further intake (Halford and Harrold, 2008). Studies over the last 20 years have identified cellular and behavioral mechanisms of these appetitive processes to include dopamine, glutamate, and their intracellular signaling webs in the limbic-corticostriatal-hypothalamic circuit (Kelley, 2004). Serotonin (5-HT) is additionally important in the control over the affective and motivational aspects of palatable food and drug reward, which has been described to occur at the level of satiety (Hewitt, et al., 2002; Lyness, et al., 1980; Blundell, et al., 1980) as well as palatability or reinforcing efficacy (Wogar, et al., 1991). In particular, there is a growing literature based upon genetic and pharmacological manipulations that the serotonin 5-HT2C receptor (5-HT2CR) signaling regulates such neurobehavioral processes which may underlie important chronic health maladies including obesity, eating disorders and drug addiction (Halford, et al., 2010; Bubar and Cunningham, 2008; Steiger, 2004).
The selective activation of signaling through the 5-HT2CR has been associated with reduced feeding and decreased body weight in animals (Fletcher, et al., 2009; Clifton, et al., 2000) and most recently in humans (Smith, et al., 2010; Smith, et al., 2009). The constitutive knockout of the 5-HT2CR in mice results in hyperphagia (Tecott, et al., 1995) and increased intake of cocaine (Rocha, et al., 1998); the phenotype also includes adult-onset obesity and depressed metabolic rate (Tecott, et al., 1995). Selective 5-HT2CR antagonists have been shown to increase baseline food intake (Bonhaus, et al., 1997; Thomsen, et al., 2008) (but see, Hewitt, et al., 2002) in the same dose range shown to block the effects of a 5-HT2CR selective agonist to decrease feeding (Thomsen, et al., 2008; Grottick, et al., 2000). Analogous to these findings with palatable food reward, rates for responding for cocaine in a self-administration task were suppressed by pretreatment with a preferential 5-HT2CR agonist and enhanced by a selective 5-HT2CR antagonist (Fletcher, et al., 2008; Grottick, et al., 2000), suggesting an important role for the 5-HT2CR in control of the overt rewarding efficacy of cocaine. A preferential 5-HT2CR agonist also suppressed intake of nicotine (Grottick, et al., 2001) and alcohol (Tomkins, et al., 2002). The mechanisms are complex but a role for the 5-HT2CR to enhance satiety and/or suppress incentive-motivational aspects of appetitive food or drug reinforcers are both supported (Fletcher, et al., 2010; Burbassi and Cervo, 2008; Rocha, et al., 2002; Vickers, et al., 1999; Hewitt, et al., 2002; Fletcher, et al., 2010).
There is much less known about the role of serotonin in general, and the 5-HT2CR in specific, to regulate drug- or food-seeking behavior upon exposure to environmental stimuli previously associated with drug or palatable food, respectively. Loss of 5-HT neurons after intraventricular infusion of a 5-HT neurotoxin decreased cocaine-seeking but enhanced sucrose-seeking during extinction in freely-fed rats previously trained to self-administer cocaine or sucrose, respectively (Tran-Nguyen, et al., 2001). The selective 5-HT reuptake inhibitor fluoxetine was shown to suppress palatable food intake most potently in freely-fed female rats pre-exposed to food cues (Cifani, et al., 2009). The preferential 5-HT2CR agonists MK 212 (Neisewander and Acosta, 2007) and Ro 60-0175 (Fletcher, et al., 2008) have been shown to suppress cue-evoked reinstatement in cocaine self-administration. However, the interpretation of findings across studies and the development of an overarching appreciation of how 5-HT2CR neurocircuitry controls drug or palatable food intake or “relapse” in the face of reward-related cues are hampered by two issues. The first challenge is that selective agonists with singular high affinity and efficacy for the 5-HT2CR have only recently become commercially available. The second challenge is that few studies have established the sensitivity of palatable reward vs. reward-related cues to a specific manipulation of 5-HT2CR function employing comparable assay methodologies in a given species [for a recent review of these methodologies, see (Nair, et al., 2009)].
The 5-HT2C receptor (5-HT2CR) shares high homology with the two other members (5-HT2AR, 5-HT2BR) of the 5-HT2R family of G-protein-coupled receptors. Until recently, only “preferential” 5-HT2CR ligands which frequently display affinity (agonists, antagonist) and/or efficacy (agonists) at the 5-HT2AR and 5-HT2BR have been available and some experimental outcomes with non-selective 5-HT2CR ligands have led to ambiguous conclusions concerning the biological roles for this receptor. Furthermore, as 5-HT2AR or 5-HT2BR agonists would be expected to evoke hallucinations (Nichols, 2004) or cardiac valvulopathy (Fitzgerald, et al., 2000; Roth, 2007), respectively, therapeutically-useful 5-HT2CR agonists must not have demonstrable efficacy at 5-HT2AR or 5-HT2BR in vivo. Such selectivity has been difficult to achieve, providing challenges to the careful preparation of preclinical analyses in support of ultimate proof-of-concept studies of selective 5-HT2CR agonists in humans for treatment of obesity, eating disorders or addiction.
In a compound series recently developed at Wyeth Research, vabicaserin (SCA-136) was identified as a selective 5-HT2CR full agonist (Ki = 3 nM; efficacy 100% relative to 5-HT), a 5-HT2BR antagonist (IC50 = 29 nM) and a very weak 5-HT2AR antagonist (IC50 = 1,650 nM) (Rosenzweig-Lipson, et al., 2007a; Tong, et al., 2010a; Tong, et al., 2010b). Vabicaserin has been in clinical trials to evaluate antipsychotic potential (www.clinicaltrials.gov). WAY 163909 is chemically-similar to vabicaserin with high affinity (Ki=10.5 nM) and full efficacy (90% relative to 5-HT) at the 5-HT2CR. WAY 163909 exhibits a low affinity (Ki=212 nM) at the 5-HT2AR, and no efficacy and is a weak partial agonist at the 5-HT2BR at concentrations 23-fold greater (Dunlop, et al., 2005). In the present study, we have evaluated the efficacy of WAY 163909 to suppress the reinforcing effects of the abused psycho stimulant cocaine and the palatable food sucrose as well as cue-evoked reinstatement in parallel experimental designs in freely-fed rats. To date, the only other preferential 5-HT2CR agonists to be profiled in a some what similar fashion are MK 212 and Ro 60-0175; Table 1 provides a comparison of affinity and efficacy at the three 5-HT2R for MK 212, Ro 60-0175 and WAY 163909.
Table 1.
Affinity and efficacy of MK 212, Ro 60-0175 and WAY 163909 for 5-HT2AR, 5-HT2BR and 5-HT2CR
| Agonist | 5-HT2 Receptor Family1 | |||||
|---|---|---|---|---|---|---|
| h5-HT2AR | h5-HT2BR | h5-HT2CR | ||||
| Affinity Ki (nM) |
Efficacy Emax |
Affinity Ki (nM) |
Efficacy Emax |
Affinity Ki (nM) |
Efficacy Emax |
|
| MK 212 | 1023 | < 50% | 617 | 75% | 98 | 100% |
| Ro 60-0175 | 36.3 | 69% | 5.4 | 79% | 6.0 | 84% |
| WAY 163909 | 212 | 0% | 2101 | 40% | 10.5 | 90% |
Published studies employed radioligand binding assays to establish the affinity (Ki) and efficacy (Emax) of MK 212 (Knight, et al., 2004; Cussac, et al., 2002; Porter, et al., 1999) and Ro 60-0175 for the 5-HT2R subtypes in h5-HT2AR- or h5-HT2CR-transfected Human Embryonic Kidney (HEK)-293 (Knight, et al., 2004) and h5-HT2AR-, h5-HT2BR-, or h5-HT2CR-transfected Chinese Hamster Ovary (CHO)-K1 clonal cell lines (Porter, et al., 1999; Cussac, et al., 2002) while the affinity of WAY 163909 for each h5-HT2R subtype was established in CHO-K1 cells (Dunlop, et al., 2005). The efficacy of MK 212 (Porter, et al., 1999), Ro 60-0175 (Porter, et al., 1999) and WAY 163909 (Dunlop, et al., 2005) was established in agonist-stimulated mobilization of intracellular calcium in CHO-K1 cells with a fluorometric imaging plate reader; the Emax is expressed as a percentage of the maximal response to 5-HT. MK 212 exhibits did not exhibit efficacy (IC50> 1 µM) for the 5-HT2AR (Knight, et al., 2004). Ro 60-0175 did not exhibit efficacy (IC50> 1 µM) for a multitude of proteins with the highest affinity for β2 adrenoceptor (β2-noradrenergic receptor; IC50= 251; Martin, et al., 1998). WAY 163909 exhibited modest affinity for the dopamine D4R (Ki=245 nM), 5-HT7R (Ki=343 nM) and α1 adrenoceptor (Ki=665 nM; Dunlop, et al., 2005).
The present study investigated the hypothesis that WAY 163909 would suppress cocaine or sucrose self-administration or cue-evoked reinstatement in freely-fed male rats under conditions that minimized potential confounds imposed by food (or water) restriction [see (Grottick, et al., 2000; Fletcher, et al., 2002; Nic Dhonnchadha, et al., 2009), for discussion] and operant pretraining for an appetitive reinforcer (Grottick, et al., 2000; Fletcher, et al., 2002; Nic Dhonnchadha, et al., 2009). The doses of cocaine for reliable self-administration and reinstatement are well-established in the literature (Nic Dhonnchadha, et al., 2009; Carroll and Lac, 1997; Nic Dhonnchadha, et al., 2009; Pentkowski, et al., 2010; Grottick, et al., 2000; Burbassi and Cervo, 2008; Fletcher, et al., 2002) and we chose to compare cocaine to a sucrose reinforcer under the same assay conditions because preliminary observations indicated that freely-fed rats would establish levels of active lever presses for sucrose pellets under an ultimate fixed ratio (FR) 5 schedule that were comparable to those seen for a cocaine self-administration. Employing these analogous assay methodologies in the rat allowed us to establish the differential sensitivity of a natural (sucrose) and drug (cocaine) reward vs. reward-related cues to pretreatment with a highly-selective and efficacious 5-HT2CR agonist and to compare with the body of literature available for the preferential 5-HT2CR agonists MK 212 (Neisewander and Acosta, 2007) and Ro 60-0175 (Higgins, et al., 2001; Grottick, et al., 2001; Fletcher, et al., 2008; Fletcher, et al., 2010). We report that cocaine-taking and especially cocaine-seeking are highly sensitive to suppression by WAY 163909. Interestingly, sucrose-taking is also potently suppressed by WAY 163909, but sucrose-seeking is relatively insensitive to the selective 5-HT2CR agonist in freely-fed rats.
2. METHODS
2.1 Animals
A total of 91 male Sprague-Dawley rats (Harlan, Inc., Indianapolis, IN, USA) weighing 225–325g at the start of the experiments were used. Rats were allowed to acclimate for 5–7 days in a colony room at a constant temperature (21–23°C) and humidity (45–50%) on a 12 hr light-dark cycle (lights on 0700–1900 hr). Rats were housed either two (self-administration studies) or four rats per cage (locomotor activity studies) with food and water ad libitum throughout all phases of the studies. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and with the approval of the Institutional Animal Care and Use Committee at University of Texas Medical Branch. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, when available.
2.2 Drugs
WAY 163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino [6,7,1hi]indole; Wyeth Research, Princeton, NJ, USA] was dissolved in 0.9% NaCl. SB242084 [6-chloro-5-methyl-1-[[2-(2-methylpyrid-3-yloxy) pyrid-5-yl]carbamoyl]indolinedihydrochloride; Sigma Chemical Co., St. Louis, MO, USA] was dissolved in 0.9% NaCl containing 10 mM citric acid (Sigma Chemical Co.) and 8% 2-hydroxypropyl-β-cyclodextrin (Trappsol® Hydroxpropyl Beta Cyclodextrin, Pharmaceutical Grade, Cyclodextrin Technologies Development Inc., High Springs, FL, USA) with the final pH of the solution adjusted to 5.6. (−)-Cocaine (National Institutes on Drug Abuse, Research Triangle Park, NC, USA) was dissolved in 0.9% NaCl.
2.3 Locomotor Activity Studies
2.3.1 Apparatus
Locomotor activity was monitored and quantified under low light conditions using a modified open field activity system (San Diego Instruments, San Diego, CA, USA). Clear Plexiglass chambers (40 × 40 × 40 cm) were surrounded by a 4 × 4 photobeam matrix positioned 4 cm from chamber floor. Consecutive photobeam breaks within the central 16 × 16 cm of the activity monitor were recorded as central ambulation. Peripheral ambulation was counted as consecutive beam breaks in the surrounding perimeter. Central and peripheral ambulations were summed to provide a measure of total horizontal ambulation. Vertical activity was also recorded using a row of 16 photobeams, positioned 16 cm from the activity monitor floor; breaks in these beams indicated vertical (rearing) activity.
2.3.2 Procedures
Rats in locomotor activity studies (n=16) were acclimated to the colony room 5–7 days prior to the start of handling protocols. Following seven days of handling, rats were habituated to the activity monitors for 60 min. Twenty four hours later, rats received the first dose of vehicle (saline, 1 ml/kg, i.p.) or WAY 163909 (0.3, 1, 3 or 10 mg/kg, i.p.) immediately prior to placement in activity monitors; activity was assessed for 60 min. Using a repeated measures design, rats were tested every three days such that all rats received vehicle and all three doses of WAY 163909 in a counterbalanced manner.
2.4 Self Administration Studies
2.4.1 Surgery
Implantations of intravenous catheters with back mounts were performed under anesthesia with a cocktail containing 8.6 mg/kg ofxylazine, 1.5 mg/kg of acepromazine, and 43 mg/kg of ketamine in bacteriostatic saline. Small incisions were made in the right posterior neck and just below the left shoulder blade to expose the jugular vein and to insert the catheter base, respectively. A subcutaneous burrow was made between the two incisions, and the catheter apparatus was pulled through this burrow. The catheter was composed of Silastic tubing (Dow Corning, Midland MI, USA) connected to a bent 22-gauge metal cannula encased within a plastic screw connector (Plastics One, Roanoke, VA, USA) at one end and affixed with a small ball of silicone 3.5 cm from the other end. The distal end of the catheter was inserted into the jugular vein until flush with the silicone ball, terminating outside the right atrium. The catheter was secured to the vein with sutures (SP116 braided silk non-absorbable 4.0; Surgical Specialties Corporation, Reading, PA, USA) on both sides of the silicone ball. The incision was then sutured (Monomid nylon non-absorbable 4.0; CP Medical, Portland, OR, USA). The cannula base was encased with dental acrylic and a small mesh circle (3 cm × 3 cm; Polypro mesh 500 micron, Small Parts, Inc., Seattle, WA, USA) affixed to the bottom, which was pulled thru the small incision in the back. The remaining opening was then sutured (Monomid nylon non-absorbable 4.0; CP Medical). Daily flushes with a solution of 0.1 ml of bacteriostatic saline containing heparin sodium (10U/mL; American Pharmaceutical Partners, East Schaumburg, IL, USA), streptokinase (0.67 mg/mL; Sigma Chemical) and ticarcillin disodium (66.67 mg/mL; Research Products International, Mt. Prospect, IL, USA) were performed after each cocaine self-administration session to maintain catheter patency. Proper catheter function was verified periodically throughout the experiment by intravenous administration of 10 mg/kg of methohexital sodium (Monarch Pharmaceuticals Inc., Bristol, TN, USA), a dose sufficient to briefly anesthetize the animal only when administered intravenously. All rats were allowed at least five days of recovery after surgery before initiation of self-administration training. Rats trained in sucrose self-administration were naïve at the start of the experiment.
2.4.2 Apparatus
Standard operant conditioning chambers (Med-Associates, Inc., St. Albans, VT, USA) housed in ventilated sound-attenuating cubicles with fans (Med-Associates, Inc.) were utilized for both the cocaine and sucrose self-administration studies. Each chamber was equipped with two retractable response levers, a stimulus light above each response lever, a house light opposite the levers, and a magazine-type pellet dispenser. Cocaine infusions were delivered by a syringe attached to an infusion pump (Med Associates, Inc.) located outside the cubicle. The infusion pumps were connected to liquid swivels (Instech, Plymouth Meeting, PA, USA) that were fastened to the catheters via polyethylene 20 tubing encased inside a metal spring leash (Plastics One). Sucrose pellets (45 mg; Bio-Serv, Frenchtown, NJ, USA) were delivered into a pellet receptacle located between the two levers.
2.4.3 Experimental Design
2.4.3.1 Effects of WAY 163909 and SB 242084 during cocaine self-administration
Cocaine self-administration training consisted of daily 60-min sessions during which rats (n=8) were trained to press the active lever to obtain a cocaine infusion (0.25 mg/kg/0.1 mL infusion). Animals were not food restricted or trained on an operant task prior to commencement of self-administration and no cocaine priming infusions were given during the experiment. Schedule completions on the active lever resulted in simultaneous illumination of the house and stimulus lights, followed 1 sec later by activation of the infusion pump. Rats received a 0.1 mL cocaine infusion delivered over a 6-sec period, after which the pump and stimulus light (conditioned stimuli paired with delivery of cocaine) were inactivated simultaneously. The house light remained illuminated for a 20-sec timeout period, during which lever presses had no scheduled consequences. Responses on the inactive lever were recorded but had no scheduled consequences. Rats were initially trained on an FR 1 schedule of cocaine reinforcement until they met a criterion of seven infusions/hr for at least three sessions with <10% variation in the number of infusions received for three consecutive sessions. After reaching this acquisition criterion and demonstrating stable cocaine self-administration on an FR1 schedule of reinforcement, rats progressed to an FR 3 schedule of reinforcement, and then on to an FR 5 schedule of reinforcement once the same criterion levels were met. Once stable cocaine self-administration on an FR 5 schedule was achieved (i.e., <10% variation in the number of infusions received for three consecutive sessions), the efficacy and selectivity of WAY 163909 to alter response for cocaine self-administration was assessed. Rats were administered vehicle (saline; 1mL/kg; 15 min) or WAY 163909 (0.5, 1 or 2 mg/kg, i.p.; 15 min) prior to self-administration sessions, according to a within-subjects design. To investigate the selectivity of WAY 163909 for the 5-HT2C receptor, the same rats were administered the 5-HT2C receptor antagonist SB 242084 (0.5 mg/kg, i.p.; 30 min) plus vehicle (saline; 1 mL/kg; 15 min) or WAY 163909 (2 mg/kg, i.p.; 15 min) prior to self-administration sessions, according to a within-subjects design. The order of injections was counterbalanced and a minimum of two intervening sessions of cocaine self-administration (0.25 mg/kg/0.1 mL infusion) occurred between each drug challenge to assure stability of baseline responding.
2.4.3.2 Effects of WAY 163909 on cue-induced reinstatement in rats trained to self-administer cocaine
A separate cohort of rats (n=34) was utilized to assess the effects of WAY 163909 on cue-induced reinstatement following cocaine self-administration. Cocaine self-administration training consisted of daily 180-min sessions during which rats were trained to press the active lever to obtain a cocaine infusion (0.75 mg/kg/0.1 mL infusion) on an FR 1 schedule of reinforcement before progressing to an FR 5 schedule. Animals were not food restricted or trained on an operant task prior to commencement of self-administration and no cocaine priming infusions were given during the experiment. Rats that met the criterion for stable self-administration (<10% variation in the number of infusions received for three consecutive sessions) were subjected to daily 60-min extinction sessions, during which active and inactive lever presses were recorded but had no scheduled consequences (i.e., did not activate the infusion pump or result in presentation of the stimulus light). Once rats achieved the extinction criterion of response rates <15 total responses/hr for three consecutive sessions, the effects of WAY 163909 on cue-induced reinstatement of cocaine-seeking behavior were examined. The criterion of <15 responses/hr as indicative of extinction level responding is commonly employed in self-administration studies and corresponds to approximately 15% of responding during active cocaine self-administration (Leri, et al., 2002; Nic Dhonnchadha, et al., 2009). To initiate reinstatement sessions, one non-contingent presentation of the cocaine-paired cues (pump and stimulus light) was given. During the 60-min test session, responses on the active lever were reinforced by presentation of the cocaine-paired cues on an FR 1 schedule. To assess the effects of WAY 163909 on cue-induced reinstatement of cocaine-seeking behavior, rats were injected with saline (1 mL/kg; 15 min) or WAY 163909 (0.5, 1, or 1.5 mg/kg, i.p.; 15 min) before the start of the reinstatement session according to a between-subjects, counterbalanced design (n=8–9/group).
2.4.3.3 Effects of WAY 163909 and SB 242084 on sucrose self-administration
Sucrose self-administration training consisted of daily 60-min sessions during which rats (n=8) were trained to press the active lever to obtain a 45 mg sucrose pellet. Experimental parameters were identical to those described in Section 2.4.3.1, above, except for substitution of a single 45 mg sucrose pellet as the reinforce. Animals were not food restricted or trained on an operant task prior to commencement of self-administration and no sucrose priming events were given during the experiment. Upon reaching stability on the FR 5 schedule, the efficacy and selectivity of WAY 163909 to alter response for sucrose self-administration was assessed, as described above (Section 2.4.3.1).
2.4.3.4 Effects of WAY 163909 on cue-induced reinstatement in rats trained to self-administer sucrose pellets
A separate cohort of rats (n=25) was utilized to assess the effects of WAY 163909 on cue-induced reinstatement following sucrose self-administration. Sucrose self-administration training consisted of daily 180-min sessions during which rats were trained to press the active lever to obtain a 45 mg sucrose pellet on an FR 1 schedule of reinforcement before progressing to an FR 5 schedule. Experimental parameters were identical to those described in Section 2.4.3.2 above, except for substitution of a single 45 mg sucrose pellet as the reinforcer. Animals were not food restricted or trained on an operant task prior to commencement of self-administration and no sucrose priming events were given during the experiment. To assess the effects of WAY 163909 on cue-induced reinstatement of sucrose-seeking behavior, rats were injected with saline (1 mL/kg; 15 min) or WAY 163909 (0.5 or 1.5 mg/kg,i.p.; 15 min) before the start of the reinstatement session according to a between-subjects, counterbalanced design (n=8–9/group).
2.5 Statistical analysis
Locomotor activity data were analyzed and are presented as mean total horizontal ambulation or vertical activity (± SEM) over the 60-min session (Figs. 1A Inset, 1B Inset) or within the 15, 30, 45 and 60 min time bins (Figs. 1A, 1B). The main effect of treatment (WAY 163909 dose) on horizontal ambulation or vertical activity was analyzed using a repeated measures, one-way analysis of variance (ANOVA) using the general linear model (GLM) procedure (SAS for Windows, Version 8.2, SAS Institute, Inc., Cary, NC, USA). A two-way ANOVA for repeated measures for the factors of treatment (WAY 163909 dose) and time (15, 30, 45 or 60 min) was also conducted. Subsequent a priori comparisons between means of total horizontal ambulation or total vertical activity were made using the Dunnett’s procedure, with vehicle as the comparator. The four parameter logistic nonlinear regression model equation (Sigma Plot, Version 11.0, Systat Software, Inc., Chicago, IL, USA) was used to estimate the dose of WAY 163909 predicted to decrease horizontal ambulation or vertical activity by 50% (ID50) (Table 2) (Ratkowsky and Reedy, 1986; Tallarida and Murray, 1987). All statistical analyses were conducted with an experiment wise error rate of α=0.05.
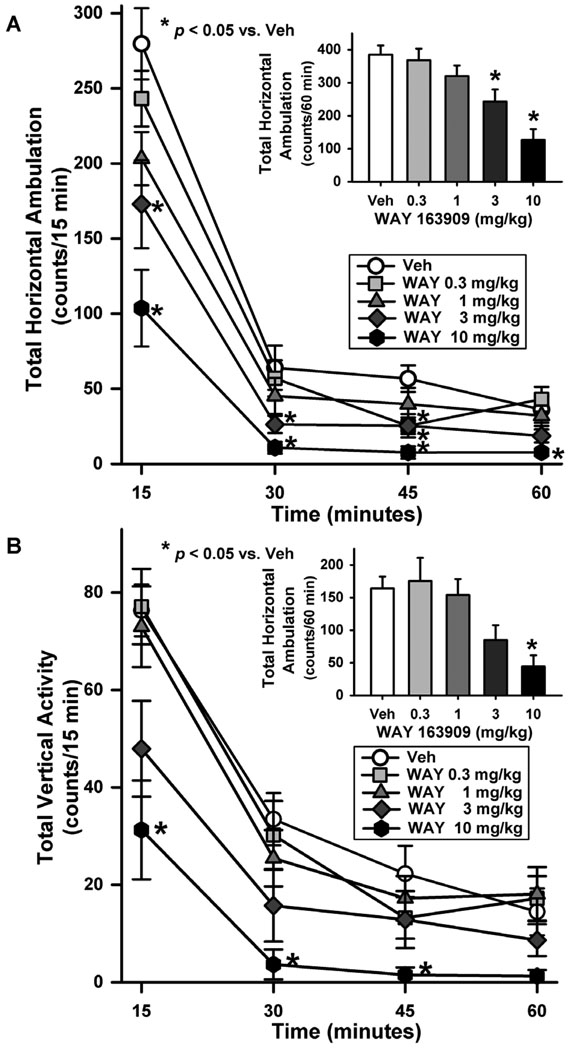
Fig. 1. WAY 163909 evokes a dose-dependent suppression of horizontal ambulation and vertical activity.
[A] The time course of horizontal ambulation is divided into 15 min time bins across the 60 min session; [A, Inset] the mean total horizontal ambulation (counts/60 min) (± SEM) is represented following administration of vehicle (saline; Veh) or WAY 163909 (WAY; 0.3, 1, 3 or 10 mg/kg, i.p.). [B] The time course of vertical activity is divided into 15 min time bins across the 60 min session; [B, Inset] the mean total vertical activity (counts/60 min) (± SEM) is represented following administration of vehicle (saline; Veh) or WAY 163909 (WAY; 0.3, 1, 3, or 10 mg/kg, i.p.). *p< 0.05 vs. Veh.
Table 2.
Efficacy of WAY 163909 on locomotor activity, cocaine and sucrose self-administration, and reinstatement
| Suppression of Horizontal Ambulation (ID50) |
Suppression of Vertical Activity (ID50) |
Suppression of Cocaine (ID50) |
Suppression of Sucrose (ID50) |
||
|---|---|---|---|---|---|
| Taking | Seeking | Taking | Seeking | ||
| 5.89 mg/kg | 2.3 mg/kg | 1.19 mg/kg | 0.5 mg/kg | 0.7 mg/kg | NE |
The dose predicted to decrease basal horizontal ambulation, vertical activity and both cocaine and sucrose –taking and seeking by 50% (ID50) was estimated using the four parameter logistic nonlinear regression model equation (see Methods). NE no effect
The data from the self-administration, extinction and reinstatement phases were analyzed separately and presented as mean ± SEM. A one-way ANOVA (SAS for Windows, V8.2) for repeated measures was used to analyze the dependent measures of the total number of 1) active lever responses/session, 2) inactive lever presses/session, and 3) number of cocaine infusions or sucrose pellets/session during the self-administration phase and the total number of responses/session on the 4) previously-active, or 5) -inactive lever during the extinction phase. A one-way ANOVA for repeated measures (cocaine- or sucrose-taking) or independent groups (cocaine- or sucrose-seeking) was used to analyze the effects of pretreatment with WAY 163909 on the total number of 1) responses/session on the previously-active, or 2) -inactive lever, and 3) cocaine infusions/session or sucrose pellets/session, and 4) the latency to respond on the previously-active lever during the self-administration and the cue-induced reinstatement phase. A priori comparisons for WAY 163909 pretreatment were carried out with Student–Newman–Keuls test with the alpha level set at p< 0.05. A repeated measures one-way ANOVA was employed to compare responding on the previously-active lever over the last three sessions of extinction with responding observed following vehicle pretreatment during reinstatement sessions. The four parameter logistic nonlinear regression model equation (Sigma Plot, Version 11, SystatSoftware, Inc.) was used to estimate the dose of WAY 163909 predicted to decrease lever presses during cocaine or sucrose self-administration and reinstatement sessions by 50% (ID50), for each (Table 2) (Ratkowsky and Reedy, 1986; Tallarida and Murray, 1987).
3. RESULTS
3.1. WAY 163909 evokes a dose-dependent suppression of locomotor activity
A main effect of treatment (F(4,66) = 10.33, p <0.001), time (F(3,66) = 253.42, p <0.001) and a treatment × time interaction (F(12,198) = 5.23, p <0.001) was observed for horizontal ambulation divided into four 15 min time bins (Fig. 1A). WAY 163909 at 0.3 mg/kg significantly reduced horizontal ambulation versus vehicle during the third interval (45 min), while 3 mg/kg of WAY 163909 significantly reduced horizontal ambulation versus vehicle at the first (15 min), second (30 min) and third intervals (45 min) (p < 0.05; Fig. 1A). WAY 163909 at 10 mg/kg significantly reduced horizontal ambulation versus vehicle during each of the four 15 min intervals examined (p < 0.05; Fig. 1A). A main effect of WAY 163909 treatment was observed for horizontal ambulation (F4,66 = 10.65, p < 0.001; Fig. 1A, inset) summed across the 60-min test session; a priori comparisons using the Dunnett’s procedure revealed that 3 and 10 mg/kg of WAY 163909 significantly reduced horizontal ambulation versus vehicle (p < 0.05; Fig. 1A, inset). The dose of WAY 163909 predicted to decrease horizontal ambulation by 50% (ID50) was 5.89 mg/kg (Table 2).
A main effect of treatment (F(4,66) = 6.16, p < 0.001), time (F3,66 = 146.02, p< 0.001) and a significant treatment × time interaction (F(12,198) = 2.73, p <0.01) was observed for vertical activity divided into four 15 min intervals (Fig. 1B). WAY 163909 at 10 mg/kg significantly reduced vertical activity versus vehicle during the first three 15 min intervals (p < 0.05; Fig. 1B). A main effect of WAY 163909 treatment was observed for vertical activity (F4,66 = 5.66, p < 0.001; Fig. 1B Inset) summed across the 60-min test session; a priori comparisons using the Dunnett’s procedure revealed that 10mg/kg of WAY 163909 significantly reduced vertical activity versus vehicle (p < 0.05; Fig. 1B, inset). The dose of WAY 163909 predicted to decrease vertical activity by 50% (ID50) was 2.3 mg/kg (Table 2).
3.2. WAY 163909 dose-dependently suppresses cocaine self-administration
Rats (n = 8) readily acquired cocaine self-administration (0.25 mg/kg/0.1ml infusion) to stability (i.e., seven infusions/hr on an FR 5 schedule for at least three sessions) and displayed <10% variation in the number of infusions received (i.e., cocaine intake) (Fig. 2A). Across the last three sessions of stable self-administration, there was no main effect of session on the number of active [F(2,21) = 0.00, ns] or inactive lever responses [F(2,17) = 1.75, ns], or the number of infusions received [F(2,21) = 0.00, ns] (Fig. 2A). Average daily cocaine intake over the last three sessions of training was 4.4 ± 0.7 mg/kg.
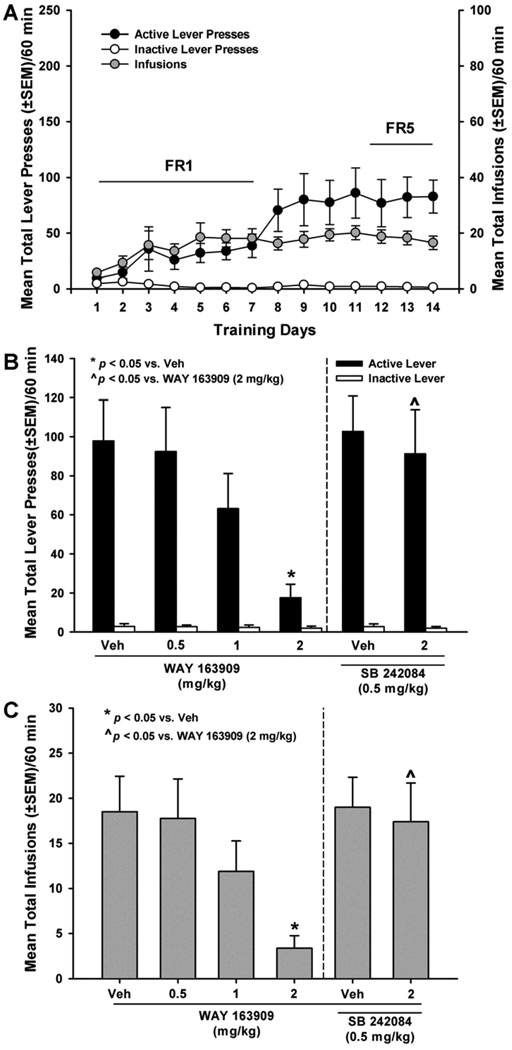
Fig. 2. WAY 163909 dose-dependently suppresses cocaine self-administration.
[A] Mean responses (+/− SEM) on the active (black circles) or inactive lever (white circles), and total number of cocaine infusions (+/− SEM; gray circles) are presented for the acquisition phase of cocaine self-administration training under FR1 – FR5 schedules of reinforcement. Active lever responses resulted in the delivery of an intravenous cocaine infusion (0.25 mg/kg/0.1 ml infusion) and simultaneous presentation of the cocaine-paired cues. [B] Mean responses (+/− SEM) on the active (black bars) or inactive lever (white bars) or [C] mean total cocaine infusions (+/− SEM; gray bars) following pretreatment with vehicle (saline; Veh) or WAY 163909 (0.5, 1 or 2 mg/kg, i.p., 15 min) (left panel) or following pretreatment with the 5-HT2CR antagonist SB 242084 (0.5 mg/kg, i.p., 15 min) plus vehicle (saline; Veh) or WAY 163909 (2 mg/kg, i.p.) (right panel) during the cocaine self-administration maintenance phase. Active lever responses resulted in the delivery of an intravenous cocaine infusion (0.25 mg/kg/0.1 ml infusion) according to an FR 5 schedule and simultaneous presentation of cocaine-paired cues. *p < 0.05 vs. Veh; ^p < 0.05 vs. WAY 163909 (2 mg/kg).
An one-way ANOVA revealed a main effect of pretreatment on the number of active lever presses [F(5,42) = 2.92; p< 0.05; Fig. 2B, left panel] and number of cocaine infusions received across the entire 60-min session [F(5,42) = 2.79; p < 0.05; Fig. 2C, left panel]. No main effect was observed for the number of inactive lever presses [F(5,42) = 0.12; ns; Fig. 2B]. A priori comparisons revealed that pretreatment with 2 mg/kg of WAY 163909 significantly reduced the number of active lever presses (p< 0.05; Fig. 2B, left panel) as well as the number of cocaine infusions (p< 0.05; Fig. 2C, left panel) without altering the number of inactive lever presses (ns; Fig. 2B). The dose of WAY 163909 predicted to decrease lever presses for cocaine by 50% (ID50) is 1.19 mg/kg (Table 2). Pretreatment with the selective 5-HT2CR antagonist SB 242084 (0.5 mg/kg i.p.) completely blocked the effects of WAY 163909 (2 mg/kg) on the number active lever presses (p < 0.05; Fig. 2B, right panel) and number of cocaine infusions received (p <0.05; Fig. 2C, right panel).
3.3. WAY 163909 dose-dependently suppresses cocaine cue-evoked reinstatement
Rats (n = 34) readily acquired cocaine self-administration (0.75 mg/kg/0.1 ml infusion) in daily 180 min sessions to stability (i.e., seven infusions/hr on an FR 5 schedule for at least three sessions) and displayed <10% variation in the number of infusions received (i.e., cocaine intake) (Fig. 3A). Across the last three sessions of stable self-administration on an FR 5 schedule, there was no main effect of session on the number of active [F(2,99) = 2.75, ns] or inactive lever responding [F(2,99) = 0.63, ns] or the number of infusions received [F(2,99) = 2.42, ns]. The rats were then separated into four groups for reinstatement sessions. Cocaine intake did not differ significantly across groups of rats assigned to receive pretreatment with vehicle (34.6 ± 1.8 infusions/session) or WAY 163909 at doses of 0.5 mg/kg (33.5 ± 1.8 infusions/session), 1 mg/kg (32 ± 1.2 infusions/session), or 1.5 mg/kg (31.9 ± 2.8 infusions/session) [F(3,30) = 0.62; ns].
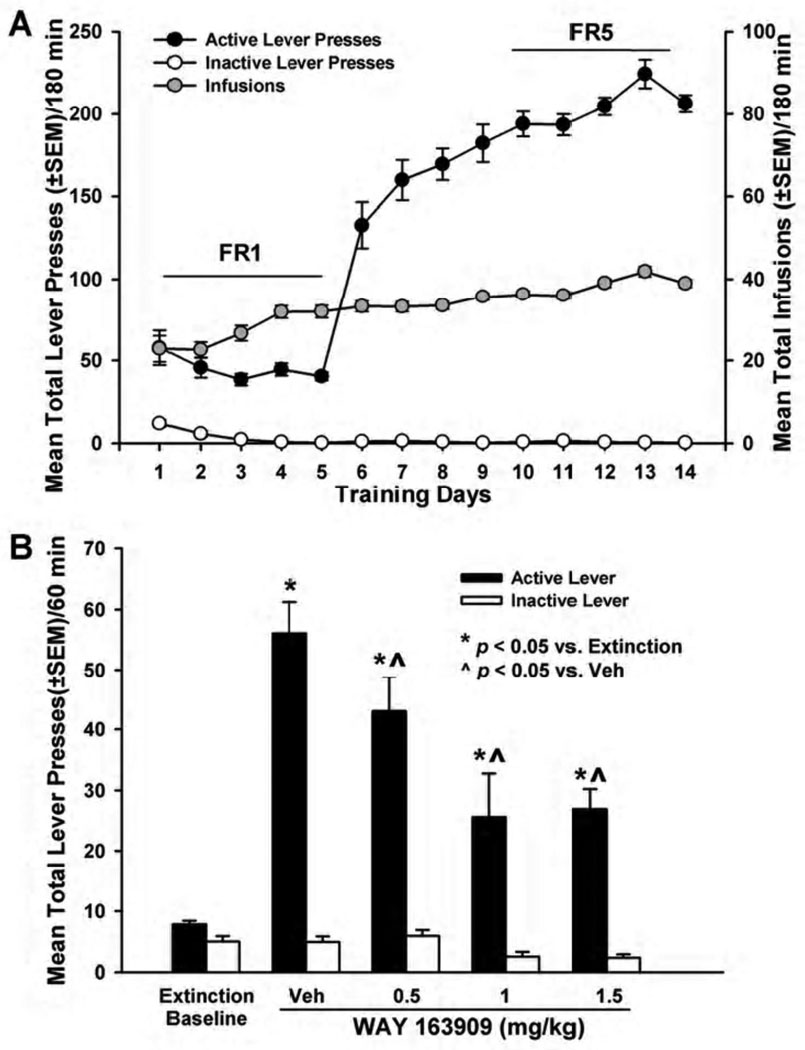
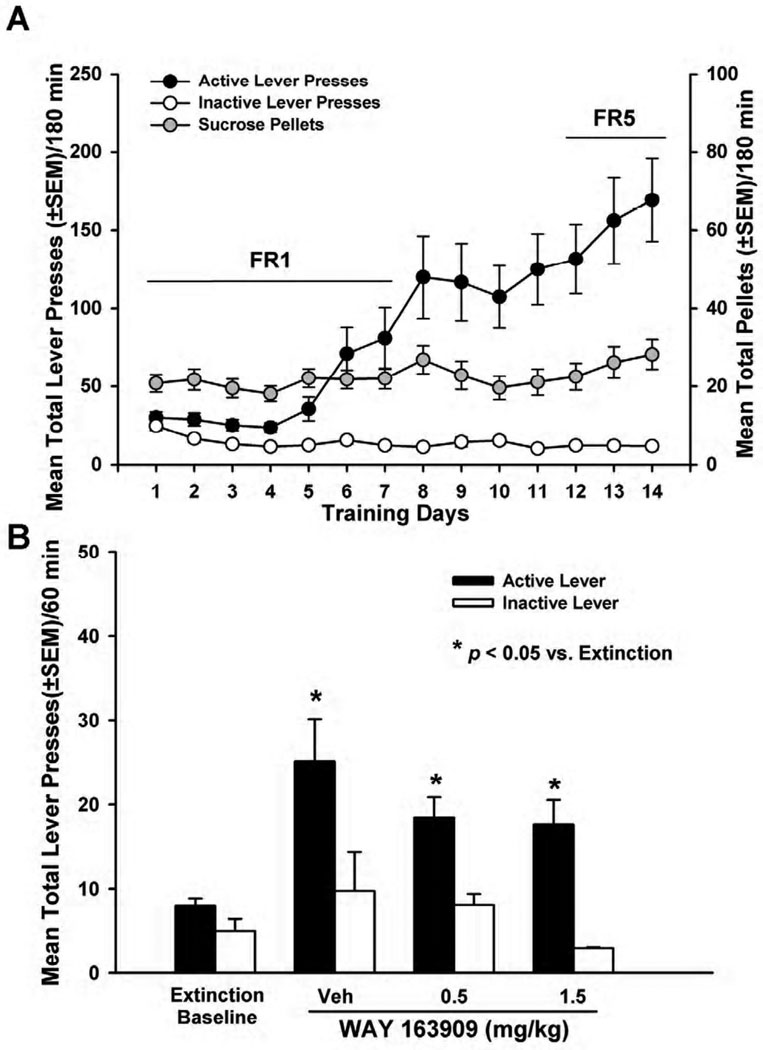
Fig. 3. WAY 163909 dose-dependently suppresses cocaine cue-evoked reinstatement.
[A] Mean responses (+/− SEM) on the active (black circles) or inactive lever (white circles), and the total number of cocaine infusions (+/− SEM; gray circles) are presented for the acquisition phase of cocaine self-administration training under FR1 – FR5 schedules of reinforcement. Active lever responses resulted in the delivery of an intravenous cocaine infusion (0.75 mg/kg/0.1 ml infusion) and simultaneous presentation of the cocaine-paired cues. [B] Mean (+/− SEM) total number of responses on the active (black bars) or inactive lever (white bars) following extinction (“Extinction Baseline”) and upon pretreatment with vehicle (saline; Veh) or WAY 163909 (0.5, 1, or 1.5 mg/kg, i.p.) on the test day for reinstatement of extinguished cocaine-seeking behavior. Reinstatement was initiated by a single non-contingent presentation of cocaine-paired cues (pump and stimulus light). Each active lever press resulted in the presentation of the conditioned stimuli in the absence of cocaine delivery. *p < 0.05 vs. Extinction; ^p < 0.05 vs. Veh.
A decrease in presses on the previously-active lever was observed across extinction sessions with all rats achieving the extinction criterion (<15 active lever responses/session for three consecutive sessions) by session 12 of extinction training (data not shown). A repeated-measures ANOVA revealed a main effect of extinction session on active [F(11,265) = 78.8; p< 0.05] and inactive lever presses [F(11,265) = 4.88; p< 0.05] for all rats (n = 34) with both active and inactive lever presses decreasing across the extinction sessions (data not shown). The “extinction baseline” was calculated as the mean total lever presses of all rats on the active (7.8 ± 0.6) or inactive lever (4.9 ± 0.9) during the final 60 min extinction session (Fig. 3B, left).
Figure 3 Billustrates the effects of vehicle or WAY 163909 (0.5–1.5 mg/kg) pretreatment on reinstatement of responding supported by cues previously associated with cocaine availability. Cocaine-trained rats readily reinstated active lever pressing across the 60-min reinstatement session (Fig. 3B). A main effect of pretreatment on active lever responding was observed [F(4,63) = 43.02; p <0.001; Fig. 3B]. Significant decreases in active lever presses relative to vehicle were observed after pretreatment with WAY 163909 at doses of 0.5, 1 and 1.5 mg/kg (p< 0.05; Fig. 3B). There was no main effect of pretreatment on inactive lever responding [F(4,61) = 1.66; ns; Fig. 3B). The latency to the first lever press following pretreatment with WAY 163909 was unaltered [F(3,30) = 0.95; ns] (data not shown). The dose of WAY 163909 predicted to decrease lever presses for cocaine-paired cues by 50% (ID50) is 0.5 mg/kg (Table 2).
3.4. WAY 163909 dose-dependently suppresses sucrose self-administration
Rats (n = 8) readily acquired sucrose self-administration (45 mg sucrose pellet/response) to stability (i.e., 10 pellets/hr on an FR 5 schedule for at least three sessions) and displayed <10% variation in the number of pellets received (i.e., pellet intake) (Fig. 4A). Across the last three sessions of stable self-administration on an FR5 schedule, there was no main effect of session on the number of active [F(2,21) = 0.01, ns] or inactive operant lever responses [F(2,21) = 1.8, ns] or the number of pellets received [F(2,21) = 0.03, ns]. Average daily sucrose intake over the last three sessions of training was 23.0 ± 3.4 pellets.
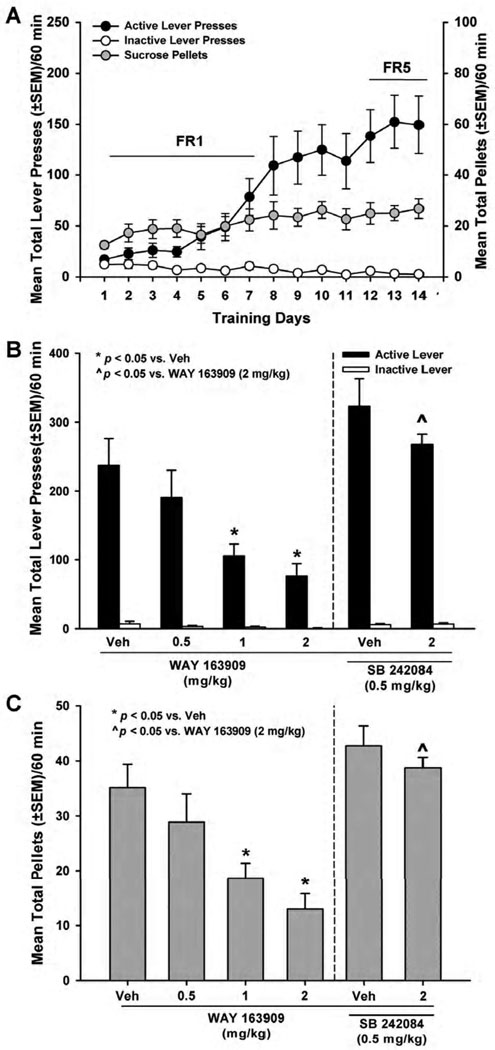
Fig. 4. WAY 163909 dose-dependently suppresses sucrose self-administration.
[A] Mean responses (+/− SEM) on the active (black circles) or inactive lever (white circles), and total number of sucrose pellets (+/− SEM; gray circles) are presented for the acquisition phase of cocaine self-administration training under FR1 – FR5 schedules of reinforcement. Active lever responses resulted in the delivery of a single sucrose pellet (45 mg) and simultaneous presentation of the sucrose-paired cues. [B] Mean responses (+/− SEM) on the active (black bars) or inactive lever (white bars) or [C] mean total sucrose pellets (+/− SEM; gray bars) following pretreatment with vehicle (saline; Veh) or WAY 163909 (0.5, 1 or 2 mg/kg, i.p., 15 min) (left panel) or following pretreatment with the 5-HT2CR antagonist SB 242084 (0.5 mg/kg, i.p., 15 min) plus vehicle (saline; Veh) or WAY 163909 (2 mg/kg, i.p.) (right panel) during the sucrose self-administration maintenance phase. Active lever responses resulted in the delivery of a single sucrose pellet (45 mg) according to an FR5 schedule of reinforcement and simultaneous presentation of the sucrose-paired cues. *p < 0.05 vs. Veh; ^p < 0.05 vs. WAY 163909 (2 mg/kg).
An one-way ANOVA revealed a main effect of pretreatment on the number of active lever presses [F(5,42) = 9.88 ; p< 0.05; Fig. 4B, left panel] and number of sucrose pellets received across the 60-min session [F(5,42) = 10.73 ; p< 0.05; Fig. 4C, left panel]. No main effect was observed for the number of inactive lever presses [F(5,42) = 1.66; ns; Fig. 4B]. A priori comparisons revealed that pretreatment with 1 or 2 mg/kg of WAY 163909 significantly reduced the number of active lever presses (p< 0.05; Fig. 4B, left panel) as well as the number of sucrose pellets received (p < 0.05; Fig. 4C, left panel), without altering the number of inactive lever presses (ns; Fig. 4B). The dose of WAY 163909 predicted to decrease lever presses for sucrose by 50% (ID50) is 0.7 mg/kg (Table 2). Pretreatment with the selective 5-HT2CR antagonist SB 242084 (0.5 mg/kg i.p.) completely blocked the effects of WAY 163909 (2 mg/kg) on the number active lever presses (p < 0.05; Fig. 4B, right panel) and number of sucrose pellets received (p < 0.05; Fig. 4C, right panel).
3.5. WAY 163909 does not alter sucrose cue-evoked reinstatement
Rats (n = 25) readily acquired sucrose self-administration (45 mg pellet) in daily 180 min sessions to stability (i.e., minimum 20 pellets/session on an FR 5 schedule for at least three sessions) and displayed <10% variation in the number of pellets received (i.e., sucrose intake) (Fig. 5A). Across the last three sessions of stable self-administration on an FR 5 schedule, there was no main effect of session on the number of active [F(2,72) = 0.54, ns] or inactive lever responding [F(2,72) = 0.42, ns] or the number of pellets received [F(2,72) = 0.32, ns]. The rats were then separated into three groups for reinstatement sessions. Sucrose intake did not differ significantly across groups of rats assigned to receive pretreatment with vehicle (26.4 ± 5.2 pellets/session) or WAY 163909 at doses of 0.5 mg/kg (24.2 ± 3.9 pellets/session), or 1.5 mg/kg (22.6 ± 3.9 pellets/session) [F(2, 22) = 0.34; ns].
Fig. 5. WAY 163909 does not alter sucrose cue-evoked reinstatement.
[A] Mean responses (+/− SEM) on the active (black circles) or inactive lever (white circles), and the total number of sucrose pellets (+/− SEM; gray circles) are presented for the acquisition phase of sucrose self-administration under FR1 – FR5 schedules of reinforcement. Active lever responses resulted in the delivery of a single sucrose pellet (45 mg) and simultaneous presentation of the sucrose-paired cues. [B] Mean (+/− SEM) total number of responses on the active (filled bars) or inactive levers (open bars) following extinction (“Extinction Baseline”) and upon pretreatment with vehicle (saline; Veh) or WAY 163909 (0.5 or 1.5 mg/kg, i.p.) on the test day for reinstatement of extinguished sucrose-seeking behavior. Reinstatement was initiated by a single non-contingent presentation of sucrose-paired cues (pump and stimulus light). Each active lever press resulted in the presentation of the conditioned stimuli in the absence of sucrose pellet delivery. *p < 0.05 vs. Extinction.
A decrease in presses on the previously-active lever was observed across extinction sessions with all rats achieving the extinction criterion (<15 active lever responses/session for three consecutive sessions) by session 34 of extinction training (data not shown). A repeated-measures ANOVA revealed a main effect of extinction session on active [F(33,311) = 19.19; p< 0.05] and inactive lever presses [F(33,311) = 3.99; p< 0.05] across the extinction sessions for all rats (n = 25; data not shown). The “extinction baseline” was calculated as the mean total lever presses of all rats on the active (7.9 ± 0.9) or inactive lever (5 ± 1.4) during the final 60 min extinction session (Fig. 5B, left).
Figure 5 Billustrates the effects of WAY 163909 (0.5 and 1.5 mg/kg) pretreatment on reinstatement of responding supported by cues previously associated with sucrose availability. Sucrose-trained rats readily reinstated active lever pressing across the 60-min reinstatement session (Fig. 5B). Amain effect of pretreatment on active lever responding was observed across the 60-min reinstatement session [F(3, 45) = 11.83; p <0.001; Fig. 5B]. A priori comparisons failed to reveal any significant differences in active lever presses relative to vehicle after pretreatment with any doses of WAY 163909 tested (ns; Fig. 5B). The one-way ANOVA revealed no main effect of pretreatment on inactive lever responding [F(3,46) = 2.61; ns; Fig. 5B]. The latency to the first lever press following pretreatment with WAY 163909 was unaltered [F(2,22) = 0.82; ns] (data not shown).
4. DISCUSSION
Our results demonstrate that the highly-selective and efficacious 5-HT2CR agonist WAY 163909 dose-dependently reduced the reinforcing efficacy of cocaine and sucrose as assessed in parallel self-administration tasks conducted in freely-fed rats. We also show for the first time that WAY 163909 dose-dependently reduced reinstatement elicited by exposure to cocaine-associated contextual cues, but failed to alter reinstatement of cue-evoked sucrose-seeking in freely-fed rats. Thus, selective stimulation of the 5-HT2CR signaling decreases the reinforcing efficacy of cocaine and sucrose, but differentially alters the incentive-salience value of cocaine-versus sucrose-associated cues.
The dose of WAY 163909 predicted to decrease the reinforcing efficacy of cocaine (ID50 = 1.19 mg/kg) by 50% was ~5-fold and ~2-fold lower than that predicted to suppress horizontal ambulation (ID50= 5.89 mg/kg) and vertical activity (ID50= 2.3 mg/kg), respectively (Table 2). Although 5-HT2CR agonists are reported to suppress both horizontal and vertical activity measures (Halford, et al., 1997; Halberstadt, et al., 2009), most pharmacological studies of 5-HT2CR agonists (e.g., Grottick, et al., 2000; Fletcher, et al., 2002) typically report horizontal activityas a comparative, independent measure in pharmacological assessments. Horizontal ambulation and vertical activity often correlate, however, these measures have been behaviorally (Walsh and Cummins, 1976; Lever, et al., 2006) and pharmacologically dissociated (e.g., Wu, et al., 2005) and appear to be mediated by different brain regions (Jackson, et al., 1975; Lever, et al., 2006). In general, vertical activity is more sensitive to pharmacological manipulation than is horizontal activity (Prut and Belzung, 2003) and, consistent with the current study, Brookshire and Jones (2009) demonstrated that the IC50 for the preferential 5-HT2CR agonist MK 212 to suppress vertical activity (IC50 = ~0.93 mg/kg) was lower than that predicted to suppress horizontal activity (IC50 = ~2.29 mg/kg). In a review of the literature, we could not identify previous publications in which doses of a selective 5-HT2CR agonist were assessed in parallel for efficacy to suppress both horizontal and vertical activity as well as operant responding for an abused drug and palatable reinforcer in freely-fed rats. We uniquely present these observations that, coupled with the failure of WAY 163909 to significantly alter inactive lever presses at any dose, rule out the interpretation that the inhibitory effects of WAY 163909 on drug-seeking were due to a generalized suppression of behavior. Thus, WAY 163909 presents an efficacy window between its effects on cocaine-taking and effects on locomotor activity which may reflect its greater selectivity as a 5-HT2CR agonist relative to 5-HT2AR and 5-HT2BR (see below). Furthermore, these data illustrate that the effects of MK 212 and Ro 60-0175 (Table 1) which have been profiled to suppress cocaine-taking are consistent with their actions preferentially as 5-HT2CR agonists.
A primary therapeutic indication for selective 5-HT2CR agonists is obesity and 5-HT2CR agonists have been shown to suppress food intake and reduce weight gain in animals (Clifton, et al., 2000; Dunlop, et al., 2005; Fletcher, et al., 2010; Hayashi, et al., 2005; Hewitt, et al., 2002) and most recently in humans (Smith, et al., 2009; Smith, et al., 2010). We observed a potent WAY 163909-induced suppression of sucrose intake in freely-fed rats. The ID50 for WAY 163909 (0.7 mg/kg) in the present study is much lower than the effective dose reported to suppress feeding on powdered chow in fasted Sprague-Dawley rats (ID50 = 2.93 mg/kg) and rats with diet-induced obesity (ID50 = 5.19 mg/kg), but closer to the effective doses in obese Zucker rats (ID50 =1.4 mg/kg) (Dunlop, et al., 2005). Similarly, Ro 60-0175 suppressed sucrose intake at doses much lower than those observed to suppress feeding in fasted rats (Hayashi, et al., 2005). Skinnerian tasks such as those employed here rely on the reinforcer (i.e., sucrose) to maintain operant responding and do not simply assay food consumption. In the present study, the motivation to lever press for sucrose is based upon the efficacy and palatability of this reinforcer and the motivational state of rats that have free access to food. Previous studies suggest that preferential 5-HT2CR agonists reduce food intake by enhancing satiety (Hewitt, et al., 2002; Somerville, et al., 2007), an effect which may account for our observations, particularly in freely-fed rats in an operant task. Stimulation of the 5-HT2CR could also reduce the reinforcing efficacy of sucrose and thus the incentive-salience value of sucrose. Most likely, stimulation of the 5-HT2CR engages neural processes which control both satiety and reinforcing efficacy (Fletcher, et al., 2010). Future studies are required to understand how the 5-HT2CR signaling integrates neural process involved in motivation, satiety, palatability and reinforcing efficacy of food.
Associations between environmental (contextual) cues and consumption of rewarding stimuli (food or drug) become encoded memories and it is theorized that such cues can play an important role in relapse to drug use (Lu, et al., 2005), and possibly also in non-homeostatic eating, i.e., food intake not driven by energy deficits (Berthoud, 2004). In the present study, WAY 163909 potently suppressed reinstatement of cocaine-seeking with an 11-fold lower ID50 dose (0.5 mg/kg) relative to the ID50 dose (5.89 mg/kg) for suppression of horizontal ambulation and 5-fold lower ID50 dose (2.3 mg/kg) relative to the ID50 for suppression of vertical activity. Interestingly, neither WAY 163909 (present study) nor Ro 60–1075 (Burbassi and Cervo, 2008) inhibited sucrose-seeking during reinstatement. However, a 5-HT2CR agonist was shown to dose-dependently suppress cue-evoked food seeking in food-restricted mice (Somerville, et al., 2007). The pharmacological suppression of drug-seeking during reinstatement is most often interpreted as reflecting decrements in the incentive-motivational value of the cue-elicited cocaine-seeking behavior (Fletcher, et al., 2008; Nic Dhonnchadha, et al., 2009). Assessment and interpretation of the incentive-motivational value of non-drug-associated cues may be dependent on the palatability of the reinforcer (i.e., sucrose vs. food) as well as motivational state of rats (i.e., freely-fed vs. food-restricted) and additional studies are needed to elucidate this complex relationship. This would suggest that while selective serotonin 5-HT2CR activation suppresses the reinforcing efficacy of cocaine and sucrose, this signaling pathway is differentially engaged in the control of the incentive-motivational value of cocaine- and sucrose-associated cues.
The attenuation of cocaine- and sucrose-taking and cocaine-seeking behavior by WAY 163909 is attributable to a selective stimulation of the 5-HT2CR based upon several lines of evidence. WAY 163909 is fully efficacious as a 5-HT2CR agonist, does not stimulate the 5-HT2AR, and is only a weak 5-HT2BR partial agonist at much greater concentrations than those that activate the 5-HT2CR (Dunlop, et al., 2005) (Table 1). A low dose of the selective 5-HT2CR antagonist SB 242084 completely reversed the inhibitory effects of WAY 163909 on cocaine-and sucrose-taking providing empirical evidence of behaviorally-relevant actions at the 5-HT2CR signaling. Rosenzweig-Lipson and colleagues have demonstrated the suppressive potency of WAY 163909 and the ability of SB 242084 to block WAY 163909-generated behavioral effects in several assay systems, including those modeling the psychiatric disorders of anxiety, depression and schizophrenia (Rosenzweig-Lipson, et al., 2007b; Navarra, et al., 2008; Grauer, et al., 2009; Dunlop, et al., 2006). In addition, WAY 163909 blocked cocaine-evoked hyperlocomotion as well as the associated enhancement of dopamine efflux in the nucleus accumbens (Hughes et al., 2006). Interestingly, the high potency of WAY 163909 (0.5–2mg/kg) to suppress cocaine-taking and -seeking, as well as sucrose-taking, is similar to that seen to suppress impulsivity assessed in a five-choice serial reaction time task (Navarra, et al., 2008) as well as aggressive behavior in rodents (Rosenzweig-Lipson, et al., 2007b). Cocaine use is associated with increased impulsivity in animals (Anastasio, et al., 2011; Stoffel and Cunningham, 2008) and cocaine-dependent subjects often present with impulsivity (Moeller, et al., 2004; Moeller, et al., 2002; Moeller, et al., 2001; Kjome, et al., 2010) and there is recent evidence to suggest that impulsivity contributes to the development and maintenance of obesity (Mobbs, et al., 2010) and binge eating disorder (Mobbs, et al., 2010). Thus, selective, high efficacy 5-HT2CR agonists may prove therapeutically useful in disorders characterized by impulsive traits.
The present observations support the assertion that selective activation of the 5-HT2CR signaling may prove therapeutically useful to reduce craving for cocaine and extend abstinence (Bubar and Cunningham, 2008). However, the progress of selective 5-HT2CR agonists from preclinical evidence of efficacy to verification of safety and efficacy as psychotherapeutic medications in humans has been slow. Clinical trials evaluating the first selective 5-HT2CR agonist, lorcaserin (APD 356, Arena Pharmaceuticals), for weight reduction were successfully completed (Smith, et al., 2009; Smith, et al., 2010). Lorcaserin, which exhibits ~15-fold and ~100-fold greater potency to stimulate the 5-HT2CR over the homologous 5-HT2AR and 5-HT2BR, respectively, effectively suppressed food intake, reduced weight gain, and was well tolerated by obese subjects (Smith, et al., 2009; Smith, et al., 2010). Further, when evaluated in polydrug users, the doses shown to be therapeutic in obesity (Smith, et al., 2009; Smith, et al., 2010) were well-tolerated, exhibited low abuse liability, and were not associated with notable neurocognitive or perceptual effects (Schram, et al., 2009). The selective 5-HT2CR agonist vabicaserin has been evaluated in clinical trials to establish its efficacy and safety in subjects with an acute exacerbation of schizophrenia. The development of two selective 5-HT2CR agonists which advanced to clinical trials opens the door to implementation of clinical research to evaluate these compounds as innovative pro-abstinence, anti-relapse therapeutics for stimulant addiction, eating disorders and obesity.
In conclusion, the 5-HT2CR is a functionally important regulator of the neural substrates that control the rewarding efficacy of a drug (cocaine) and natural (sucrose) reinforcer as well as the incentive-salience value of cocaine-associated cues. WAY 163909 is a potent, selective and efficacious 5-HT2CR agonist which presents a favorable efficacy index between doses useful in inhibiting self-administration/reinstatement and doses which suppress locomotor activity. This compound will prove useful to further tease apart the precise involvement of 5-HT2CR neurocircuitry in reward and motivation as well as the learning and memory processes that carry the encoding for associations between environmental (contextual) cues and consumption of rewarding stimuli (food or drug). A more complete preclinical evaluation of these questions will ultimately allow educated proof-of-concept trials to test the concept that selective 5-HT2CR agonists may be useful as adjunctive therapy in such chronic disorders marked by impulsive drug and/or food intake.
Research Highlights.
Selective 5-HT2CR activation potently decreases the reinforcing efficacy of cocaine and sucrose in freely-fed rats
The novel, selective 5-HT2CR agonist WAY 163909 differentially alters cue-evoked cocaine-, but not sucrose-, reinstatement
WAY 163909 suppresses these behaviors at doses ~5-12-fold lower than those predicted to suppress horizontal ambulation
ACKNOWLEDGEMENTS
This research was supported by the National Institute on Drug Abuse grants P20 DA024157 (KAC, SRG, FGM), R01 DA006511 (KAC), K02 DA000403 (FGM), K05 DA020087 (KAC), and the Jeane B. Kempner Postdoctoral Scholar Award (NCA).
Abbreviations
- 5-HT
serotonin
- 5-HT2XR
5-HT2X receptor
- CHO
Chinese Hamster Ovary
- FR
fixed ratio
- i.p.
intraperitoneal
- Veh
vehicle
- WAY
WAY 163909
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG, Cunningham KA. The serotonin 5-HT2A receptor: Association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol. 2011 Apr 14; doi: 10.1097/FBP.0b013e328345f90d. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43:315–317. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Tombros E, Rogers PJ, Latham CJ. Behavioural analysis of feeding: implications for the pharmacological manipulation of food intake in animals and man. Prog Neuropsychopharmacol. 1980;4:319–326. doi: 10.1016/0364-7722(80)90002-8. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, Mcneeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: A novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- Brookshire BR, Jones SR. Direct and indirect 5-HT receptor agonists produce gender-specific effects on locomotor and vertical activities in C57 BL/6J mice. Pharmacol Biochem Behav. 2009;94:194–203. doi: 10.1016/j.pbb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin(2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of IV amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology. 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Cifani C, Zanoncelli A, Tessari M, Righetti C, Di FC, Ciccocioppo R, Massi M, Melotto S. Pre-exposure to environmental cues predictive of food availability elicits hypothalamic-pituitary-adrenal axis activation and increases operant responding for food in female rats. Addict Biol. 2009;14:397–407. doi: 10.1111/j.1369-1600.2009.00152.x. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Dourish CT. Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 2000;152:256–267. doi: 10.1007/s002130000504. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Quentric Y, Carpentier N, Poissonnet G, Parmentier JG, Goldstein S, Millan MJ. Characterization of phospholipase C activity at h5-HT2C compared with h5-HT2B receptors: influence of novel ligands upon membrane-bound levels of [3H]phosphatidylinositols. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:242–252. doi: 10.1007/s00210-001-0505-y. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Marquis KL, Lim HK, Leung L, Kao J, Cheesman C, Rosenzweig-Lipson S. Pharmacological profile of the 5-HT(2C) receptor agonist WAY-163909; therapeutic potential in multiple indications. CNS Drug Rev. 2006;12:167–177. doi: 10.1111/j.1527-3458.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, Sukoff S, Vogel RL, Stack G, Schechter L, Harrison BL, Rosenzweig-Lipson S. WAY-163909 ((7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[ 6,7,1hi]indole): A novel 5-HT2C receptor selective agonist with anorectic activity. J Pharmacol Exp Ther. 2005;313:862–869. doi: 10.1124/jpet.104.075382. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacol. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) receptor agonist RO 60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine and contextual cues. Neuropsychopharmacol. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA. Genetic and pharmacological evidence that 5-HT2C receptor activation, but not inhibition, affects motivation to feed under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2010;97:170–178. doi: 10.1016/j.pbb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology. 2009;57:259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Grauer SM, Graf R, Navarra R, Sung A, Logue SF, Stack G, Huselton C, Liu Z, Comery TA, Marquis KL, Rosenzweig-Lipson S. WAY-163909, a 5-HT2C agonist, enhances the preclinical potency of current antipsychotics. Psychopharmacology (Berl) 2009;204:37–48. doi: 10.1007/s00213-008-1433-z. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Corrigall WA, Higgins GA. Activation of 5-HT2C receptors reduces the locomotor and rewarding effects of nicotine. Psychopharmacology (Berl) 2001;157:292–298. doi: 10.1007/s002130100801. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Halberstadt AL, van dH, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacol. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol. 2010;6:255–269. doi: 10.1038/nrendo.2010.19. [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA. Neuropharmacology of human appetite expression. Dev Disabil Res Rev. 2008;14:158–164. doi: 10.1002/ddrr.20. [DOI] [PubMed] [Google Scholar]
- Halford JC, Lawton CL, Blundell JE. The 5-HT2 receptor agonist MK-212 reduces food intake and increases resting but prevents the behavioural satiety sequence. Pharmacol Biochem Behav. 1997;56:41–46. doi: 10.1016/S0091-3057(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki M, Sasamata M, Miyata K. Agonist diversity in 5-HT(2C) receptor-mediated weight control in rats. Psychopharmacology (Berl) 2005;178:241–249. doi: 10.1007/s00213-004-2019-z. [DOI] [PubMed] [Google Scholar]
- Hewitt KN, Lee MD, Dourish CT, Clifton PG. Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol Biochem Behav. 2002;71:691–700. doi: 10.1016/s0091-3057(01)00709-2. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ouagazzal AM, Grottick AJ. Influence of the 5-HT(2C) receptor antagonist SB242,084 on behaviour produced by the 5-HT(2) agonist Ro60-0175 and the indirect 5-HT agonist dexfenfluramine. Br J Pharmacol. 2001;133:459–466. doi: 10.1038/sj.bjp.0704082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DM, Anden NE, Dahlstrom A. A functional effect of dopamine in the nucleus accumbens and in some other dopamine-rich parts of the rat brain. Psychopharmacologia. 1975;45:139–149. doi: 10.1007/BF00429052. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, Swann AC, Moeller FG. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res. 2010;178:299–304. doi: 10.1016/j.psychres.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell KR, Revell DF, Kennett GA, Bickerdike MJ. Pharmacological characterisation of the agonist radioligand binding site of 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn-Schmiedeberg's Arch Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the striaterminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Burton S, O'Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17:111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Friedle NM, Moore KE. Increased self-administration of d-amphetamine after destruction of 5-hydroxytryptaminergic neurons. Pharmacol Biochem Behav. 1980;12:937–941. doi: 10.1016/0091-3057(80)90456-6. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bos M, Jenck F, Moreau JL, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HHG, Broekkamp CLE, Ruigt GSF, Kohler C, Van Delft AML. 5-HT2C receptor agonists: Pharmacological characteristics and therapeutic potential. Journal of Pharmacology & Experimental Therapeutics. 1998;286:913–924. [PubMed] [Google Scholar]
- Mobbs O, Crepin C, Thiery C, Golay A, Van der LM. Obesity and the four facets of impulsivity. Patient Educ Couns. 2010;79:372–377. doi: 10.1016/j.pec.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Fischer CJ, Dougherty DM, Reilly EL, Mathias CW, Swann AC. P300 event-related potential amplitude and impulsivity in cocaine-dependent subjects. Neuropsychobiology. 2004;50:167–173. doi: 10.1159/000079110. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Nair SG, ms-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Comery TA, Graf R, Rosenzweig-Lipson S, Day M. The 5-HT(2C) receptor agonist WAY-163909 decreases impulsivity in the 5-choice serial reaction time test. Behav Brain Res. 2008;188:412–415. doi: 10.1016/j.bbr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. Stimulation of medial prefrontal cortex serotonin 2C 5-HT2C receptors attenuates cocaine-seeking behavior. Neuropsychopharmacol. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ratkowsky DA, Reedy TJ. Choosing near-linear parameters in the four-parameter logistic model for radioligand and related assays. Biometrics. 1986;42:575–582. [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH. Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci. 2002;22:10039–10045. doi: 10.1523/JNEUROSCI.22-22-10039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Dunlop J, Marquis KL. 5-HT2C receptor agonists as an innovative approach for psychiatric disorders. Drug News Perspect. 2007a;20:565–571. doi: 10.1358/dnp.2007.20.9.1162244. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE, Grauer S, Brennan J, Cryan JF, Sukoff Rizzo SJ, Dunlop J, Barrett JE, Marquis KL. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berl) 2007b;192:159–170. doi: 10.1007/s00213-007-0710-6. [DOI] [PubMed] [Google Scholar]
- Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Schram MJ, Schoedel KA, Barlett C, Shazer RL, Sellers EM. Abuse potential evaluation of lorcaserin, a selective and potent agonist at the serotonin 2C (5-HT2C) receptor, in healthy male and female recreational polydrug users. Amer. Coll. Neuropsychopharmacol 48th Annual Meeting; American College of Neuropsychopharmacology Annual Meeting 48th Annual Meeting.2009. [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan Me, Anderson CM, Shanahan WR Study Group. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 2009;17:494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Somerville EM, Horwood JM, Lee MD, Kennett GA, Clifton PG. 5-HT(2C) receptor activation inhibits appetitive and consummatory components of feeding and increases brain c-fosimmunoreactivity in mice. Eur J Neurosci. 2007;25:3115–3124. doi: 10.1111/j.1460-9568.2007.05567.x. [DOI] [PubMed] [Google Scholar]
- Steiger H. Eating disorders and the serotonin connection: state, trait and developmental effects. J Psychiatry Neurosci. 2004;29:20–29. [PMC free article] [PubMed] [Google Scholar]
- Stoffel EC, Cunningham KA. The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend. 2008;92:69–78. doi: 10.1016/j.drugalcdep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. New York: Springer-Verlag; 1987. [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors [see comments] Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA. An investigation of the role of 5-HT(2C) receptors in modifying ethanol self-administration behaviour. Pharmacol Biochem Behav. 2002;71:735–744. doi: 10.1016/s0091-3057(01)00710-9. [DOI] [PubMed] [Google Scholar]
- Tong Z, Chandrasekaran A, Demaio W, Espina R, Lu W, Jordan R, Scatina J. Metabolism of vabicaserin in mice, rats, dogs, monkeys, and humans. Drug Metab Dispos. 2010a;38:2266–2277. doi: 10.1124/dmd.110.033670. [DOI] [PubMed] [Google Scholar]
- Tong Z, Chandrasekaran A, Demaio W, Jordan R, Li H, Moore R, Poola N, Burghart P, Hultin T, Scatina J. Species differences in the formation of vabicaserincarbamoylglucuronide. Drug Metab Dispos. 2010b;38:581–590. doi: 10.1124/dmd.109.028639. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Bellew JG, Grote KA, Neisewander JL. Serotonin depletion attenuates cocaine seeking but enhances sucrose seeking and the effects of cocaine priming on reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;157:340–348. doi: 10.1007/s002130100822. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT2C receptor mutant mice. Psychopharmacology. 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The open-field test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Evidence for an involvement of 5-hydroxytryptaminergic neurones in the maintenance of operant behaviour by positive reinforcement. Psychopharmacology. 1991;105:119–124. doi: 10.1007/BF02316873. [DOI] [PubMed] [Google Scholar]
- Wu J, Zou H, Strong JA, Yu J, Zhou X, Xie Q, Zhao G, Jin M, Yu L. Bimodal effects of MK-801 on locomotion and stereotypy in C57BL/6 mice. Psychopharmacology. 2005;177:256–263. doi: 10.1007/s00213-004-1944-1. [DOI] [PubMed] [Google Scholar]