Abstract
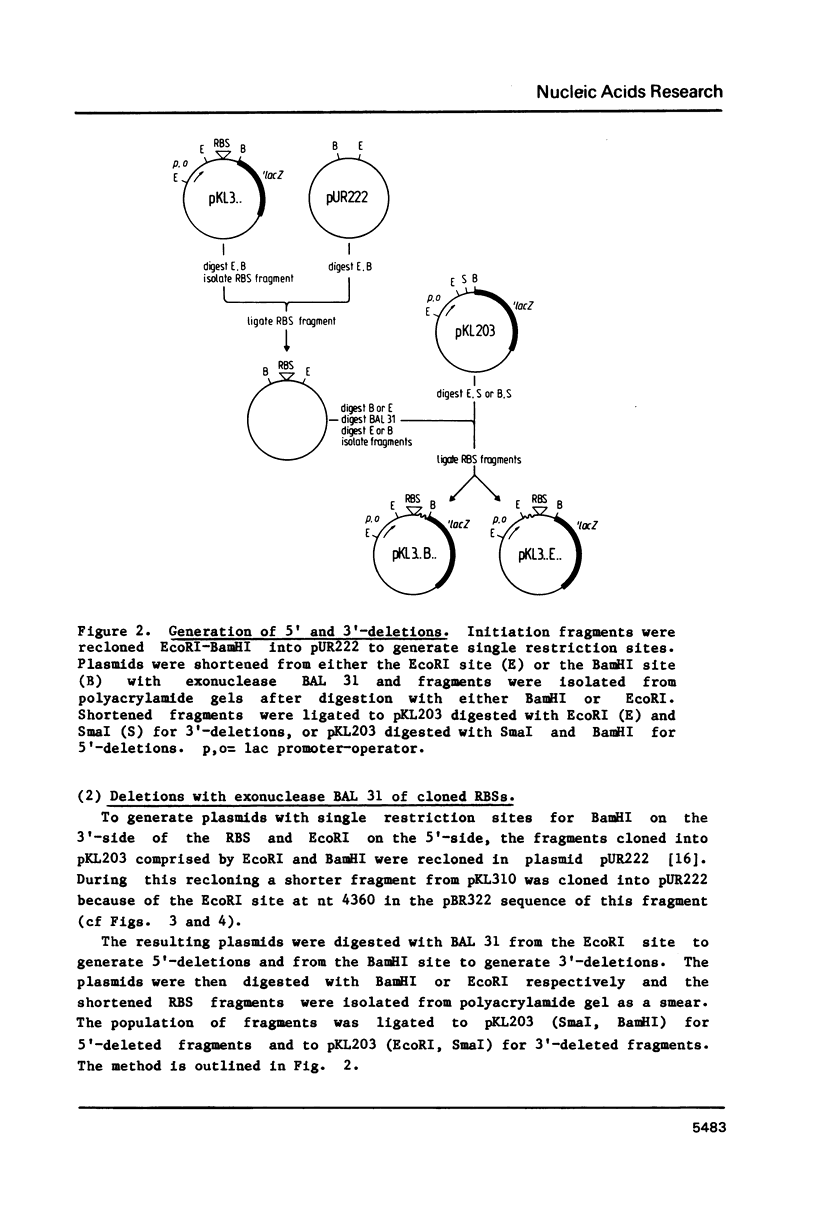
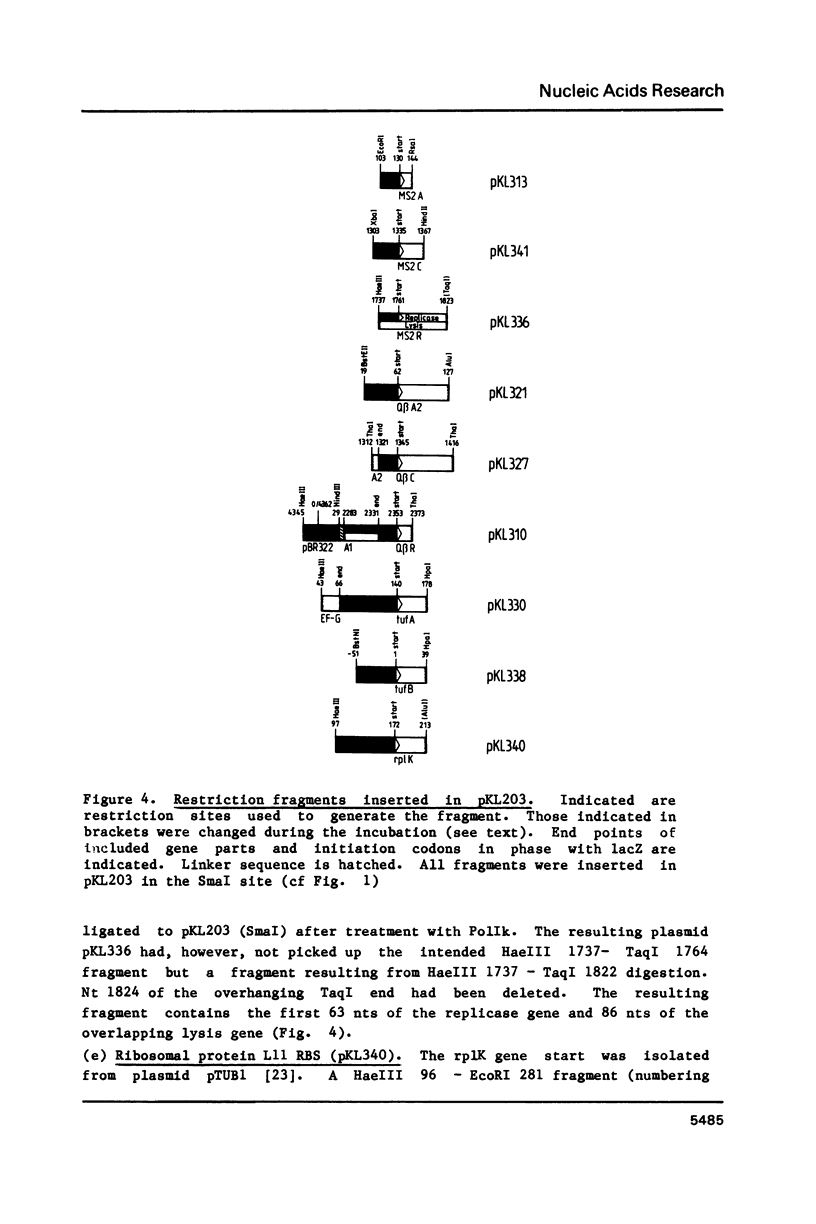
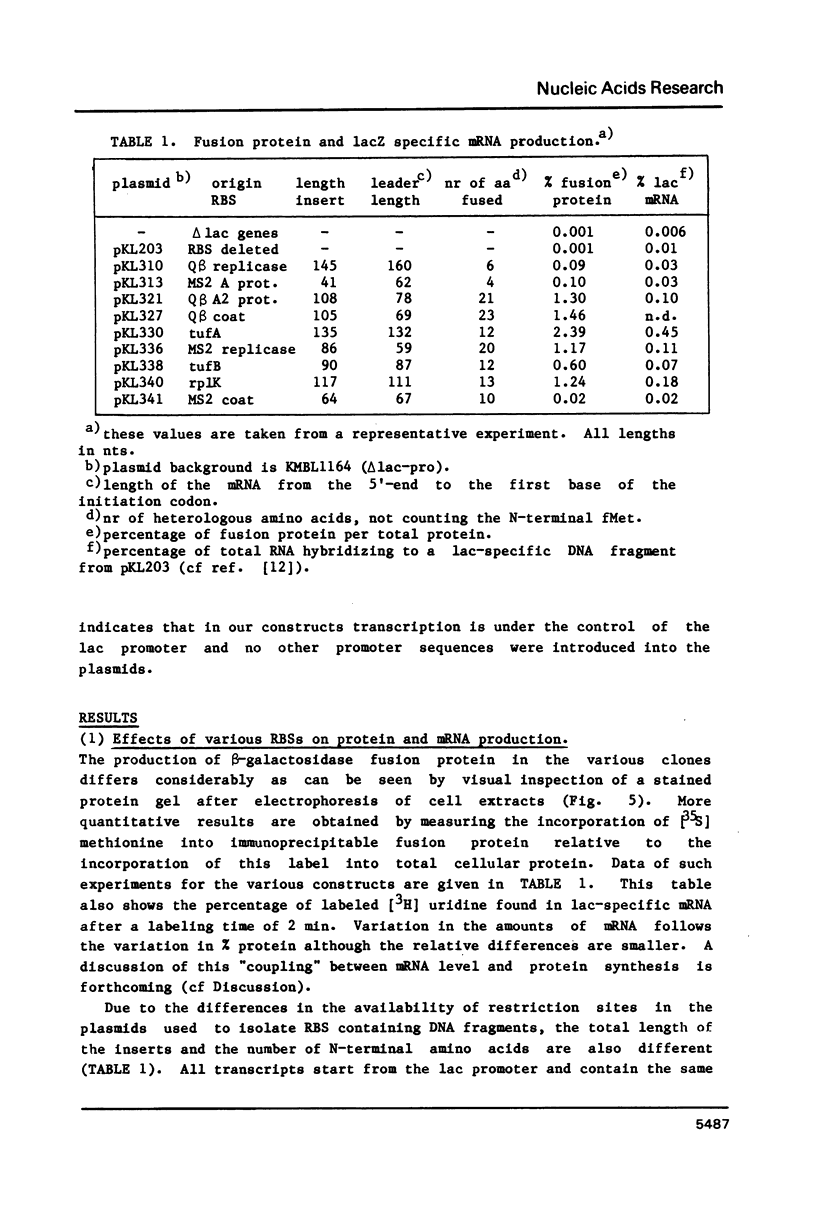
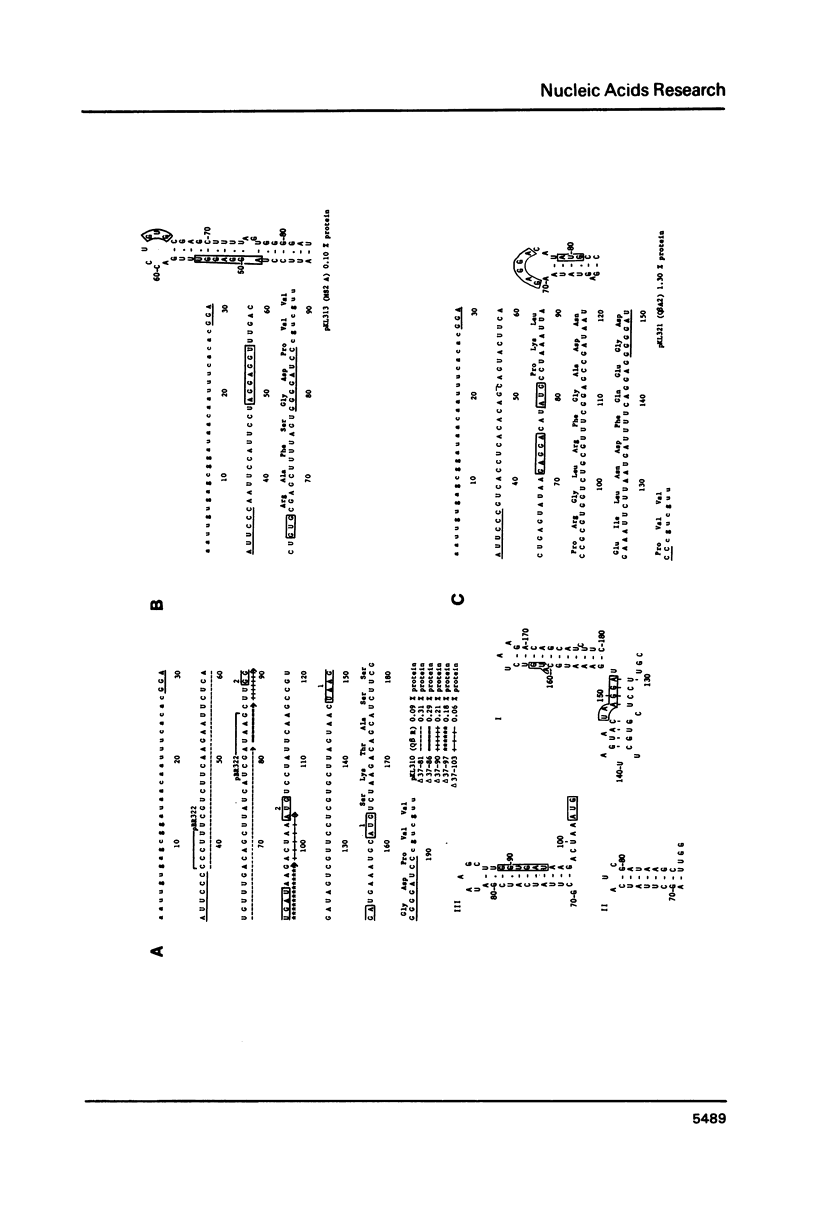
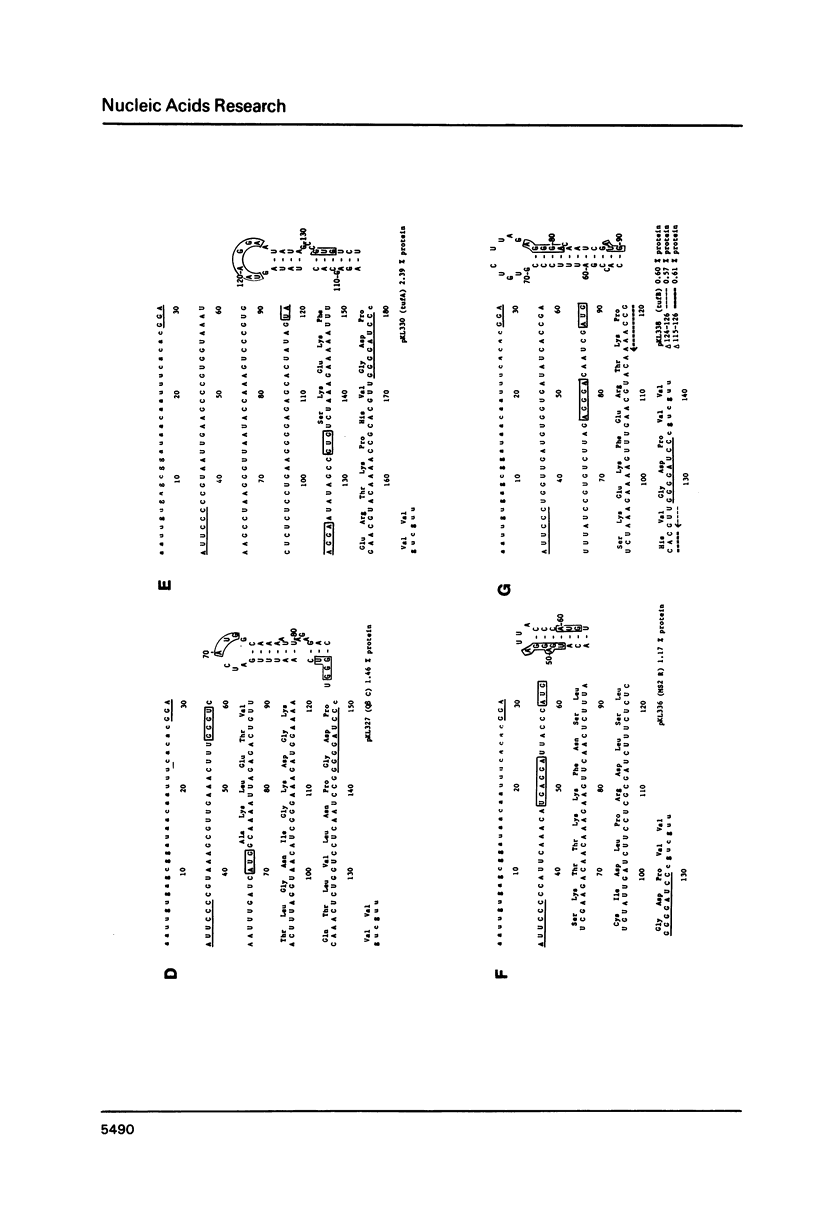
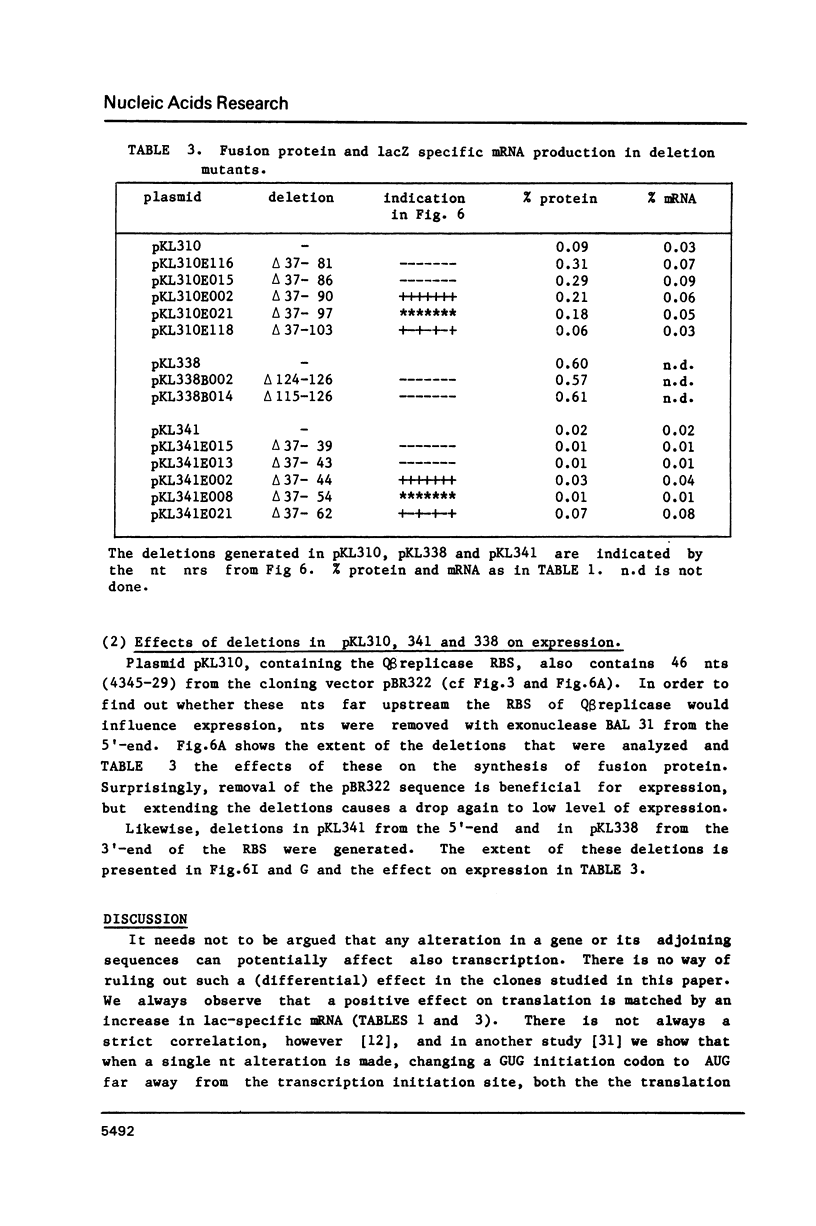
Using a previously described vector (pKL203) we fused several heterologous ribosomal binding sites (RBSs) to the lacZ gene of E. coli and then studied the variation in expression of the fusions. The RBSs originated from bacteriophage Q beta and MS2 genes and the E. coli genes for elongation factor EF-Tu A and B and ribosomal protein L11 (rplK). The synthesis of the lacZ fusion proteins was measured by an immuno precipitation method and found to vary at least 100-fold. Lac-specific mRNA synthesis follows the variation in protein production. It appears that there is a correlation between the efficiency of an RBS to function in the expression of the fused gene and the lack of secondary structure, involving the Shine and Dalgarno nucleotides (SDnts) and/or the initiation codon. This efficiency is context dependent. The sequence of the SD nts and the length and sequence of the spacer region up to the initiation codon alone are not able to explain our results. Deletion mutations, created in the phage Q beta replicase RBS, reveal a complex pattern of control of expression, probably involving the use of a "false" initiation site.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonekamp F., Clemmesen K., Karlström O., Jensen K. F. Mechanism of UTP-modulated attenuation at the pyrE gene of Escherichia coli: an example of operon polarity control through the coupling of translation to transcription. EMBO J. 1984 Dec 1;3(12):2857–2861. doi: 10.1002/j.1460-2075.1984.tb02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G., Schulz M. F., Selzer G., Chollet A., Movva N. R., Semon D., Escanez S., Kawashima E. Optimizing the expression in E. coli of a synthetic gene encoding somatomedin-C (IGF-I). Nucleic Acids Res. 1985 Mar 25;13(6):1923–1938. doi: 10.1093/nar/13.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S., Dreyfus M. Segment-specific mutagenesis of the regulatory region in the Escherichia coli galactose operon: isolation of mutations reducing the initiation of transcription and translation. Gene. 1983 Jan-Feb;21(1-2):121–131. doi: 10.1016/0378-1119(83)90154-3. [DOI] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. Escherichia coli lac operator mRNA affects translation initiation of beta-galactosidase mRNA. Nature. 1979 Feb 1;277(5695):407–409. doi: 10.1038/277407a0. [DOI] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. Evidence that the 5' end of lac mRNA starts to decay as soon as it is synthesized. J Bacteriol. 1985 Feb;161(2):820–822. doi: 10.1128/jb.161.2.820-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt H., Lührmann R. Recognition by initiator transfer ribonucleic acid of a uridine 5' adjacent to the AUG codon: different conformational states of formylatable methionine-accepting transfer ribonucleic acid at the ribosomal peptidyl site. Biochemistry. 1981 Apr 14;20(8):2075–2080. doi: 10.1021/bi00511a002. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Fraser A. R., Neilson T. Nucleotides contiguous to AUG affect translational initiation. Biochemistry. 1978 Jul 11;17(14):2769–2775. doi: 10.1021/bi00607a011. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gren E. J. Recognition of messenger RNA during translational initiation in Escherichia coli. Biochimie. 1984 Jan;66(1):1–29. doi: 10.1016/0300-9084(84)90188-3. [DOI] [PubMed] [Google Scholar]
- Grundström T., Normark S. Initiation of translation makes attenuation of ampC in E. coli dependent on growth rate. Mol Gen Genet. 1985;198(3):411–415. doi: 10.1007/BF00332931. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Hui A., Hayflick J., Dinkelspiel K., de Boer H. A. Mutagenesis of the three bases preceding the start codon of the beta-galactosidase mRNA and its effect on translation in Escherichia coli. EMBO J. 1984 Mar;3(3):623–629. doi: 10.1002/j.1460-2075.1984.tb01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Karnik S., Billeter M. The lysis function of RNA bacteriophage Qbeta is mediated by the maturation (A2) protein. EMBO J. 1983;2(9):1521–1526. doi: 10.1002/j.1460-2075.1983.tb01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R., Carey J., Yanofsky C. Translation activates the paused transcription complex and restores transcription of the trp operon leader region. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4663–4667. doi: 10.1073/pnas.82.14.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looman A. C., de Gruyter M., Vogelaar A., van Knippenberg P. H. Effects of heterologous ribosomal binding sites on the transcription and translation of the lacZ gene of Escherichia coli. Gene. 1985;37(1-3):145–154. doi: 10.1016/0378-1119(85)90267-7. [DOI] [PubMed] [Google Scholar]
- Looman A. C., van Knippenberg P. H. Effects of GUG and AUG initiation codons on the expression of lacZ in Escherichia coli. FEBS Lett. 1986 Mar 3;197(1-2):315–320. doi: 10.1016/0014-5793(86)80349-0. [DOI] [PubMed] [Google Scholar]
- Matteucci M. D., Heyneker H. L. Targeted random mutagenesis: the use of ambiguously synthesized oligonucleotides to mutagenize sequences immediately 5' of an ATG initiation codon. Nucleic Acids Res. 1983 May 25;11(10):3113–3121. doi: 10.1093/nar/11.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Kaziro Y. The expression of the cloned tufB gene in vivo. FEBS Lett. 1980 Oct 6;119(2):215–218. doi: 10.1016/0014-5793(80)80255-9. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. V. Analysis of the proteins synthesized in ultraviolet light-irradiated Escherichia coli following infection with the bacteriophages lambdadrifd 18 and lambdadfus-3. Mol Gen Genet. 1976 Mar 30;144(3):339–343. doi: 10.1007/BF00341733. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Waele P. D., Marmenout A., Stanssens P., Fiers W. Functional expression of individual plasmid-coded RNA bacteriophage MS2 genes. EMBO J. 1982;1(2):205–209. doi: 10.1002/j.1460-2075.1982.tb01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland K. L., Powell F. E., Turnbough C. L., Jr Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):991–999. doi: 10.1128/jb.163.3.991-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J. L., Sninsky J. J., Cohen S. N. Effects of alterations in the translation control region on bacterial gene expression: use of cat gene constructs transcribed from the lac promoter as a model system. Gene. 1984 May;28(2):177–193. doi: 10.1016/0378-1119(84)90255-5. [DOI] [PubMed] [Google Scholar]
- Shepard H. M., Yelverton E., Goeddel D. V. Increased synthesis in E. coli of fibroblast and leukocyte interferons through alterations in ribosome binding sites. DNA. 1982;1(2):125–131. doi: 10.1089/dna.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Weissmann C. Inhibition of Qbeta RNA 70S ribosome initiation complex formation by an oligonucleotide complementary to the 3' terminal region of E. coli 16S ribosomal RNA. Nature. 1978 Oct 26;275(5682):770–772. doi: 10.1038/275770a0. [DOI] [PubMed] [Google Scholar]
- Varenne S., Buc J., Lloubes R., Lazdunski C. Translation is a non-uniform process. Effect of tRNA availability on the rate of elongation of nascent polypeptide chains. J Mol Biol. 1984 Dec 15;180(3):549–576. doi: 10.1016/0022-2836(84)90027-5. [DOI] [PubMed] [Google Scholar]
- Wood C. R., Boss M. A., Patel T. P., Emtage J. S. The influence of messenger RNA secondary structure on expression of an immunoglobulin heavy chain in Escherichia coli. Nucleic Acids Res. 1984 May 11;12(9):3937–3950. doi: 10.1093/nar/12.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Hui A., Wong E., Vasser M. Portable Shine-Dalgarno regions; nucleotides between the Shine-Dalgarno sequence and the start codon affect the translation efficiency. Gene Amplif Anal. 1983;3:103–116. [PubMed] [Google Scholar]
- van der Meide P. H., Vijgenboom E., Talens A., Bosch L. The role of EF-Tu in the expression of tufA and tufB genes. Eur J Biochem. 1983 Feb 1;130(2):397–407. doi: 10.1111/j.1432-1033.1983.tb07166.x. [DOI] [PubMed] [Google Scholar]